Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 17
Practical Use of Upadacitinib in Patients with Severe Atopic Dermatitis in a Real-World Setting: A Systematic Review
Authors Ibba L, Gargiulo L, Vignoli CA, Fiorillo G, Valenti M, Costanzo A, Narcisi A
Received 10 January 2024
Accepted for publication 24 February 2024
Published 12 March 2024 Volume 2024:17 Pages 593—604
DOI https://doi.org/10.2147/CCID.S329442
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Luciano Ibba,1,2 Luigi Gargiulo,1,2 Carlo Alberto Vignoli,1,2 Giovanni Fiorillo,1,2 Mario Valenti,1,2 Antonio Costanzo,1,2 Alessandra Narcisi1
1Dermatology Unit, IRCCS Humanitas Research Hospital, Milan, Italy; 2Department of Biomedical Sciences, Humanitas University, Milan, Italy
Correspondence: Luciano Ibba, Department of Biomedical Sciences, Humanitas University, Rozzano, Milan, 20089, Italy, Email [email protected]
Abstract: Upadacitinib is a selective Janus kinase inhibitor approved for the treatment of severe atopic dermatitis (AD). This systematic review aims to summarize the most recent data in terms of effectiveness and safety of upadacitinib in the treatment of severe AD in a real-world setting. The review included a comprehensive search of databases, including PubMed, Google Scholar and Web of Science, according to Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guidelines. The literature search initially identified 242 studies. Of these, 214 were excluded after reviewing their titles and abstracts. We then conducted a full-text review of 25 studies, of which 17 met our inclusion criteria and were therefore included in our systematic review. The analysis of real-world studies showed high effectiveness of upadacitinib, in terms of both clinical signs and subjective symptoms, in different patient populations, including those resistant to other treatments. No new significant safety concerns have emerged as compared to randomized clinical trials.
Keywords: JAKi, real-life, upadacitinib, atopic dermatitis, systematic review
Introduction
Atopic Dermatitis
Atopic dermatitis (AD) is one of the most prevalent inflammatory dermatological conditions, affecting both children and adults with a significant impairment of quality of life and work productivity.1,2 During the last ten years, the pathogenesis of AD has been deeply explored, and interleukins (ILs) such as IL-4, IL-13 and IL-31 have emerged as the pivotal cytokines in the inflammatory cascade of AD, along with barrier defects such as filaggrin mutations.3
Novel Treatment Options for Severe Atopic Dermatitis
A better understanding of AD pathophysiology has led to the development of several new drugs that selectively target the key molecules responsible for this disease. In particular, monoclonal antibodies, which block IL-13 and IL-4 (dupilumab) or IL-13 only (tralokinumab and lebrikizumab), are currently indicated for the treatment of severe AD.4 Another class of systemic treatments for adults with severe AD is represented by the inhibitors of Janus Kinase (JAKi), including abrocitinib, baricitinib and upadacitinib.4 These three drugs have shown efficacy and safety in phase-III clinical trials, compared with placebo and therefore have been approved for the treatment of severe AD.5–7 Upadacitinib and abrocitinib have also demonstrated superior rapidity and effectiveness compared with dupilumab in head-to-head clinical trials.8–10 To date, several real-world experiences are available regarding the effectiveness and safety of upadacitinib, while only a few studies have been published on abrocitinib and baricitinib in the treatment of severe AD.11,12 Regarding baricitinib, real-life studies have been recently published on its effectiveness also in the treatment of alopecia areata.13,14 According to European Guidelines, both JAKi and IL inhibitors, along with cyclosporine, are recommended in adults with severe AD.4 Abrocitinib, baricitinib, cyclosporin, dupilumab, tralokinumab and upadacitinib are all indicated as systemic treatments for severe AD in adult patients, with the same grade of recommendation, according to European Guidelines.4 However, there is no specific indication regarding the place in therapy of JAKi, including their possible role as a first or second-line systemic treatment. Moreover, recently the Pharmacovigilance Risk Assessment Committee (PRAC) of EMA published a reassessment (EMA/PRAC/68283/2022) of the benefit–risk balance of oral JAKi, with increasing concerns regarding their safety profiles in patients with AD.15 In addition, current guidelines do not give enough evidence on patients’ management during treatment with JAKi regarding laboratory and clinical monitoring or how to handle concomitant medical procedures. In this setting, the summary of product characteristics (SmPC) of each drug can give the clinicians a little information. However, of course, these are very limited and do not cover all the possible issues that can emerge when managing these patients.16–18
Upadacitinib
Upadacitinib, a selective JAK inhibitor primarily targeting JAK1, has been approved for the treatment of severe AD in both adolescents and adults at two different daily dosages, 15 and 30 mg. This approval followed its evaluation in multiple phase-3 clinical trials where it demonstrated efficacy superior to both placebo and dupilumab after 16 weeks.5,8,19
Materials and Methods
Search Strategy
We conducted a systematic review of the English-language medical literature utilizing PubMed, Google Scholar and Web of Science databases from the earliest records through October 2023.
