Back to Journals » Clinical Ophthalmology » Volume 16
Practical Guidance for the Use of Loteprednol Etabonate Ophthalmic Suspension 0.25% in the Management of Dry Eye Disease
Authors Venkateswaran N, Bian Y, Gupta PK
Received 14 December 2021
Accepted for publication 24 January 2022
Published 9 February 2022 Volume 2022:16 Pages 349—355
DOI https://doi.org/10.2147/OPTH.S323301
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Nandini Venkateswaran,1 Yandong Bian,1 Preeya K Gupta2
1Massachusetts Eye and Ear Infirmary, Harvard Medical School, Boston, MA, USA; 2Triangle Eye Consultants, Cary, NC, USA
Correspondence: Preeya K Gupta, Email [email protected]
Abstract: Dry eye disease (DED) is a prevalent ocular surface disease. Like with any chronic disease, patients with DED can experience episodic flares. There are many existing and upcoming treatments for the chronic treatment of DED, yet treatments for DED flares are limited. Loteprednol etabonate 0.25% is an FDA approved treatment modality for the short-term treatment of the signs and symptoms of DED. This medication is formulated with the customized mucus-penetrating particle (MPP) technology, which has a greater ability to penetrate the ocular surface and more effectively deliver the active steroid to the ocular surface tissues as compared with conventional steroid preparations. There is also increasing utility of loteprednol etabonate 0.25% in the treatment of DED before and/or after cataract or refractive surgery or as induction therapy prior to starting chronic immunomodulatory medication for DED.
Keywords: dry eye disease, dry eye disease flares, loteprednol etabonate 0.25%, mucus penetrating particle technology, ocular surface inflammation
Introduction
Dry eye disease (DED), or keratoconjunctivitis sicca, is one of the most common ocular problems. A large cross-sectional, population-based study utilizing the National Health and Wellness Survey estimated the prevalence of DED in US adults to be about 7% or 16 million people.1 Notable factors associated with higher prevalence of DED include female sex and older age. Studies have found that individuals aged 75 or older were five times more likely to have a diagnosis of DED compared to individuals aged 18 to 34 years old, and that the risk of DED was two times higher among females than males.1–3
DED can be quite symptomatic, with patients reporting a myriad of symptoms including pain, dryness, and irritation, all of which can negatively impact the general quality of life. Miljanovic et al found that compared to patients without DED, patients with DED were more likely to report difficulty with activities of daily living including computer use, professional work, and driving.4 Given the significant prevalence and morbidity of DED, there has been a focus on understanding the pathophysiology of the disease and identifying effective treatment modalities.
DED as an Inflammatory Disorder and Exploring the Unmet Need to Treat DED Flares
DED is now well recognized to be a chronic inflammatory disease. As defined by TFOS DEWS II, DED is a multifactorial disease characterized by a loss of homeostasis of the tear film, and accompanied by ocular symptoms, in which tear film instability and hyperosmolarity, ocular surface inflammation and damage, and neurosensory abnormalities play etiological roles.5 Both extrinsic (ie desiccative environment, contact lenses, eye drops) and intrinsic factors (ie aging and female sex) that disturb the homeostasis of the ocular surface can negatively affect tear film stability and tear osmolarity, both which have been shown to have important roles in stimulation of the inflammatory pathways and activation of stress-associated mitogen-activated protein kinase (MAPK) pathways.6–8 MAPK stimulation results in the production and release of proinflammatory cytokines (IL-1, IL-6, TNF-alpha), chemokines (IL-8), and matrix metalloproteinases (MMPs). These then in turn promote the maturation and migration of antigen-presenting cells and activation of T cells.7,8 Ultimately, a self-perpetuating cycle of inflammation develops, driving the pathophysiology of DED.
