Back to Journals » International Journal of Nephrology and Renovascular Disease » Volume 16
Practical Considerations for the Use of Sparsentan in the Treatment of Patients with IgAN in Clinical Practice
Authors Campbell KN, Griffin S, Trachtman H, Geletka R, Wong MG
Received 13 July 2023
Accepted for publication 12 December 2023
Published 22 December 2023 Volume 2023:16 Pages 281—291
DOI https://doi.org/10.2147/IJNRD.S430377
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Pravin Singhal
Video abstract presented by Campbell.
Views: 203
Kirk N Campbell,1 Siân Griffin,2 Howard Trachtman,3 Rob Geletka,4 Muh Geot Wong5,6
1Department of Medicine, Icahn School of Medicine at Mount Sinai, New York, NY, USA; 2Department of Nephrology, University Hospital of Wales, Cardiff, UK; 3Department of Pediatrics, University of Michigan, Ann Arbor, MI, USA; 4Travere Therapeutics, Inc., San Diego, CA, USA; 5Department of Renal Medicine, Concord Repatriation General Hospital, Concord, NSW, Australia; 6Concord Clinical School, University of Sydney, Concord, NSW, Australia
Correspondence: Kirk N Campbell, Icahn School of Medicine at Mount Sinai, One Gustave L. Levy Place, Box 1243, New York, NY, 10029, USA, Tel +1 212-241-6271, Fax +1 212-987-0389, Email [email protected]
Abstract: Immunoglobulin A nephropathy (IgAN) is the most common primary glomerulonephritis worldwide. It is characterized by the mesangial deposition of IgA-containing immune complexes, triggering damage to the glomerular filtration barrier that is amplified by the tandem action of endothelin-1 and angiotensin II at their receptors. Proteinuria and progressive glomerular damage cause loss of kidney function in up to 50% of patients within 10– 20 years. The risk of progression is strongly associated with persistent proteinuria (> 0.75– 1 g/day). Current standard of care involves interventions to decrease proteinuria and control blood pressure. Immunosuppressive agents, used in selected patients at high risk for progression, can be associated with significant side effects. Sparsentan, a novel non-immunosuppressive single-molecule Dual Endothelin Angiotensin Receptor Antagonist (DEARA), received FDA accelerated approval based on interim results from the PROTECT trial, which demonstrated that sparsentan-treated patients achieved a significantly greater reduction in proteinuria from baseline versus the active control irbesartan and that sparsentan was generally safe and well tolerated. Sparsentan is the first non-immunosuppressive treatment to be FDA-approved for the reduction of proteinuria in adults with IgAN at high risk of disease progression. We provide practical guidance for the clinical use of sparsentan in adults with IgAN.
Plain Language Summary: Immunoglobulin A nephropathy (IgAN) is a type of kidney disease that most commonly affects young adults. IgAN can get worse over time and lead to kidney failure within 10– 20 years after being diagnosed. People with IgAN who leak protein in their urine (ie, proteinuria) at high levels are likely to lose their kidneys faster. The Kidney Disease Improving Global Outcomes 2021 clinical practice guidelines recommend reducing the amount of protein in the urine and keeping blood pressure in check to help protect the kidneys. Doctors sometimes give medications that can weaken the immune system to patients with IgAN who are at high risk for faster loss of kidney function; however, these drugs can have troublesome side effects. Sparsentan is a new treatment that is taken in one pill each day. It targets two important pathways (endothelin-1 and angiotensin II) that lead to the loss of kidney function in IgAN. More than 1200 patients have tried sparsentan in clinical trials, and it seems to be safe and well tolerated. It also reduces protein in the urine much better than irbesartan, a blood pressure medicine often used to treat IgAN. For adults with IgAN at high risk of worsening disease, sparsentan is the first medication approved by the US Food and Drug Administration (FDA) that can reduce protein in the urine without hurting the immune system. This article gives practical advice on how to use sparsentan in adults with IgAN, including who should get it, how to start treatment, and how to check if treatment is working for the patient.
Keywords: immunoglobulin A nephropathy, sparsentan, treatment, dual endothelin angiotensin receptor antagonist
Introduction
When a new drug becomes available following US Food and Drug Administration (FDA) approval, physicians may have questions and concerns regarding its safety and appropriate clinical use in individual patients. This may impede the uptake of novel effective treatments in routine practice despite the substantial unmet need for new treatments in immunoglobulin A nephropathy (IgAN), the most common primary glomerulonephritis worldwide.1,2 IgAN is a leading cause of kidney failure (KF), with approximately 30–40% of patients reaching KF within 10 years and >50% reaching KF within 20 years.3,4
Sparsentan received accelerated approval by the FDA in February 2023 as the first non-immunosuppressive treatment for the reduction of proteinuria in adults with IgAN at high risk of disease progression and is currently under review by the European Medicines Agency, with approval expected in the second half of 2023. Additionally, there are plans for the FDA to evaluate sparsentan for the treatment of focal segmental glomerulosclerosis (FSGS) in adults and children >8 years old.