The search strategy was performed using the following key terms: “upadacitinib” and “atopic dermatitis or atopic eczema” not “Review”. Titles and abstracts of all identified studies underwent screening with the established selection criteria.
This systematic review follows the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram from the PRISMA statement (Figure 1).20
 |
Figure 1 PRISMA Flowchart of study selection. The diagram illustrates the process used to retrieve, assess, and either select or exclude relevant studies from the databases. Adapted from Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. Open Med. 2009;3(3):e123–e130. doi:10.1136/bmj.327.7423.1083.20 |
Selection Criteria
Articles were selected for full-text review by evaluating their titles and abstracts, with selection criteria based on their relevance to the issue of interest. The following inclusion criteria were applied with no restriction to the dosage of upadacitinib: patients with severe atopic dermatitis; identification of effectiveness data from real-world studies or case series; identification of safety data from real-world studies or case series. In particular, effectiveness was assessed by the reduction in mean/median EASI (Eczema Area and Severity Index), the proportion of patients who achieved a reduction of 75%, 90% and 100% of EASI compared with baseline (EASI 75, EASI 90, EASI 100, respectively) and the mean/median reduction in itch-Numerical Rating Scale (NRS).
We excluded case series in which the effectiveness outcomes were not reported. Clinical trials and post hoc analyses were excluded. Systematic reviews and meta-analyses were excluded from the research.
Data Extraction
Two researchers (L.I. and C.A.V.) extracted data independently from the refined papers (Tables 1 and 2), and any discrepancies were resolved by a third author (L.G.). The following information was extracted from each article: name of first author, year of publication, type of study, sample size, effectiveness outcomes, safety findings and relevant comments about the paper. Authors were not contacted to provide further information.
 |
Table 1 Baseline Characteristics of Reviewed Studies |
 |
Table 2 Effectiveness Outcomes of Reviewed Studies |
Results
A total of 242 studies were retrieved from the literature search after removal of duplicates (Figure 1). Two hundred and fourteen were excluded based on their title and abstracts. The full text of 25 studies was reviewed, of which 17 met the inclusion criteria, and were included in our study. The main reason for the exclusion of articles was the lack of clear effectiveness outcomes. Data from 660 patients were included in the review. The most common type of study was observational retrospective, and most of the articles were published in 2023 (Table 1).
Effectiveness
Only five studies reached a follow-up of at least 48/52 weeks, while the other 12 reached a follow-up of 16 weeks (Table 2).
An observational retrospective multicenter study by Melgosa Ramos et al21 showed high effectiveness of upadacitinib through 52 weeks of treatment. In particular, the mean EASI decreased from 19.8 to 3.1 after 4 weeks, 0.9 after 16 weeks, 0.6 after 24 weeks, 0.8 after 32 weeks and 0.6 after one year of follow-up. At the same time points, the Authors also observed improvement in subjective symptoms with an itch-NRS that went from 7.6 at baseline to 1.5 after 4 weeks, 1.1 after 16 weeks, 0.9 after 24 weeks, 0.8 after 32 weeks and 1.4 after 52 weeks of follow-up. Moreover, all patients in this study achieved an EASI 75 response after just 4 weeks of treatment with upadacitinib.
De Greef et al22 conducted a retrospective cohort study including patients with severe atopic dermatitis treated with upadacitinib 15 or 30 mg. In this study, an EASI 75 response was obtained by 58.6% and 82.70% of patients after 12/16 weeks and 52 weeks of treatment, respectively. In addition, 58.60% of patients achieved an EASI 90 after one year.
In a prospective multicenter study of 146 patients conducted by Chiricozzi et al.23 EASI 75 was reached by 78.2%, 92.0%, and 87.6% of patients after 16, 32, and 48 weeks of treatment. At the same time points, 47.6%, 71.4% and 69.1% of patients achieved EASI 90. A complete skin clearance was observed in 28.2%, 51.80%, and 44.3% of patients after 16, 32, and 48 weeks, respectively. In this study, the mean EASI decreased consistently throughout the study period (from 25.0 at baseline to 5.4 after 16 weeks, 2.0 after 32 weeks, and 2.6 after 48 weeks). Moreover, the mean itch-NRS decreased significantly, from 7.1 before the start of upadacitinib to 1.4 after 48 weeks of follow-up.