It is less well recognized that DED can present with periodic flares or exacerbation (Figure 1). The ocular surface is constantly exposed to outdoor and indoor environments. Desiccating environmental conditions of low humidity and high airflow can negatively impact the ocular surface and result in an acute worsening of DED symptoms. Additionally, lifestyle habits, especially those that demand prolonged visual focus like reading or using a computer can also worsen DED symptoms. Iyer et al found that increased time spent doing daily activities such as watching television, reading, and using air conditioning were associated with worse clinical signs and symptoms of DED.9 Other studies have found similar results of worsening of DED signs and symptoms with visual activities such as reading and computer use.10,11
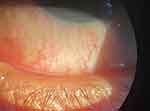 |
Figure 1 Slit lamp photo showing conjunctival injection suggestive of ocular surface inflammation during a dry eye flare. |
Controlled adverse environment studies, where participants are exposed to environmental triggers like low humidity and increased airflow, also support the existence of DED flares. A study of DED and non-DED patients exposed to a controlled desiccating environment for two hours found a significant increase in fluorescein staining and decreased tear break up time.12 In a similar study with a controlled environmental chamber meant to mimic air plane cabins (typically characterized by low humidity and high air flow), patients reported increased incidence of symptoms of burning, itching sensation, and were found to have increased corneal fluorescein staining and decreased tear break up time.13 These studies also found elevated levels of matrix metalloproteinase (MMP)-9 after exposure, demonstrating environmental activation as an inflammatory process well studied in the pathophysiology of DED.12,13
Previously available immunomodulatory agents, cyclosporine A and lifitegrast, are intended for use in patients with chronic DED and have a slow onset of action, often taking weeks to reach peak efficacy. Thus, there exists an unmet need for treatment of these patients who suffer from periodic flares of DED.
Role of Steroids in Treatment of DED
Ocular lubrication is often the initial management of DED. While still considered a mainstay of therapy, more symptomatic DED patients often require additional treatment modalities. Previously, topical corticosteroids were only used off-label to treat DED.14 Potent anti-inflammatory agents, topical corticosteroids are thought to help break the cycle of inflammation in DED. Animal models have shown that topical steroids significantly reduce expression of MMP-9 and activation of MAPK signaling pathways, both which have been implicated in the pathogenesis of DED.15 Steroids also inhibit production of inflammatory cytokine and chemokine production such as IL-1, IL-6, and TNF-alpha, and decrease expression of cell adhesion molecules such as ICAM-1, all of which are involved in the inflammatory pathogenesis of DED.15 There are many clinical studies supporting the efficacy of topical corticosteroids in treatment of DED patients.14 However, long-term use of topical corticosteroids has been associated with a number of ocular complications including cataract formation, glaucoma development, and increased risk of infections.
Loteprednol etabonate is a topical corticosteroid retro-metabolically synthesized from an inactive metabolite of prednisolone acetate where the ketone at the carbon-20 position is replaced by a cleavable 17B-chloromethyl ester.4 After exerting its therapeutic effects, it is rapidly de-esterified to inactive metabolites reducing risk of adverse reactions.16 Accordingly, it has been shown to have lower side effect risk of clinically significant intraocular pressure (IOP) elevations with both short-term and long-term use.17
There have been several studies on the utility of loteprednol etabonate for the treatment of DED. Loteprednol etabonate with a concentration of 0.5% monotherapy or in conjunction with artificial tears and cyclosporine A has been shown to improve signs and symptoms of DED, particularly in patients with a more pronounced inflammatory component.17–19
How Does Loteprednol Etabonate 0.25% Work and What is the Mucus-Penetrating Particle (MPP) Technology?
Delivery of topical medications to the anterior segment tissues can be challenging due to anatomic and physiologic barriers that normally protect the eye and negatively affect the bioavailability of these topical formulations. These barriers can be static such as the corneal and conjunctival epithelium and stroma, and dynamic such as conjunctival blood and lymphatic flow and tear film.