Our purpose in this paper is to provide clinically relevant practical guidance on the use of sparsentan for the treatment of IgAN in adults. This is based on our collective experience of treating patients with sparsentan as clinical investigators in the Phase 3 PROTECT (NCT03762850) and DUPLEX (NCT03493685) trials, and the Phase 2 DUET trial (NCT01613118) with an ongoing open-label extension (OLE). First, we briefly describe the pathophysiology of IgAN, the role of endothelin-1 (ET-1) and angiotensin II (Ang II) in kidney disease, and treatment goals in IgAN. Next, we discuss sparsentan’s mechanism of action, selection of appropriate patients for treatment, sparsentan dose initiation and increase to target dose, and monitoring of safety and efficacy. We hope the dissemination of this information will expedite the incorporation of sparsentan into the treatment pathway for patients with IgAN.
Mechanisms of Disease and Treatment Goals
Mechanism of Disease
The pathogenesis of IgAN is best considered as a multi-hit disease model.5,6 The initial hit of increased production of mucosal-derived galactose-deficient IgA1, triggered by genetic and/or environmental factors, leads to formation of anti-glycan antibodies (second hit) that generate circulating immune complexes (third hit).5,6 Deposition of immune complexes in the kidney mesangial region (fourth hit) leads to inflammation, extracellular matrix (ECM) production, mesangial cell dysfunction, and podocyte injury.5–7 This damage is amplified by ET-1 and Ang II upregulation that occurs with the deposition of immune complexes.8,9 For example, ET-1 expression in kidney biopsies from patients with IgAN directly correlated with the degree of proteinuria.9
ET-1 and Ang II in Kidney Disease
ET-1, the effector peptide of the endothelin system, is involved in glomerular hemodynamics and cell signaling.10,11 ET-1 binding with ET-1 type A receptors (ETAR) results in vasoconstriction, production of ECM, inflammation, stimulation of renal cell proliferation and hypertrophy, and podocyte damage.8,11,12 These changes disrupt the glomerular filtration barrier and contribute to the development of proteinuria and glomerulosclerosis.8,12 Ang II acts via the Ang II type 1 (AT1R) receptors on glomerular arterioles, inducing vasoconstriction and producing similar effects to ET-1 at the ETAR.11,13 Under pathological conditions, ET-1 and Ang II act in tandem via ETAR and AT1R to promote adverse effects on kidney structure and function (Figure 1).8,9,11,14–22
 |
Figure 1 Role of ET-1 and Ang II in IgAN disease process and sparsentan’s mechanism of action.8,9,11,15–22 Abbreviations: Ang II, angiotensin II; AT1R, angiotensin II receptor type 1; ETAR, endothelin receptor type A; ET-1, endothelin 1; IgA, immunoglobulin A. Notes: The activity of endothelin and angiotensin II via ETAR and AT1R may have additive injury effects on glomeruli, tubulointerstitium, and vasculature. The nephroprotective potential of sparsentan’s dual antagonism of ETAR and AT1R (step 3) in patients with IgAN is being examined in the PROTECT trial. |
Treatment Goals
The Kidney Disease Improving Global Outcomes (KDIGO) 2021 clinical practice guidelines recommend preserving kidney function by decreasing proteinuria and controlling blood pressure.20 Risk of progression to KF is associated with persistent proteinuria >0.75–1 g/day despite ≥90 days of maximized supportive care.3,20,23 Currently, the standard of care for IgAN is renin-angiotensin-aldosterone system inhibition (RAASi) with an angiotensin-converting enzyme inhibitor (ACEi) or angiotensin receptor blocker (ARB) at the maximally tolerated or allowed dose, additional agents as needed to lower blood pressure to target, and lifestyle modification.4,20 Despite optimal supportive care, it is estimated that up to 50% of patients will progress to KF within 20 years, experiencing significant impacts on quality of life and increased risk of premature death.4,6 This risk is higher in patients with residual heavy proteinuria despite maximum RAASi. A recent report highlighted the elevated risk of progressive kidney disease in patients with IgAN and persistent proteinuria in the range of 0.44 to <0.88 g/g, underscoring the need for novel antiproteinuric agents like sparsentan.3
Until recently, there were no FDA-approved treatments for IgAN. Immunosuppressant agents have been shown to delay onset of poor kidney outcomes but are associated with unwanted side effects. Moreover, treatment results are generally disappointing; although a course of oral corticosteroids may be indicated in selected patients at high risk for progression, it is associated with increased risk of infection.20 Targeted-release budesonide, a corticosteroid, recently received accelerated approval for use in adults with IgAN at risk of rapid disease progression, with a recommended duration of therapy of 9 months.24 Emerging immunosuppressive approaches, including B-cell therapies and complement inhibitors, are currently being investigated, but data on their effectiveness remain limited,6 and there are limited data supporting proteinuria reduction with hydroxychloroquine treatment.25 There is developing support for sodium-glucose cotransporter-2 (SGLT2) inhibitors in the treatment of IgAN, as indicated by a subgroup analysis of patients with IgAN in the dapagliflozin chronic kidney disease (DAPA-CKD) trial that suggested a reduced risk of CKD progression.26 Ongoing sparsentan clinical studies are examining concomitant treatment with SGLT2i.27,28