Gargiulo et al24 showed an excellent response to upadacitinib in adults with severe atopic dermatitis over 52 weeks of follow-up. In particular, after 16 weeks of treatment, 85.7%, 71.4% and 55.6% of patients achieved EASI 75, EASI 90, and EASI 100, respectively. After one year of treatment, 87.9%, 75.8% and 57.6% reached EASI 75, EASI 90 and EASI 100, respectively. Subjective symptom improvement was also excellent, with 81.8% of patients achieving at least a 4-point reduction in itch-NRS from baseline after 52 weeks of treatment.
Gori et al25 performed a retrospective analysis of eight patients with AD involving sensitive areas (head/neck, genitalia and hands) who were resistant to dupilumab and treated with upadacitinib 15 or 30 mg. At baseline, the mean EASI score was 17.30, which decreased to 1.2 after 4 weeks and 0.5 after 1 year of treatment. Complete skin clearance (EASI 100) was observed in five patients (62.5%) at weeks 4 and 52, while the remaining three patients had minimal residual disease (EASI ≤ 1). In terms of subjective symptoms, the mean itch-NRS decreased from 6.1 at baseline to 0.7 at week 4 and 0 at week 52.
According to a real-life study by Napolitano et al,26 upadacitinib showed a rapid improvement in pruritus after 4 and 16 weeks of treatment. In particular, the mean itch-NRS at baseline was 8.9, which decreased to 0.3 at week 4 and 0.2 at week 16. The Authors also found excellent effectiveness of upadacitinib in terms of mean EASI, which decreased from 27.2 at baseline to 7.3 at week 4 and 3.3 at week 16. In addition, 77.78% of patients achieved EASI 75 after 16 weeks of treatment with upadacitinib.
Patruno et al27 published a case series of four adolescents with severe AD treated with upadacitinib 15 mg. The mean EASI score decreased from 27.5 at baseline to 9.75 at week 4 and 2.25 after 16 weeks of treatment. Mean itch-NRS decreased from 9.25 to 1.50 and 0.75 at the same time points.
In an Australian retrospective cohort study by Tran et al,28 the mean EASI score at baseline was 27.3. After 3 months of treatment with upadacitinib, the mean EASI score was 6.8, and 60% of patients achieved EASI 75.
In a 16-week prospective multicenter study by Chiricozzi et al.29 After 4 weeks of treatment with upadacitinib, 88.5%, 42.3% and 23.1% of patients reached EASI 75, EASI 90 and EASI 100, respectively. At week 16, almost all patients (97.5%) achieved EASI 75, while 82.1% and 69.2% of patients achieved EASI 90 and EASI 100, respectively.
Similar results were reported in a 16-week retrospective study by Gargiulo et al30 in which the EASI score decreased from a mean of 17.48 at baseline to 2.56 at week 8 and 1.45 after 16 weeks. At week 8, the percentages of EASI 75, EASI 90 and EASI 100 responses were 76.32%, 55.26%, and 42.11%, respectively. At week 16, out of 35 patients, 91.43%, 74.29%, and 60% of them achieved EASI 75, EASI 90, and EASI 100, respectively. At the same time points, the Authors also observed a significant impact on itch-NRS, which decreased from a mean of 8.42 at baseline to 1.05 at week 8 and 1.03 at week 16.
In a multicenter retrospective study, Georgakopoulos et al31 showed high effectiveness of upadacitinib even in patients who had previously failed dupilumab. The mean EASI score decreased from 16.7 at baseline to 1.4 at week 16. At the same time point, EASI 75 or IGA 0/1 was achieved by 76.9% of patients. Complete skin clearance (EASI 100 or IGA 0) was achieved by 56.4% of patients after 16 weeks of treatment with upadacitinib.
Boesjes et al32 conducted a multicenter prospective study to evaluate the effectiveness of upadacitinib in patients with severe AD, including those who have failed dupilumab and/or baricitinib. The mean EASI score improved from 16.6 at baseline to 7.5 after 4 weeks and 5.7 at week 16, while an IGA of 0 or 1 was reached by 36.2% and 32.9% of patients at the same time points. Regarding subjective symptoms, the authors observed a significant reduction of mean itch-NRS, which decreased from 7.0 at baseline to 3.9 at week 4 and 3.7 at week 16.