Drugs can be carried away by the conjunctival lymphatic and blood circulation. The conjunctiva also secretes hydrophilic mucin which is present in the tear film and can trap and clear topical drug formulations from the ocular surface. The cornea contains both a hydrophobic epithelium and hydrophilic stroma which will restrict agents that are either too hydrophilic or hydrophobic. Both the cornea and conjunctiva also contain efflux pumps. The tear film which has a rapid restoration time of two to three minutes and can wash away most topically administered solutions within 15 to 30 seconds after instillation, with only less than 5% of applied dose being available for absorption.20,21
Innovation in drug delivery technologies such as nanoparticles may have the potential to improve ocular tissue penetration, but the ability of nanoparticles to penetrate the ocular surface may still be undermined by the adhesive ocular mucus layer. The viscous, adhesive mucus layer, primarily composed of crosslinked and entangled mucin fibers, normally forms a protective layer, trapping and removing foreign particles. For nanoparticles to move through mucus, they must have surface properties like hydrophilicity and neutral charge to avoid adhesion and be small enough to avoid steric hindrance by the dense fiber mesh.22
Mucus-penetrating particle (MPP) technology involves engineering nanoparticles designed to effectively penetrate mucus and prevent entrapment of drug particles by mucins (Figure 2). These nanoparticles are polymer coated with a high density of low-molecular-weight polymer.23 Recently, MPP technology has seen the advent of drug-core MPPs, which no longer require encapsulation of the drugs in a polymeric matrix, but rather are composed purely drug with nanometer-scale particle size and a coating that prevents adherence to mucins. Drug-core MPPs can also be stored at room temperature and are stable in ready-to-use aqueous suspensions, such as aqueous suspension eye drops.24
 |
Figure 2 Schematic diagram showing the difference between conventional micro and nanoparticles gaining access to surface epithelial cells as compared with mucus penetrating particle technology. Mucus penetrating particles are engineered to effectively penetrate mucus and prevent entrapment of drug particles by mucins. Reproduced with permission from Mary Ann Liebert, Inc. publishers. Popov A. Mucus-penetrating particles and the role of ocular mucus as a barrier to micro- and nanosuspensions. J Ocul Pharmacol Ther. 2020;36(6):366–375.24 |
Schopf et al have conducted preclinical studies investigating ocular delivery of topical loteprednol etabonate formulated as MPPs.25,26 Utilizing a formulation of loteprednol etabonate-MPP, Schopf et al found that in rabbits that received topical administration of loteprednol etabonate MMP suspension 0.4%, there was a 3.6-fold increase in maximum drug concentration at the cornea as well as a 1.5-fold higher total drug availability at the cornea and 2.6-fold higher total drug availability at the conjunctiva, as compared with rabbits that received topical administration of traditional loteprednol etabonate ophthalmic suspension 0.5%.26 These studies showed that loteprednol etabonate formulated with MMP technology had a greater ability to penetrate the ocular surface and more effectively deliver the active steroid to the ocular surface tissues, even though the concentration of the drug was lower in the MPP formulation.
KPI-121 0.25% (EYSUVIS, Kala Pharmaceuticals, Inc) is the commercially available MMP formulation of loteprednol etabonate ophthalmic suspension that was FDA approved in 2020 for short-term treatment (up to 2 weeks) of DED. KPI-121 0.25% was investigated for treatment in DED in one phase-2 (NCT02188160), and three phase-3 clinical trials (STRIDE 1 NCT02813265; STRIDE 2 NCT02819284; and STRIDE 3 NCT03616899). All studies were multicenter, double-masked, randomized, and vehicle (placebo) controlled. Total enrollment across the four trials consisted of more than 2800 DED patients. Patients received KPI-121 or vehicle drops four times a day for two weeks. Main efficacy outcome measures were conjunctival hyperemia and ocular discomfort severity scores at the end of the 2 weeks. Significant improvements in conjunctival hyperemia and ocular discomfort severity scores were seen in patients who received KPI-121 compared to those who received vehicle drops. KPI-121 was well tolerated with the most frequently reported adverse effect being instillation site pain, and notably there was no difference in intraocular pressure increase between the KPI-121 and vehicle arms.27
KPI-121 with MPP technology was first approved by the Food and Drug Administration (FDA) in 2019 for the treatment of postoperative inflammation and pain following ocular surgery but at a concentration of 1%.28 The 0.25% concentration was studied and selected for treatment of DED because it had an improved pharmacokinetic profile in the reduced dose strength compared with that in conventional loteprednol etabonate suspension 0.5% without the MPP drug delivery technology.