Sparsentan
Mechanism of Action
Sparsentan is a novel, non-immunosuppressive, single-molecule dual endothelin angiotensin receptor antagonist (DEARA) with high selectivity for ETAR and AT1R.6 While sparsentan has high affinity at ETAR (12.8 nM) and AT1R (0.36 nM), its affinity at ET-1 type B and Ang II type 2 receptors is negligible.29 The antiproteinuric and potentially nephroprotective effects of sparsentan in IgAN are likely due to dual ETAR and AT1R antagonism.6,11,18,30,31 This is supported by the effects of sparsentan in models of IgAN, such as the grouped Deutschland, Denken, Yoken (gddY) mouse model, as well as in other models of kidney disease. These effects include decreased albuminuria, amelioration of the development of mesangial hypercellularity, reduced glomerulosclerosis and tubulointerstitial fibrosis, and protection of podocytes and glomerular glycocalyx.32–36
Efficacy and Safety
Sparsentan treatment of adults with IgAN is being assessed in the phase 3 PROTECT clinical trial. The trial randomized 404 patients with IgAN to sparsentan treatment versus the active comparator irbesartan with more than 95% titrated to maximal label irbesartan dose and managed under ideal study conditions, including full optimization of medication adherence.37,38 Eligible patients had proteinuria ≥1 g/day despite maximally tolerated RAASi that was at least one-half of the maximum labeled dose for ≥12 weeks at enrollment (median [interquartile range] urine protein/creatinine ratio [UP/C] was 1.2 [0.8–1.8] g/g for the 404 patients).37,38 The study met its primary efficacy endpoint (prespecified interim analysis) of sparsentan-treated patients showing significantly greater reduction from baseline in UP/C at week 36 based on a 24-hour urine sample (primary analysis set)39 and the proteinuria reduction was maintained throughout the 2-year study period (sparsentan −42.8% geometric least-squares mean reduction of UP/C from baseline at week 110 vs irbesartan −4.4%).40 Greater proteinuria reduction with sparsentan versus irbesartan was consistent across patient subgroups of demographic (eg, age, sex, race) and baseline clinical characteristics (eg, estimated glomerular filtration rate [eGFR] and proteinuria levels). Rates of complete (urinary protein excretion <0.3 g/day; 31% vs 11% of patients) and partial (urinary protein excretion <1.0 g/day; 78% vs 53% of patients) proteinuria remission at any time and at each follow-up visit were higher with sparsentan versus irbesartan.40 The proteinuria endpoint was selected based on the Kidney Health Initiative recommendation that reduction in proteinuria may be used as a surrogate marker to assess a drug’s effect on progression to KF in IgAN.37 Specifically, a 30% reduction in proteinuria is associated with a hazard ratio of 0.5 (95% confidence interval, 0.34–0.73) for progression of kidney disease.37,41,42 The 2-year PROTECT trial supported preservation of kidney function with sparsentan treatment as shown in a slower rate of eGFR decline versus irbesartan.40
Treatment-emergent adverse events (TEAEs) in sparsentan-treated versus irbesartan-treated patients in the PROTECT trial of particular clinical relevance were hyperkalemia (16% vs 13%), peripheral edema (15% vs 12%), dizziness (15% vs 6%), hypotension (13% vs 4%), and anemia (8% vs 4%).40 There were no discontinuations due to heart failure or edema. Overall, the sparsentan safety outcomes in PROTECT were consistent with the DUPLEX and DUET trials and long-term treatment during the DUET OLE in FSGS.43–46
Patient Selection for Sparsentan Treatment
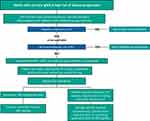
In clinical practice with adult patients, sparsentan treatment should be added to standard-of-care interventions of lifestyle changes such as smoking cessation and exercise, cholesterol lowering, and following a low-salt diet (daily intake <2 g/day). Sparsentan replaces optimized RAASi therapy, which should be stopped upon sparsentan initiation. Sparsentan is only available in the United States through a Risk Evaluation and Mitigation Strategy (REMS) program that is in place as a precautionary measure to ensure patient safety (see Monitoring Safety section). The efficacy and safety of sparsentan treatment in pediatric patients with IgAN is not yet known but is being examined in the ongoing Evaluating Problematic Proteinuria in Kids (EPPIK; NCT05003986) trial.
Initiating Sparsentan Treatment
Pretreatment Baseline Labs
For assessing proteinuria, we recommend adopting the sample and analytic method employed routinely in the individual practice (eg, urine spot or 24-hour urine collection). Consistent laboratory methods enable reliable determination of the effect of sparsentan on the percentage reduction in proteinuria. Baseline liver function tests and pregnancy testing should be completed in line with the REMS program. Serum potassium should be normal (ie, <5.5 mEq/L) prior to sparsentan initiation. We recommend caution before prescribing sparsentan to patients who have hematocrit <27% or hemoglobin <9 g/dL in pretreatment laboratory assessments or who have a history of allergic reaction or a serious adverse event in response to any Ang II antagonist or endothelin receptor antagonist (ERA). If sparsentan is prescribed under these circumstances, more frequent monitoring should be implemented.