Two studies by Hagino et al33,34 were included in this systematic review. The first one was a retrospective analysis of 31 patients affected by severe AD treated with upadacitinib 15 mg plus twice/daily topical corticosteroids.33 At baseline, the mean EASI score was 23.4. An EASI75 achievement rate of 51.6% and 67.7% was observed at weeks 4 and 12, respectively, and an EASI 90 in 22.6% and 41.9% at weeks 4 and 12, respectively. The median IGA score at baseline was 3, and a reduction to IGA 0/1 was observed in 16.7% and 36.7% at weeks 4 and 12, respectively. The second publication by Hagino et al34 consisted of a retrospective study conducted on 39 Japanese adolescent patients (aged 12 to 17 years) suffering from severe AD treated with oral upadacitinib 15mg/day plus twice daily topical corticosteroids. The median EASI at baseline amounted to 21.3. It reached 4.4 at weeks 4 and 12. The median IGA at baseline was 3, and it decreased to 1 at weeks 4 and 12. A reduction of median PP-NRS was observed, descending from 8 at baseline to 3 and 2 at weeks 4 and 12, respectively. The achievement rates at weeks 4 and 12 were respectively 64.1% and 62.5% for EASI 75, 12.8% and 15.6% for EASI 90, 12.8% and 15.6% for EASI 100, and 11.6% and 18.6% for IGA 0/1.
A retrospective two-center study by Napolitano et al35 of 16 adults with severe AD treated with upadacitinib 30 mg showed a significant reduction in mean EASI score (from 25.88 at baseline to 9.56 at week 4 and 4.31 at week 16). The improvement in clinical signs was accompanied by a reduction in subjective symptoms. The mean itch-NRS decreased from 8.44 at baseline to 2.69 and 1.19 at the same time points.
A retrospective multicentric study on a population of 43 patients affected by AD treated with 15- or 30-mg daily doses of upadacitinib was conducted by Pereyra-Rodriguez et al.36 Upadacitinib 30mg was prescribed in the majority of patients (60.4%), 39.5% received topical corticosteroids, 6.9% received oral corticosteroids during weeks 0–4, and 2.3% of the patients received oral corticosteroids during weeks 4–16. The mean EASI was 24.9 at baseline and decreased to 4.1 at week 16. The EASI 75 was achieved by 76.7% of patients, and the EASI 90 was achieved by 51.1% after 16 weeks of treatment with upadacitinib. At baseline, the mean PP-NRS amounted to 8.0 and reached 2.5 at week 16. Most patients had an IGA score of 4 at baseline (62.7%), while after 16 weeks, most patients had an IGA score of 0/1 (62.8%).
Vanlerberghe et al37 published a multicenter French real-world study to evaluate the effectiveness of JAK inhibitors for the treatment of severe AD. After three months of treatment, the Authors found that 35.7% and 7.1% of patients achieved EASI 75 and EASI 90, respectively, with the dosage of 15 mg, while 57.1% reached EASI 75 and EASI 90 with upadacitinib 30 mg. Only a small proportion of patients reached 6 months of treatment, with 37.5% and 100% of patients achieving EASI 75 with upadacitinib 15 and 30 mg, respectively.
Safety
A safety evaluation was performed in 16 articles out of 17 included in this review, as a retrospective study was not included because it did not report AEs.26 Among the articles analyzed, a total of 359 AEs were reported (Tables 3 and 4).
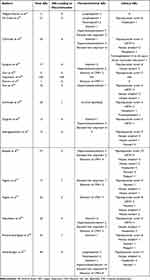 |
Table 3 Safety Evaluation of Reviewed Studies. The Table Specified the Most Common and Relevant Adverse Events |
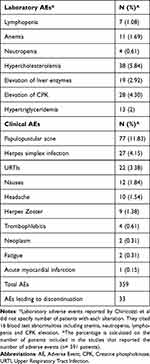 |
Table 4 Most Frequent Clinical and Laboratory Adverse Events |
The most common clinical AE reported was papulopustular acne, which was found in 77 patients, followed by Herpes Simplex infections (n = 27), upper respiratory tract infections (n = 22), nausea (n = 12), headache (n = 10) and Herpes Zoster (n = 9). Moreover, four patients experienced thrombophlebitis, including three patients with multiple risk factors for thrombosis.
Among laboratory AEs, hypercholesterolemia was the most reported (n = 38), followed by elevation of creatine phosphokinase (CPK) (n = 28), elevation of liver enzymes (n = 19), hypertriglyceridemia (n = 13), anemia (n = 11) and lymphopenia (n = 7). One study by Chiricozzi et al reported 16 blood test abnormalities, including anemia, neutropenia, lymphopenia and CPK elevation, without specifying the number of patients with each alteration.29
Moreover, 33 AEs led to discontinuation of the treatment with upadacitinib.