Treatments for DED
It is difficult to formulate one treatment algorithm for DED given the heterogeneity and fluctuating nature of the disease. In general, the majority of interventions are long-term treatments, likely due to the usually chronic nature of this disease.
The first step for the clinician is counseling, especially regarding lifestyle changes and environmental modifications that can help ameliorate symptoms.14 Artificial tear substitutes are also often first-line treatments. There are numerous different over-the-counter formulations available. A Cochrane systematic review concluded that the majority of over-the-counter formulations likely have similar efficacies in treating DED.27
There are three FDA approved medications aimed at decreasing inflammation in chronic DED, lifitegrast (Xiidra, Novartis) cyclosporine A 0.05% (Restasis, Allergan) and cyclosporine A 0.09% (Cequa, Sun Pharma). Cyclosporine A is an anti-inflammatory, immunomodulatory drug that inhibits IL-2 activation of lymphocytes. There have been many studies that have shown cyclosporine A 0.05%’s efficacy in treating DED with improvement in tear function, improvement in the ocular surface, and improvement in DED symptoms.29 In contrast, cyclosporine A 0.09% is the highest dose of cyclosporine approved by the FDA for dry eye treatment, and is formulated with a novel NCELL technology to improve the ocular penetration and absorption of cyclosporine.30 Its use has been shown to lead to an increase in Schirmer’s test scores as well as improved corneal and conjunctival staining as compared with a vehicle drug and has improved dry eye symptom scores by 30%.30,31 Lifitegrast is a lymphocyte functional associated antigen-1 (LFA-1) antagonist. It blocks the interaction of LFA-1 and the intracellular adhesion molecule-1 (ICAM-1) to inhibit T-cell mediated inflammation in chronic DED. Three Phase III clinical trials (OPUS 1-3) showed improvement in signs and symptoms of DED, notably significant improvement in eye dryness score within 14 days.32
Tear conservation with punctal occlusion either through punctal plugs or permanent surgical occlusion can be used in patients who would benefit from aqueous retention on the ocular surface (ie contact lens wearer, refractive surgery). The use of punctal occlusion in the presence of concurrent ocular surface inflammation remains controversial and is generally not recommended as it may prolong the presence of pro-inflammatory cytokines on the ocular surface. A Cochrane review of punctal plugs found while in general punctal plugs provided symptomatic improvement, few studies demonstrated a benefit of punctal plugs over a comparison intervention and in general there is a lack of well-designed clinical trials.33
Autologous serum tears have been shown to improve signs and symptoms of DED. They have been shown to enhance corneal epithelial wound healing and inhibit release of inflammatory cytokines. Many small trials support the efficacy of autologous serum tears in treatment of DED. In general patients showed significant improvement in symptoms, and improved tear break up time and corneal fluorescein staining.14 However, lack of a universally accepted methodology for preparation of autologous serum tears and high cost limit its availability to patients.
Tetracyclines have been used off-label to treat DED and associated inflammatory disorders of DED including rosacea and blepharitis.14 Tetracyclines have been shown to decrease the activity of collagenase, MMPs, and the production of inflammatory cytokines like IL-1 and TNF-alpha.15 However, the optimal dosing schedule of tetracyclines has not been established and can vary from provider to provider.
The Role of Loteprednol Etabonate 0.25% in the Treatment of DED
Clinicians can very commonly encounter DED patients who have recurrent flares of their DED despite use of the chronic therapies. DED flares are characterized by episodes of worsening ocular surface discomfort, with an increase in ocular surface inflammation, keratopathy or tear deficiency noted on clinical exam. This can then occur several times a year despite baseline DED therapy.