Sparsentan Dose
Upon sparsentan initiation, discontinue all use of RAASi (including ACEIs, ARBs), ERAs, and aliskiren. There is no need for a washout period prior to initiating sparsentan. Start sparsentan at 200 mg once daily (Figure 2). After 14 days, increase the dose to the recommended 400 mg once daily, as tolerated by the patient. The dose increase from 200 to 400 mg/day does not usually require an office visit. Patients should be instructed to contact their doctor if they experience difficulties such as dizziness, which may indicate hypotension, including orthostatic hypotension. If intolerable side effects occur, the dose should be reduced by 50% or stopped if they are taking 200 mg/day. At a later time, based on tolerability, restarting or increasing the dose to 400 mg/day can be attempted again. Follow-up office visits and laboratory monitoring should be conducted within 2–4 weeks after reaching the target dose of sparsentan, according to the REMS program timing, and whenever deemed clinically necessary by the treating physician. Sparsentan tablets should be swallowed whole with water prior to either the morning or evening meal.
Monitoring Safety
The REMS program includes monitoring of liver function tests to mitigate the potential risk of hepatotoxicity and monitoring of pregnancy due to embryo-fetal toxicity with sparsentan (full details available at www.filsparirems.com). Other safety-related outcomes to monitor during sparsentan treatment include blood pressure, fluid retention, kidney function, and potassium levels. Key considerations for potential drug–drug interactions are shown in Box 1.47
 |
Box 1 Sparsentan in Clinical Practice |
Monitoring Liver Function
In the PROTECT 2-year trial, 2% of sparsentan-treated patients had an alanine aminotransferase or aspartate aminotransferase elevation of at least 3× upper limit of normal (ULN) versus 3% of irbesartan-treated patients. There were no cases of drug-induced liver injury.40 Although the risk of hepatotoxicity is low, the REMS for monitoring liver function tests is required for all patients. Serum aminotransferase levels and total bilirubin should be measured prior to initiating sparsentan, monthly for the first 12 months, and every 3 months thereafter during treatment. Advise patients to report symptoms suggestive of hepatotoxicity (eg, nausea, vomiting, right upper quadrant pain, fatigue, anorexia, jaundice, dark urine, fever, or itching). For patients with abnormal aminotransferase levels at any time during treatment, interrupt sparsentan and monitor as recommended in the “Dosage Adjustment and Monitoring in Patients Developing Aminotransferase Elevations >3× ULN” table in the Prescribing Information.
Managing Decreased Blood Pressure
In the PROTECT trial interim analysis, the mean decline in systolic blood pressure at 4 to 6 weeks with sparsentan was comparable to irbesartan (−2.6 to −4.5 mmHg versus −2.6 to −3.3 mmHg, respectively).39 There was a modest difference between the sparsentan and irbesartan treatment groups in the mean decline in diastolic blood pressure at 4 to 6 weeks (−3.2 to −4.2 mmHg versus −0.5 to −0.4 mmHg, respectively). Blood pressure should be monitored and patients reporting tolerability issues related to decreased blood pressure, for example orthostatic hypotension or dizziness, should be evaluated for volume depletion. For patients at risk for hypotension who are receiving additional antihypertensive medications, consideration should be given to discontinuation or dose adjustment of the other antihypertensive agents. If hypotension develops despite adjustments, consider reducing sparsentan or temporarily discontinuing the drug.
Managing Fluid Retention and Edema
In the PROTECT trial, weight change through 2 years with sparsentan was similar to irbesartan.40 New use of any diuretics (24% versus 27%, respectively), and of thiazides, the most frequently used class (17% versus 21%, respectively), were similar with sparsentan and irbesartan treatment.40 There were no serious TEAEs of study drug-related edema and no patients discontinued sparsentan due to edema. If clinically significant fluid retention develops, with or without associated weight gain, we recommend determining the cause including changes in diet or medications such as calcium channel blockers and strong vasodilators. Evaluate whether treatment modifications are needed, such as initiation or increased dose of a diuretic, then consider modifying the dose of sparsentan.
Managing Decreased Kidney Function
RAASi drugs may cause changes in kidney function, including acute kidney injury (AKI). As is typical with RAASi, at initiation of sparsentan, there is an expected acute decline in eGFR that then stabilizes, and treatment should not be stopped. The acute eGFR change with sparsentan is qualitatively similar to ARBs.40,45 Similar to RAASi guidance, if a patient is sick with vomiting, diarrhea, or viral illness, it is advisable to withhold sparsentan. Patients should be counseled to be more careful of the risk of AKI during hot weather months. Monitor kidney function periodically and consider withholding or discontinuing sparsentan in patients who develop a clinically significant decrease in kidney function.