In the two studies by Chiricozzi et al, six patients discontinued the treatment due to AEs. Two patients had thrombophlebitis, two were diagnosed with a neoplasm (one with metastatic pancreatic carcinoma and one with bladder metaplasia), one had a myocardial infarction, and one patient experienced limb heaviness and pain associated with diffuse swelling.23,29
In a French retrospective observational study, four patients discontinued treatment with upadacitinib due to cytolysis, hypercholesterolemia, dyspnea, and a facial papular eruption.37 In the cohort studied by Napolitano et al,35 three patients discontinued treatment due to anemia, restless leg syndrome, and elevated blood CPK. In a Spanish retrospective observational study, one patient discontinued treatment due to asthenia and weakness.36 AD flares led to discontinuation in three patients within the cohorts of subjects studied by Hagino et al33,34 Among the 47 patients from the Dutch BioDay Registry, seven patients interrupted the treatment with upadacitinib for various reasons, including dyspnea, nausea, recurrent herpes simplex infections, reactive lymphoid infiltrate, the elevation of liver enzymes, a combination of headache and acne, and finally, a combination of headache, nausea, and acne.32
In an Australian real-world study by Tran et al, three patients experienced AEs that led to discontinuation of upadacitinib (one because of eczema herpeticum, one because of herpes zoster ophthalmicus and one because of worsening of his acne).28
In the 52-week retrospective study by Gargiulo et al, six patients discontinued upadacitinib due to AEs.24 Two patients experienced a severe manifestation of herpes zoster, three patients interrupted the treatment because of blood test abnormalities and one due to unplanned pregnancy.
Discussion
To the best of our knowledge, this is the first systematic review aiming to provide real-world data, specifically on patients’ characteristics, treatment regimens, clinical effectiveness and safety related to upadacitinib for the treatment of severe AD.
In most real-world studies, the patient cohorts were slightly older than in randomized clinical trials (RCTs), except for two studies that included adolescent patients.27,34 Moreover, the baseline level of disease activity was lower, as the mean EASI scores in Measure Up 1 and 2 were higher than in the studies included in this systematic review.5 The number of patients treated with upadacitinib 15 mg was comparable to the number of patients treated with upadacitinib 30 mg.
In terms of effectiveness, the percentage of patients achieving EASI 75 in most of the real-world studies analyzed was higher compared to the data from the RCTs. In the Measure Up 1 and Measure 2 clinical trials, EASI 75 was achieved by 69.6% and 60.1% of patients treated with upadacitinib 15 mg and 79.7% and 72.9% of patients treated with upadacitinib 30 mg, respectively, after 16 weeks of treatment.5 The percentages of patients achieving EASI 75 in the AD Up clinical trial were 64.6% and 77.1% for upadacitinib 15mg and 30mg, respectively.19 In the Phase 3b Heads Up clinical trial comparing the efficacy of upadacitinib 30 mg with dupilumab, the rate of EASI 75 after 16 weeks of treatment was comparable to other RCTs (71% of patients).8
The percentage of patients reaching EASI 90 and complete skin clearance (EASI 100) was also comparable or slightly better in most real-world studies than in RCTs. After 16 weeks of treatment with upadacitinib 30 mg, EASI 90 was achieved by 63.1% and 60.6% of patients in the AD Up and Heads Up clinical trials, respectively. Complete skin clearance was achieved by 22.6% and 27.9% of patients in the same clinical trials.8,19
In this systematic review, there was significant heterogeneity regarding the effectiveness data, likely due to differences in study methods, such as baseline patient characteristics and sample size. However, improvement in disease activity was often observed within 16 weeks of starting upadacitinib and was sustained over time, as also confirmed by an open-label extension study of the Heads Up trial.38
Real-world studies also confirmed the effectiveness of upadacitinib among different patient phenotypes, as it showed comparable responses in those with prominent involvement of the head/neck district or hands.24,25 Recently, upadacitinib has also been successfully used for the treatment of concomitant psoriasis and AD due to its broader action on inflammatory cytokines involved in both diseases.39
Regarding safety, papulopustular acne, herpes simplex infection, and upper respiratory tract infection were the most common AEs observed in the real-world setting. No new safety concerns have been identified despite the large number of patients who are older and have more comorbidities than those enrolled in RCTs. The number of AEs of special interest (including malignancies, major adverse cardiovascular events, deep vein thrombosis and serious infections) also remained stable, with only two real-world studies reporting thrombophlebitis in patients older than 65 years.23,29 According to EMA recommendations, JAKi should be used cautiously in patients older than 65 years, and IL-13 or IL-4/13 inhibitors should be primarily considered in this cohort due to their different safety profiles, according to clinical trials and real-world experiences.40–42 However, some of the studies analyzed in this review also included patients older than 65 without significant safety findings, which is consistent with a recent case series on seven over-65 patients treated with upadacitinib for at least 32 weeks.43
According to this systematic review, patients who may benefit most from upadacitinib are those with high levels of pruritus and AD affecting sensitive areas such as the face/neck, hands and genitalia. Upadacitinib has been effective in patients with features of both psoriasis and AD, likely due to the broad spectrum of action of JAKi on cytokines involved in the pathogenesis of both conditions. Although data are limited, JAKi, and in particular upadacitinib, could be considered for patients with concomitant asthma or allergic rhinitis, especially those with mild to moderate symptoms.44 This systematic review has a few limitations. First, the reliability of the summary of a systematic review depends on the strength of the methods used in each underlying study. Second, real-world studies have inherent biases, such as the selection bias and the presence of confounders. Third, the heterogeneity of all included studies, in terms of concomitant AD treatment, wash-out from previous therapies and dosage of upadacitinib, could possibly affect the conclusion of our review. Last, given the inclusion of several real-world experiences from the same geographic area, it is likely that some patients could have been enrolled in multiple studies.