When patients present with a DED flare, KP-121 0.25% therapy is an excellent adjunct.34 Its favorable pharmacologic profile allows for delivery of loteprednol etabonate onto the ocular surface with high concentration and rapid breakdown, decreasing adverse effects such as elevated intraocular pressure or cataract formation. KP-121 0.25% should be administered four times a day for two weeks during the course of a DED flare to relieve the patient’s acute symptomatology and also quell the cycle of ocular surface inflammation. Flares occur a few times per year in most patients, and KP-121 0.25% can be used safely during these episodes. It is, however, important to realize that if a patient is having very frequent flares, their underlying DED is poorly controlled. As such, alterations or augmentations need to be made in their baseline DED therapy to reduce the frequency and severity of episodic flares.
Similarly, KP-121 0.25% can also be used to treat significant ocular surface disease prior to surgery. KPI-121 0.25% can be used in place of traditional topical ophthalmic corticosteroids to optimize the ocular surface before cataract surgery or refractive surgery as a stable ocular surface is critical when obtaining biometric measurements for intraocular lens power calculations or when obtaining refractions for refractive surgery treatment planning to ensure patients obtain excellent postoperative visual outcomes.34,35 Similarly, ocular surface disease can worsen after cataract or refractive surgery, especially if it is not treated adequately pre-operatively, and KP-121 0.25% can be used for short-term treatment of DED in the pre- and post-surgical time frames.
Lastly, KP-121 0.25% can be used as an induction therapy for immunomodulatory therapy in DED. When a patient starts topical immunomodulatory therapy, KPI-121 0.25% can be used for two weeks to quell ocular surface inflammation and DED symptoms until the new therapeutic agent takes effect, which is often after four to six weeks.
Conclusion
KP-121 0.25% with its custom engineered MPP technology allows for the effective and efficient delivery of loteprednol etabonate onto the ocular surface for the short-term treatment of signs and symptoms of DED with minimal side effects. Clinical trials have demonstrated the utility of this medication in DED flares and clinicians should incorporate this medication into their DED treatment armamentarium.
Abbreviations
DED, dry eye disease; MAPK, mitogen-activated protein kinase; MMP, matrix metalloproteinases; MPP, mucus-penetrating particle; FDA, Food and Drug Administration.
Ethics Approval and Informed Consent
No informed consent was needed for this review paper.
Consent for Publication
The authors have received consent to publish all figures in this manuscript.
Funding
There was no funding for this study.
Disclosure
Dr Preeya K Gupta reports personal fees from Kala Pharmaceuticals and B+L, during the conduct of the study. The authors report no other conflicts of interest in this work.