Managing Hyperkalemia
In PROTECT, mean potassium concentration was stable over the 2-year trial.40 Periodically monitor serum potassium and treat appropriately. Patients with advanced kidney disease, taking concomitant drugs that raise serum potassium levels, and/or using potassium-containing salt substitutes are at increased risk for developing hyperkalemia (serum potassium ≥5.5 mmol/L). More frequent monitoring of serum potassium may be required in these patients. Hyperkalemia should be treated based on the patient’s presentation and may include low potassium diet, discontinued use of any potassium supplements or salt substitutes that contain potassium, and medications that lower serum potassium. The availability of new well-tolerated oral agents to manage hyperkalemia should enable safe treatment of hyperkalemia in patients treated with sparsentan.
Managing an Intercurrent Illness
While there are not specific clinical trial data for use of sparsentan during intercurrent illnesses, the current phase 3 trials were executed during the COVID-19 pandemic and suggest a favorable safety profile with sparsentan use during the pandemic which was comparable to irbesartan. As with ARB/ACEi treatment, there is a risk of AKI during intercurrent illness (eg, upper respiratory tract infection, gastrointestinal infection, vomiting, diarrhea) in patients who are elderly, are volume depleted, those on diuretic therapy, or with compromised kidney function. It may be advisable to temporarily hold sparsentan treatment under these conditions. When restarting sparsentan, one could consider restarting at 200 mg/day. Immediate management of AKI should focus on supportive care.20 If there is visible hematuria or increasing proteinuria in association with decline in kidney function, it could be due to the underlying condition rather than treatment with sparsentan and warrants appropriate investigation.
Special Populations
Pregnancy, Breastfeeding, and Patients of Childbearing Potential
The REMS program for monitoring pregnancy is required for all patients with childbearing potential. Because of the risk of embryo-fetal toxicity, pregnancy must be excluded before the start of sparsentan, monthly during treatment, and 1 month after discontinuation of sparsentan. Patients should be advised not to breastfeed during sparsentan treatment due to the potential for adverse effects in the infant. In animal chronic toxicity studies, male reproductive organ toxicity was not evident with sparsentan at exposures up to 10 times and 1.3 times the area under the curve at the maximum recommended human dose in rats and monkeys, respectively.
Hepatic Impairment
Due to the potential risk of serious liver injury, avoid sparsentan treatment in patients with any hepatic impairment (Child-Pugh class A-C).
Advanced CKD
In IgAN, a hypothesized “point of no return” is described to occur within CKD stage 4 and indicates certain progression to KF in the natural history of disease in the absence of treatment.48,49 The proposed concept of a “point of no return” emphasizes the importance of early treatment to reduce proteinuria and blood pressure to delay progression to KF.49 As discussed earlier, sparsentan has demonstrated efficacy in the early onset and sustained lowering of blood pressure and proteinuria in patients with IgAN.39 Additional data in the PROTECT study that examine the delay to KF with sparsentan treatment during CKD stage 4 are expected to become available as a small number of patients with eGFR ranging from 15 to 30 mL/min/1.73 m2 were enrolled in PROTECT and some of these patients are continuing treatment in the PROTECT OLE. The risks for glomerular filtration rate decline and hyperkalemia associated with RAASis have led to concerns regarding their use in patients with advanced CKD. A recent study of patients with advanced CKD (eGFR <30 mL/min/1.73 m2) did not detect differences in risk for progression to KF or mortality based on patterns of ACEi or ARB use.50 Physician’s discretion should be used, based on the patient’s clinical status (eg, susceptibility to hyperkalemia, volume status, frequency of monitoring, and compliance with CKD stage 4–5 management) to determine continuation or cessation of sparsentan.
Chronic Heart Failure
No patients discontinued treatment due to heart failure in the PROTECT 2-year trial.40 Sparsentan has not been evaluated in patients with chronic heart failure.
Elderly Patients
In the PROTECT study, 15 (7.4%) patients were 65 years of age or older. No overall differences in effectiveness or safety were observed between these patients and younger patients.29 Practitioners should exercise clinical judgement in the frequency of monitoring of older adults due to their higher susceptibility to AKI and postural hypotension in general.
Patients in Medical Intensive Care Unit (ICU)
Sparsentan was not evaluated in patients in the medical ICU during clinical trials. Practical guidance for the use of sparsentan in this setting is to withhold sparsentan during severe acute illness, especially with the presence of AKI. With clinical improvement and resolution of AKI, sparsentan can be restarted, with consideration for restarting at 200 mg/day.