Conclusion
Upadacitinib represents one of the most recent treatment options for severe AD. This systematic review evaluated available real-world data in order to offer an overview of the practical use of this drug in a clinical setting.
In the future, it will be critical to conduct longer studies to further assess the effectiveness and safety of upadacitinib since only very few experiences reported a follow-up longer than 4 months.
Disclosure
L. Gargiulo has been a consultant for Almirall. L. Ibba has been a consultant for Almirall.
A. Costanzo has served as an advisory board member, consultant and has received fees and speaker’s honoraria or has participated in clinical trials for AbbVie, Almirall, Biogen, LEO Pharma, Lilly, Janssen, Novartis, Pfizer, Sanofi Genzyme, and UCB-Pharma. A. Narcisi has served on advisory boards, received honoraria for lectures and research grants from Almirall, AbbVie, Leo Pharma, Celgene, Eli Lilly, Janssen, Novartis, Sanofi‐Genzyme, Amgen and Boehringer Ingelheim. M Valenti reports personal fees from AbbVie, personal fees from Eli-Lilly, personal fees from Almirall, personal fees from Novartis, personal fees from UCB, personal fees from Leopharma, personal fees from Boehringer-Ingelheim, personal fees from Sanofi, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Sacotte R, Silverberg JI. Epidemiology of adult atopic dermatitis. Clin Dermatol. 2018;36(5):595–605. doi:10.1016/j.clindermatol.2018.05.007
2. Chung J, Simpson EL. The socioeconomics of atopic dermatitis. Ann Allergy Asthma Immunol. 2019;122(4):360–366. doi:10.1016/j.anai.2018.12.017
3. Sroka-Tomaszewska J, Trzeciak M. Molecular mechanisms of atopic dermatitis pathogenesis. Int J Mol Sci. 2021;22(8):4130. doi:10.3390/ijms22084130
4. Wollenberg A, Kinberger M, Arents B, et al. European guideline (EuroGuiDerm) on atopic eczema: part I - systemic therapy. J Eur Acad Dermatol Venereol. 2022;36(9):1409–1431. doi:10.1111/jdv.18345
5. Guttman-Yassky E, Teixeira HD, Simpson EL, et al. Once-daily upadacitinib versus placebo in adolescents and adults with moderate-to-severe atopic dermatitis (Measure Up 1 and Measure Up 2): results from two replicate double-blind, randomised controlled phase 3 trials. Lancet. 2021;397(10290):2151–2168. doi:10.1016/S0140-6736(21)00588-2
6. Simpson EL, Sinclair R, Forman S, et al. Efficacy and safety of abrocitinib in adults and adolescents with moderate-to-severe atopic dermatitis (JADE MONO-1): a multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet. 2020;396(10246):255–266. doi:10.1016/S0140-6736(20)30732-7
7. Reich K, Kabashima K, Peris K, et al. Efficacy and safety of baricitinib combined with topical corticosteroids for treatment of moderate to severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2020;156(12):1333–1343. doi:10.1001/jamadermatol.2020.3260
8. Blauvelt A, Teixeira HD, Simpson EL, et al. Efficacy and safety of upadacitinib vs dupilumab in adults with moderate-to-severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2021;157:1047–1055. doi:10.1001/jamadermatol.2021.3023
9. Bieber T, Simpson EL, Silverberg JI, et al. Abrocitinib versus placebo or dupilumab for atopic dermatitis. N Engl J Med. 2021;384(12):1101–1112. doi:10.1056/NEJMoa2019380
10. Simpson EL, Silverberg JI, Thyssen JP, et al. Efficacy and safety of abrocitinib in patients with severe and/or difficult-to-treat atopic dermatitis: a post hoc analysis of the randomized phase 3 JADE COMPARE trial. Am J Clin Dermatol. 2023;24(4):609–621. doi:10.1007/s40257-023-00785-5
11. Olydam JI, Schlösser AR, Custurone P, Nijsten TEC, Hijnen D. Real-world effectiveness of abrocitinib treatment in patients with difficult-to-treat atopic dermatitis. J Eur Acad Dermatol Venereol. 2023;37:2537–2542. doi:10.1111/jdv.19378
12. Vittrup I, Elberling J, Skov L, et al. Short-term real-world experience with baricitinib treatment in Danish adults with moderate-severe atopic dermatitis. J Eur Acad Dermatol Venereol. 2023;37(4):e543–e546. doi:10.1111/jdv.18804