References
1. Farrand KF, Fridman M, Stillman I, Schaumberg DA. Prevalence of diagnosed dry eye disease in the United States among adults aged 18 years and older. Am J Ophthalmol. 2017;182:90–98. doi:10.1016/j.ajo.2017.06.033
2. Moss SE, Klein R, Klein BE. Long-term incidence of dry eye in an older population. Optom Vis Sci. 2008;85(8):668–674. doi:10.1097/OPX.0b013e318181a947
3. Schaumberg DA, Sullivan DA, Buring JE, Dana MR. Prevalence of dry eye syndrome among US women. Am J Ophthalmol. 2003;136(2):318–326. doi:10.1016/S0002-9394(03)00218-6
4. Miljanović B, Dana R, Sullivan DA, Schaumberg DA. Impact of dry eye syndrome on vision-related quality of life. Am J Ophthalmol. 2007;143(3):409–415. doi:10.1016/j.ajo.2006.11.060
5. Craig JP, Nichols KK, Akpek EK, et al. TFOS DEWS II definition and classification report. Ocul Surf. 2017;15(3):276–283. doi:10.1016/j.jtos.2017.05.008
6. Yamaguchi T. Inflammatory response in dry eye. Invest Ophthalmol Vis Sci. 2018;59(14):Des192–des199. doi:10.1167/iovs.17-23651
7. Bron AJ, de Paiva CS, Chauhan SK, et al. TFOS DEWS II pathophysiology report. Ocul Surf. 2017;15(3):438–510. doi:10.1016/j.jtos.2017.05.011
8. Stevenson W, Chauhan SK, Dana R. Dry eye disease: an immune-mediated ocular surface disorder. Arch Ophthalmol. 2012;130(1):90–100. doi:10.1001/archophthalmol.2011.364
9. Iyer JV, Lee SY, Tong L. The dry eye disease activity log study. ScientificWorldJournal. 2012;2012:589875. doi:10.1100/2012/589875
10. Karakus S, Agrawal D, Hindman HB, Henrich C, Ramulu PY, Akpek EK. Effects of prolonged reading on dry eye. Ophthalmology. 2018;125(10):1500–1505. doi:10.1016/j.ophtha.2018.03.039
11. Yazici A, Sari ES, Sahin G, et al. Change in tear film characteristics in visual display terminal users. Eur J Ophthalmol. 2015;25(2):85–89. doi:10.5301/ejo.5000525
12. López-Miguel A, Tesón M, Martín-Montañez V, et al. Dry eye exacerbation in patients exposed to desiccating stress under controlled environmental conditions. Am J Ophthalmol. 2014;157(4):788–798.e782. doi:10.1016/j.ajo.2014.01.001
13. Tesón M, González-García MJ, López-Miguel A, et al. Influence of a controlled environment simulating an in-flight airplane cabin on dry eye disease. Invest Ophthalmol Vis Sci. 2013;54(3):2093–2099. doi:10.1167/iovs.12-11361
14. Jones L, Downie LE, Korb D, et al. TFOS DEWS II management and therapy report. Ocul Surf. 2017;15(3):575–628. doi:10.1016/j.jtos.2017.05.006
15. De Paiva CS, Corrales RM, Villarreal AL, et al. Corticosteroid and doxycycline suppress MMP-9 and inflammatory cytokine expression, MAPK activation in the corneal epithelium in experimental dry eye. Exp Eye Res. 2006;83(3):526–535. doi:10.1016/j.exer.2006.02.004
16. Beckman K, Katz J, Majmudar P, Rostov A. Loteprednol etabonate for the treatment of dry eye disease. J Ocul Pharmacol Ther. 2020;36(7):497–511. doi:10.1089/jop.2020.0014
17. Sheppard JD, Comstock TL, Cavet ME. Impact of the topical ophthalmic corticosteroid loteprednol etabonate on intraocular pressure. Adv Ther. 2016;33(4):532–552. doi:10.1007/s12325-016-0315-8
18. Sheppard JD, Donnenfeld ED, Holland EJ, et al. Effect of loteprednol etabonate 0.5% on initiation of dry eye treatment with topical cyclosporine 0.05%. Eye Contact Lens. 2014;40(5):289–296. doi:10.1097/ICL.0000000000000049
19. Pflugfelder SC, Maskin SL, Anderson B, et al. A randomized, double-masked, placebo-controlled, multicenter comparison of loteprednol etabonate ophthalmic suspension, 0.5%, and placebo for treatment of keratoconjunctivitis sicca in patients with delayed tear clearance. Am J Ophthalmol. 2004;138(3):444–457. doi:10.1016/j.ajo.2004.04.052