Summary
Sparsentan is a first-in-class, novel, single-molecule dual endothelin and angiotensin receptor antagonist that is highly selective for ETAR and AT1R. Sparsentan is the first non-immunosuppressive treatment with accelerated approval by the FDA for the reduction of proteinuria in adults with IgAN who are at high risk of disease progression. Clinical experience with sparsentan, including more than 1200 patients treated in clinical trials and the ongoing PROTECT, DUPLEX, and DUET OLE (with long-term treatment ≥5 years) clinical trials supports the significantly greater antiproteinuric effect of sparsentan versus irbesartan and that the drug is well tolerated with a consistent and manageable safety profile.40,46 The PROTECT trial supported long-term nephroprotection with sparsentan in patients with IgAN over a 2-year double-blind period.40 For the treatment of patients with IgAN with sparsentan, we have outlined a program for patient identification, treatment initiation, efficacy and safety monitoring including REMS monitoring, and considerations for special populations. We are optimistic that the clinical benefits of sparsentan will be apparent to patients and physicians and anticipate smooth and rapid uptake of the drug into clinical practice.
Ethics Statement
This review paper does not include any new data collection or patient interaction and thus did not require ethical approval.
Acknowledgments
The authors thank June Stevens, Lynanne McGuire, Courtney Breuel and Stephen Bublitz of MedVal Scientific Information Services, LLC, for medical writing and editorial assistance, which were funded by Travere Therapeutics, Inc. This manuscript was prepared according to the International Society for Medical Publication Professionals’ “Good Publication Practice for Communicating Company-Sponsored Medical Research: GPP 2022.”
Funding
Travere Therapeutics, Inc.
Disclosure
Dr Campbell served as a consultant to Travere Therapeutics, Inc., Chinook, Calliditas, Sanofi and has received grants from Aurinia. Dr Griffin has served as a consultant, received honoraria for scientific presentations or is a member of a data monitoring committee Alexion, Bayer, Chemocentryx, Eledon Pharmaceuticals, Inc., Genentech, Hansa and for Travere Therapeutics. Dr Trachtman has served as a consultant to and/or a member of a data monitoring committee for Akebia, Chemocentryx, Goldfinch Bio, Inc., Natera, Otsuka, Travere Therapeutics, Inc., and Walden. Dr Geletka is an employee and stockholder of Travere Therapeutics, Inc. Dr Wong has received honoraria for scientific presentations from Alpine, Amgen, AstraZeneca, Baxter, Chinook, CSL Behring, Dimerix, Eledon, George Clinical, Horizon, Otsuka, and Travere Therapeutics, Inc.
References
1. McGrogan A, Franssen CF, de Vries CS. The incidence of primary glomerulonephritis worldwide: a systematic review of the literature. Nephrol Dial Transplant. 2011;26(2):414–430. doi:10.1093/ndt/gfq665
2. Schena FP, Nistor I. Epidemiology of IgA nephropathy: a global perspective. Semin Nephrol. 2018;38(5):435–442. doi:10.1016/j.semnephrol.2018.05.013
3. Pitcher D, Braddon F, Hendry B, et al. Long-term outcomes in IgA nephropathy. Clin J Am Soc Nephrol. 2023;18(6):727–738. doi:10.2215/CJN.0000000000000135
4. Kwon CS, Daniele P, Forsythe A, Ngai C. A systematic literature review of the epidemiology, health-related quality of life impact, and economic burden of immunoglobulin a nephropathy. J Health Econ Outcomes Res. 2021;8(2):36–45. doi:10.36469/jheor.2021.26129
5. Magistroni R, D’Agati VD, Appel GB, Kiryluk K. New developments in the genetics, pathogenesis, and therapy of IgA nephropathy. Kidney Int. 2015;88(5):974–989. doi:10.1038/ki.2015.252
6. Trachtman H, Hogan JJ, Tesar V, Komers RS. Sparsentan. dual angiotensin II AT1 receptor blocker and endothelin ETA receptor antagonist, treatment of focal segmental glomerulosclerosis, treatment of IgA nephropathy. Drugs Future. 2020;45(2):79–98. doi:10.1358/dof.2020.45.2.3058863
7. Rodrigues JC, Haas M, Reich HN. IgA nephropathy. Clin J Am Soc Nephrol. 2017;12(4):677–686. doi:10.2215/CJN.07420716
8. Kohan DE, Barton M. Endothelin and endothelin antagonists in chronic kidney disease. Kidney Int. 2014;86(5):896–904. doi:10.1038/ki.2014.143
9. Lehrke I, Waldherr R, Ritz E, Wagner J. Renal endothelin-1 and endothelin receptor type B expression in glomerular diseases with proteinuria. J Am Soc Nephrol. 2001;12(11):2321–2329. doi:10.1681/ASN.V12112321