13. De Greef A, Thirion R, Ghislain PD, Baeck M. Real-life effectiveness and tolerance of baricitinib for the treatment of severe alopecia areata with 1-year follow-up data. Dermatol Ther. 2023;13(11):2869–2877. doi:10.1007/s13555-023-01030-x
14. Gargiulo L, Ibba L, Vignoli CA, et al. Effectiveness and safety of baricitinib in patients with severe alopecia areata: a 36-week multicenter real-world experience. J DermatolTreat. 2023;34(1):2268764. doi:10.1080/09546634.2023.2268764
15. Reguiai Z, Becherel PA, Fougerousse AC, et al. Janus kinase inhibitors for the treatment of atopic dermatitis: real-life data on efficacy and safety in light of the pharmacovigilance risk assessment committee recommended measures. J Eur Acad Dermatol Venereol. 2023;37(11):e1307–e1309. doi:10.1111/jdv.19302
16. European Medicines Agency. Rinvoq (upadacitinib): summary of product characteristics; 2019. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/rinvoq.
17. European Medicines Agency. Cibinqo (abrocitinib): summary of product characteristics. 2021. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/cibinqo.
18. European Medicines Agency. Olumiant (baricitinib): summary of product characteristics. 2017. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/olumiant.
19. Reich K, Teixeira HD, de Bruin-Weller M, et al. Safety and efficacy of upadacitinib in combination with topical corticosteroids in adolescents and adults with moderate-to-severe atopic dermatitis (AD Up): results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2021;397(10290):2169–2181. doi:10.1016/S0140-6736(21)00589-4
20. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. Open Med. 2009;3(3):e123–e130. doi:10.1136/bmj.327.7423.1083
21. Melgosa Ramos FJ, González-Delgado V, Motilla JMS, Marta GP, Mateu Puchades A, Sergio SA. Upadacitinib effectiveness in moderate-to-severe atopic dermatitis: a real-life multicentre and retrospective study. Australas J Dermatol. 2023;64(4):e361–e364. doi:10.1111/ajd.14164
22. De Greef A, Ghislain PD, de Montjoye L, Baeck M. Real-life effectiveness and tolerance of upadacitinib for severe atopic dermatitis in adolescents and adults. Adv Ther. 2023;40(5):2509–2514. doi:10.1007/s12325-023-02490-5
23. Chiricozzi A, Ortoncelli M, Schena D, et al. Long-term effectiveness and safety of upadacitinib for atopic dermatitis in a real-world setting: an interim analysis through 48 weeks of observation. Am J Clin Dermatol. 2023;24:953–961. doi:10.1007/s40257-023-00798-0
24. Gargiulo L, Ibba L, Piscazzi F, et al. Effectiveness and safety of upadacitinib for moderate-to-severe atopic dermatitis in a real-world setting: a 52-week retrospective study. J Eur Acad Dermatol Venereol. 2023. doi:10.1111/jdv.19507
25. Gori N, Ippoliti E, Peris K, Chiricozzi A. Head and neck atopic dermatitis: still a challenging manifestation in the biologic era. Expert Opin Biol Ther. 2023;23(7):575–577. doi:10.1080/14712598.2023.2224499
26. Napolitano M, Fabbrocini G, Genco L, Martora F, Potestio L, Patruno C. Rapid improvement in pruritus in atopic dermatitis patients treated with upadacitinib: a real-life experience. J Eur Acad Dermatol Venereol. 2022;36(9):1497–1498. doi:10.1111/jdv.18137
27. Patruno C, Fabbrocini G, Potestio L, Genco L, Napolitano M. Real-life efficacy and safety of upadacitinib in adolescents with moderate-to-severe atopic dermatitis unresponsive to dupilumab: a case series. J Eur Acad Dermatol Venereol. 2023;37(7):e901–e903. doi:10.1111/jdv.19011
28. Tran V, Ross G. A real-world Australian experience of upadacitinib for the treatment of severe atopic dermatitis. Australas J Dermatol. 2023;64(4):e352–e356. doi:10.1111/ajd.14139
29. Chiricozzi A, Gori N, Narcisi A, et al. Effectiveness and safety of upadacitinib in the treatment of moderate-severe atopic dermatitis: a multicentric, prospective, real-world, cohort study. Drugs R D. 2022;22(3):245–252. doi:10.1007/s40268-022-00396-1