20. Gaudana R, Ananthula HK, Parenky A, Mitra AK. Ocular drug delivery. Aaps J. 2010;12(3):348–360. doi:10.1208/s12248-010-9183-3
21. Cholkar K, Patel SP, Vadlapudi AD, Mitra AK. Novel strategies for anterior segment ocular drug delivery. J Ocul Pharmacol Ther. 2013;29(2):106–123. doi:10.1089/jop.2012.0200
22. Lai SK, Wang YY, Hanes J. Mucus-penetrating nanoparticles for drug and gene delivery to mucosal tissues. Adv Drug Deliv Rev. 2009;61(2):158–171. doi:10.1016/j.addr.2008.11.002
23. Yang M, Lai SK, Wang YY, et al. Biodegradable nanoparticles composed entirely of safe materials that rapidly penetrate human mucus. Angew Chem Int Ed Engl. 2011;50(11):2597–2600. doi:10.1002/anie.201006849
24. Popov A. Mucus-penetrating particles and the role of ocular mucus as a barrier to micro- and nanosuspensions. J Ocul Pharmacol Ther. 2020;36(6):366–375. doi:10.1089/jop.2020.0022
25. Schopf L, Enlow E, Popov A, Bourassa J, Chen H. Ocular pharmacokinetics of a novel loteprednol etabonate 0.4% ophthalmic formulation. Ophthalmol Ther. 2014;3(1–2):63–72. doi:10.1007/s40123-014-0021-z
26. Schopf LR, Popov AM, Enlow EM, et al. Topical ocular drug delivery to the back of the eye by mucus-penetrating particles. Transl Vis Sci Technol. 2015;4(3):11. doi:10.1167/tvst.4.3.11
27. Korenfeld M, Nichols KK, Goldberg D, et al. Safety of KPI-121 ophthalmic suspension 0.25% in patients with dry eye disease: a pooled analysis of 4 multicenter, randomized, vehicle-controlled studies. Cornea. 2021;40(5):564–570. doi:10.1097/ICO.0000000000002452
28. Kim T, Sall K, Holland EJ, Brazzell RK, Coultas S, Gupta PK. Safety and efficacy of twice daily administration of KPI-121 1% for ocular inflammation and pain following cataract surgery. Clin Ophthalmol. 2019;13:69–86. doi:10.2147/OPTH.S185800
29. Sacchetti M, Mantelli F, Lambiase A, Mastropasqua A, Merlo D, Bonini S. Systematic review of randomised clinical trials on topical ciclosporin A for the treatment of dry eye disease. Br J Ophthalmol. 2014;98(8):1016–1022. doi:10.1136/bjophthalmol-2013-304072
30. Goldberg DF, Malhotra RP, Schechter BA, Justice A, Weiss SL, Sheppard JD. A Phase 3, randomized, double-masked study of OTX-101 ophthalmic solution 0.09% in the treatment of dry eye disease. Ophthalmology. 2019;126(9):1230–1237. doi:10.1016/j.ophtha.2019.03.050
31. Hovanesian JA, Berdy GJ, Epitropoulos A, Holladay JT. Effect of cyclosporine 0.09% treatment on accuracy of preoperative biometry and higher order aberrations in dry eye patients undergoing cataract surgery. Clin Ophthalmol. 2021;15:3679–3686. doi:10.2147/OPTH.S325659
32. Donnenfeld ED, Perry HD, Nattis AS, Rosenberg ED. Lifitegrast for the treatment of dry eye disease in adults. Expert Opin Pharmacother. 2017;18(14):1517–1524. doi:10.1080/14656566.2017.1372748
33. Ervin AM, Law A, Pucker AD. Punctal occlusion for dry eye syndrome. Cochrane Database Syst Rev. 2017;6(6):Cd006775. doi:10.1002/14651858.CD006775.pub3
34. Gupta PK, Venkateswaran N. The role of KPI-121 0.25% in the treatment of dry eye disease: penetrating the mucus barrier to treat periodic flares. Ther Adv Ophthalmol. 2021;13:25158414211012797. doi:10.1177/25158414211012797
35. Gupta PK, Drinkwater OJ, VanDusen KW, Brissette AR, Starr CE. Prevalence of ocular surface dysfunction in patients presenting for cataract surgery evaluation. J Cataract Refract Surg. 2018;44(9):1090–1096. doi:10.1016/j.jcrs.2018.06.026
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