10. Kohan DE, Pritchett Y, Molitch M, et al. Addition of atrasentan to renin-angiotensin system blockade reduces albuminuria in diabetic nephropathy. J Am Soc Nephrol. 2011;22(4):763–772. doi:10.1681/ASN.2010080869
11. Komers R, Plotkin H. Dual inhibition of renin-angiotensin-aldosterone system and endothelin-1 in treatment of chronic kidney disease. Am J Physiol Regul Integr Comp Physiol. 2016;310(10):R877–R884. doi:10.1152/ajpregu.00425.2015
12. Barton M, Tharaux PL. Endothelin and the podocyte. Clin Kidney J. 2012;5(1):17–27. doi:10.1093/ckj/sfs001
13. Siragy HM, Carey RM. Role of the intrarenal renin-angiotensin-aldosterone system in chronic kidney disease. Am J Nephrol. 2010;31(6):541–550. doi:10.1159/000313363
14. Kohan DE, Rossi NF, Inscho EW, Pollock DM. Regulation of blood pressure and salt homeostasis by endothelin. Physiol Rev. 2011;91(1):1–77. doi:10.1152/physrev.00060.2009
15. Lai KN, Tang SC, Schena FP, et al. IgA nephropathy. Nat Rev Dis Primers. 2016;2:16001. doi:10.1038/nrdp.2016.1
16. Raina R, Chauvin A, Chakraborty R, et al. The role of endothelin and endothelin antagonists in chronic kidney disease. Kidney Dis. 2020;6(1):22–34. doi:10.1159/000504623
17. Chan LY, Leung JC, Tang SC, Choy CB, Lai KN. Tubular expression of angiotensin II receptors and their regulation in IgA nephropathy. J Am Soc Nephrol. 2005;16(8):2306–2317. doi:10.1681/ASN.2004121117
18. Benigni A, Buelli S, Kohan DE. Endothelin-targeted new treatments for proteinuric and inflammatory glomerular diseases: focus on the added value to anti-renin-angiotensin system inhibition. Pediatr Nephrol. 2021;36(4):763–775. doi:10.1007/s00467-020-04518-2
19. Lin YJ, Kwok CF, Juan CC, et al. Angiotensin II enhances endothelin-1-induced vasoconstriction through upregulating endothelin type A receptor. Biochem Biophys Res Commun. 2014;451(2):263–269. doi:10.1016/j.bbrc.2014.07.119
20. Kidney Disease: Improving Global Outcomes (KDIGO) Glomerular Diseases Work Group. KDIGO 2021 clinical practice guideline for the management of glomerular diseases. Kidney Int. 2021;100(4s):S1–S276. doi:10.1016/j.kint.2021.05.021
21. Sharma S, Smyth B. From proteinuria to fibrosis: an update on pathophysiology and treatment options. Kidney Blood Press Res. 2021;46(4):411–420. doi:10.1159/000516911
22. Kohan DE, Inscho EW, Wesson D, Pollock DM. Physiology of endothelin and the kidney. Compr Physiol. 2011;1(2):883–919.
23. Reich HN, Troyanov S, Scholey JW, Cattran DC. Toronto glomerulonephritis registry. Remission of proteinuria improves prognosis in IgA nephropathy. J Am Soc Nephrol. 2007;18(12):3177–3183. doi:10.1681/ASN.2007050526
24. Calliditas Therapeutics AB. TARPEYO (budesonide) delayed release capsules, for oral use [prescribing information]. Stockholm, Sweden; 2021.
25. Stefan G, Mircescu G. Hydroxychloroquine in IgA nephropathy: a systematic review. Ren Fail. 2021;43(1):1520–1527. doi:10.1080/0886022X.2021.2000875
26. Wheeler DC, Toto RD, Stefánsson BV, et al. A pre-specified analysis of the DAPA-CKD trial demonstrates the effects of dapagliflozin on major adverse kidney events in patients with IgA nephropathy. Kidney Int. 2021;100(1):215–224. doi:10.1016/j.kint.2021.03.033
27. Ayoub I, Komers R, Mercer A, Preciado P, Tang SC, Rovin BH. Sparsentan and sodium-glucose cotransporter 2 inhibitors (SGLT2i) in the PROTECT open-label extension (OLE) substudy and SPARTACUS: trials in progress [Abstract]. J Am Soc Nephrol. 2023;24:979.
28. Kooienga L, Malecki R, Preciado P, He P, Mercer A. On behalf of the DUPRO steering committee. Concomitant sparsentan and sodium-glucose cotransporter-2 inhibitors (SGLT2i) in patients with IgA nephropathy (IgAN) in the PROTECT open-label extension (OLE) [Abstract]. J Am Soc Nephrol. 2023;24:979.
29. Travere Therapeutics, Inc. FILSPARI™ (sparsentan) tablets, for oral use [prescribing information]. San Diego, CA; 2023.
30. Coppo R, Amore A, Gianoglio B, et al. Angiotensin II local hyperreactivity in the progression of IgA nephropathy. Am J Kidney Dis. 1993;21(6):593–602. doi:10.1016/S0272-6386(12)80031-X
31. Boels MG, Avramut MC, Koudijs A, et al. Atrasentan reduces albuminuria by restoring the glomerular endothelial glycocalyx barrier in diabetic nephropathy. Diabetes. 2016;65(8):2429–2439. doi:10.2337/db15-1413
32. Nagasawa H, Suzuki H, Jenkinson C, et al. MO261: sparsentan, the dual endothelin angiotensin receptor antagonist (DEARA), attenuates albuminuria and protects from the development of renal injury to a greater extent than losartan in the GDDY mouse model of IgA nephropathy: a 16-week study [abstract]. Nephrol Dial Transplant. 2022;37(suppl_3):gfac067–060.