30. Gargiulo L, Ibba L, Cortese A, et al. Real-life effectiveness and safety of upadacitinib in adults and adolescents with moderate-to-severe atopic dermatitis: a single-center 16-week study. Dermatol Ther. 2023;13(2):651–660. doi:10.1007/s13555-022-00882-z
31. Georgakopoulos JR, Sheka D, Rankin B, et al. Real-world effectiveness and safety of upadacitinib for the treatment of atopic dermatitis in adult patients switched from dupilumab: a multicenter retrospective study. J Am Acad Dermatol. 2023;89(6):1308–1311. doi:10.1016/j.jaad.2023.08.059
32. Boesjes CM, Van der Gang LF, Zuithoff NPA, et al. Effectiveness of upadacitinib in patients with atopic dermatitis including those with inadequate response to dupilumab and/or baricitinib: results from the BioDay registry. Acta Derm Venereol. 2023;103:adv00872. doi:10.2340/actadv.v103.5243
33. Hagino T, Saeki H, Kanda N. The efficacy and safety of upadacitinib treatment for moderate to severe atopic dermatitis in real-world practice in Japan. J Dermatol. 2022;49(11):1158–1167. doi:10.1111/1346-8138.16549
34. Hagino T, Hamada R, Yoshida M, Fujimoto E, Saeki H, Kanda N. Effectiveness and safety of upadacitinib in combination with topical corticosteroids in adolescent patients with moderate-to-severe atopic dermatitis. Clin Cosmet Invest Dermatol. 2023;16:3201–3212. doi:10.2147/CCID.S439053
35. Napolitano M, Potestio L, Hansel K, et al. Efficacy and safety of upadacitinib in adult patients affected by moderate to severe atopic dermatitis: a 16-week real-life dual-centre experience. Clin Exp Dermatol. 2023;48(3):247–249. doi:10.1093/ced/llac078
36. Pereyra-Rodriguez JJ, Herranz P, Figueras-Nart I, et al. Treatment of severe atopic dermatitis with upadacitinib in clinical practice: short-term efficacy and safety results. J Investig Allergol Clin Immunol. 2023;33(3):211–213. doi:10.18176/jiaci.0831
37. Vanlerberghe J, Dezoteux F, Martin C, et al. Effectiveness and tolerance of Janus kinase inhibitors for the treatment of recalcitrant atopic dermatitis in a real-life French multicenter adult cohort. J Am Acad Dermatol. 2023;88(4):900–904. doi:10.1016/j.jaad.2022.10.034
38. Blauvelt A, Ladizinski B, Prajapati VH, et al. Efficacy and safety of switching from dupilumab to upadacitinib versus continuous upadacitinib in moderate-to-severe atopic dermatitis: results from an open-label extension of the phase 3, randomized, controlled trial (Heads Up). J Am Acad Dermatol. 2023;89(3):478–485. doi:10.1016/j.jaad.2023.05.033
39. Gargiulo L, Ibba L, Pavia G, et al. Upadacitinib for the treatment of concomitant psoriasis and atopic dermatitis: a case series. J DermatolTreat. 2023;34(1):2183729. doi:10.1080/09546634.2023.2183729
40. Merola JF, Butler DC, Mark T, Schneider S, Kim Y, Abuabara K. Safety and efficacy of tralokinumab in older adults with moderate-to-severe atopic dermatitis: a secondary analysis. JAMA Dermatol. 2023;159(10):1119–1123. doi:10.1001/jamadermatol.2023.2626
41. Gargiulo L, Ibba L, Vignoli CA, et al. Tralokinumab rapidly improves subjective symptoms and quality of life in patients with moderate-to-severe atopic dermatitis: a real-life 16-week experience. J DermatolTreat. 2023;34(1):2216815. doi:10.1080/09546634.2023.2216815
42. Blauvelt A, de Bruin-Weller M, Gooderham M, et al. Long-term management of moderate-to-severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): a 1-year, randomised, double-blinded, placebo-controlled, phase 3 trial. Lancet. 2017;389(10086):2287–2303. doi:10.1016/S0140-6736(17)31191-1
43. Piscazzi F, Gargiulo L, Ibba L, et al. Upadacitinib for the treatment of atopic dermatitis in the elderly: an Italian case series of seven patients. J DermatolTreat. 2023;34(1):2245510. doi:10.1080/09546634.2023.2245510
44. Gargiulo L, Ibba L, Piscazzi F, et al. Upadacitinib improves symptoms of concomitant allergic rhinitis or allergic asthma in patients with severe atopic dermatitis: a 16-week multicentre retrospective study. J Eur Acad Dermatol Venereol. 2024;2024:1. doi:10.1111/jdv.19862
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