33. Reily C, Moldoveanu Z, Pramparo T, et al. The dual endothelin angiotensin receptor antagonist (DEARA) sparsentan protects from glomerular hypercellularity and associated immune/inflammatory gene-network-activity in a model of IgA nephropathy (IgAN) [abstract]. J Am Soc Nephrol. 2021;32:459. doi:10.1681/ASN.2020060833
34. Gyarmati G, Deepak SK, Shroff UN, et al. Sparsentan improves glomerular endothelial and podocyte functions and augments protective tissue repair in a mouse model of focal segmental glomerulosclerosis (FSGS) [abstract]. J Am Soc Nephrol. 2022;33:839–79.
35. Bedard P, Jenkinson C, Komers R. MO255: sparsentan protects the glomerular basement membrane and glycocalyx, and attenuates proteinuria in a rat model of focal segmental glomerulosclerosis (FSGS) [abstract]. Nephrol Dial Transplant. 2022;37(suppl_3):
36. Cosgrove D, Gratton MA, Madison J, et al. Dual inhibition of the endothelin and angiotensin receptor ameliorates renal and inner ear pathologies in Alport mice. J Pathol. 2023;260(3):353–364. doi:10.1002/path.6087
37. Barratt J, Rovin B, Diva U, Mercer A, Komers R. Implementing the kidney health initiative surrogate efficacy endpoint in patients with IgA nephropathy (the PROTECT trial). Kidney Int Rep. 2019;4(11):1633–1637. doi:10.1016/j.ekir.2019.08.007
38. Barratt J, Rovin B, Wong MG, et al. IgA nephropathy patient baseline characteristics in the sparsentan PROTECT study. Kidney Int Rep. 2023;8(5):1043–1056. doi:10.1016/j.ekir.2023.02.1086
39. Heerspink HJL, Radhakrishnan J, Alpers CE, et al. Sparsentan in patients with IgA nephropathy: a prespecified interim analysis from a randomised, double-blind, active-controlled clinical trial. Lancet. 2023;401(10388):1584–1594. doi:10.1016/S0140-6736(23)00569-X
40. Rovin BH, Barratt J, Heerspink HJL, et al. Efficacy and safety of sparsentan versus irbesartan in patients with IgA nephropathy (PROTECT): 2-year results from a randomised, active-controlled, phase 3 trial. Lancet. 2023;402:2077–90.
41. Thompson A, Carroll K, Inker LA, et al. Proteinuria reduction as a surrogate end point in trials of IgA nephropathy. Clin J Am Soc Nephrol. 2019;14(3):469–481. doi:10.2215/CJN.08600718
42. Carroll K, Conley L, Mercer A, Saleem MA, Barratt J. Estimating delay in time to ESKD for treatment effects on proteinuria in IGA nephropathy and FSGS [abstract]. Nephrol Dial Transplant. 2021;36(suppl 1):
43. Hogan J, Derebail VK, Murphy E, et al. Long-term effects of sparsentan, a dual angiotensin and endothelin receptor antagonist in primary focal segmental glomerulosclerosis (FSGS): interim 84-week analysis of the DUET trial [abstract]. J Am Soc Nephrol. 2018;29(suppl):61.
44. Hogan J, Diva U, Murphy E, Rosenberg N, Trachtman H, Komers R. Complete remission of proteinuria in patients with focal segmental glomerulosclerosis treated with sparsentan, a dual endothelin and angiotensin receptor antagonist, in the DUET trial. J Am Soc Nephrol. 2020;31(suppl):55.
45. Trachtman H, Nelson P, Adler S, et al. DUET: a phase 2 study evaluating the efficacy and safety of sparsentan in patients with FSGS. J Am Soc Nephrol. 2018;29(11):2745–2754. doi:10.1681/ASN.2018010091
46. Rheault MN, Alpers CE, Barratt J, et al. Sparsentan versus irbesartan in focal segmental glomerulosclerosis. N Engl J Med. 2023. doi:10.1056/NEJMoa2308550
47. Chen S-C, Cai D, Winnett C, et al. Effect of multiple doses of sparsentan on the single-dose pharmacokinetics of dapagliflozin: an open-label drug–drug interaction study in healthy adults. Clin Pharmacol Drug Dev. 2023;12(5):535–541. doi:10.1002/cpdd.1231
48. Schöll U, Wastl U, Risler T, et al. The ”point of no return” and the rate of progression in the natural history of IgA nephritis. Clin Nephrol. 1999;52(5):285–292.
49. Komatsu H, Fujimoto S, Sato Y, et al. ”Point of no return (PNR)” in progressive IgA nephropathy: significance of blood pressure and proteinuria management up to PNR. J Nephrol. 2005;18(6):690–695.
50. Arora N, Katz R, Bansal N. ACE inhibitor/angiotensin receptor blocker use patterns in advanced CKD and risk of kidney failure and death. Kidney Med. 2020;2(3):248–257. doi:10.1016/j.xkme.2019.12.007
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.