Back to Journals » International Journal of General Medicine » Volume 8
Practical and clinical considerations in assessing patients with atrial fibrillation for switching to non-vitamin K antagonist oral anticoagulants in primary care
Authors Guimarães P, Kaatz S, Lopes RD
Received 1 May 2015
Accepted for publication 20 June 2015
Published 7 September 2015 Volume 2015:8 Pages 283—291
DOI https://doi.org/10.2147/IJGM.S62760
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Patrícia O Guimarães,1 Scott Kaatz,2 Renato D Lopes1
1Duke Clinical Research Institute, Duke Medicine, Durham, NC, 2Hurley Medical Center, Flint, MI, USA
Abstract: Atrial fibrillation (AF) is an important risk factor for thromboembolic events, and anticoagulation therapy can reduce this risk. Vitamin K antagonists (VKAs), such as warfarin, have been used for decades in patients with AF for stroke prevention. Currently, non-VKA oral anticoagulants (NOACs) are approved and available for non-valvular AF patients who are at increased risk of stroke. These agents are safe and effective and have important advantages over VKAs, such as significant reduction in intracranial hemorrhage and no need for routine laboratory monitoring. Thus, should all VKA-treated patients be switched to a NOAC? The aims of this article are: 1) to review the advantages of NOACs over VKAs; 2) to identify the group of patients who most benefit from receiving a NOAC and, therefore, are higher priority to be switched from VKAs; and 3) to provide clinical and practical guidance on how to switch patients safely from VKAs to NOACs.
Keywords: anticoagulation, atrial fibrillation, clinical practice, stroke prevention
Introduction
In the past decades, attention has been given to the importance of maintaining adequate anticoagulation in patients with atrial fibrillation (AF) who are at increased risk of stroke. Oral anticoagulants, such as warfarin and non-vitamin K antagonist oral anticoagulants (NOACs), play an important role in preventing thromboembolic events in this population.1 Currently, there are four NOACs approved and available for patients with non-valvular AF (NVAF), defined as patients with AF without moderate/severe mitral stenosis and/or prosthetic heart valves. However, with several options available, physicians need to be aware of each drug’s attributes so, based on each individual patient’s characteristics, the most appropriate treatment can be chosen.
Warfarin, a vitamin K antagonist (VKA), has been the main anticoagulant used for stroke prevention for patients with AF in the last decades, based on a significant efficacy in reducing the risk of stroke.2 However, some important challenges are associated with warfarin treatment. It is difficult to achieve and maintain the international normalized ratio (INR) within a therapeutic range (2.0–3.0). A meta-analysis with more than 20,000 warfarin-treated patients in the United States has shown that the average time in therapeutic range (TTR) was only 55%.3 Variations in diet and use of concomitant drugs that interact with warfarin’s liver metabolism comprise some of the difficulties of using this drug. In addition, when using warfarin, there is need for monitoring its effect through regular INR measures and frequent dose adjustments, especially in the first weeks of initiating treatment. More important, warfarin use is associated with increased risk of bleeding, particularly intracranial hemorrhage.4
NOACs have been studied in large randomized clinical trials with over 70,000 patients from multiple countries. Dabigatran, a direct thrombin inhibitor, was the first agent without need for laboratory monitoring approved by regulatory agencies for anticoagulation in NVAF patients in the United States. Rivaroxaban, apixaban, and edoxaban, direct inhibitors of factor Xa, were studied thereafter. All of these therapies are safe and efficacious and have important advantages over warfarin.5–8 Thus, an important question has inevitably been raised: should we switch all VKA-treated patients to a NOAC? The aims of this article are: 1) to review the advantages of NOACs over VKAs; 2) to identify the group of patients who most benefit from receiving a NOAC and, therefore, are higher priority to be switched from VKAs; and 3) to provide clinical and practical guidance on how to switch patients safely from VKAs to NOACs.
Efficacy of NOACs
The Randomized Evaluation of Long-Term Anticoagulation Therapy (RE-LY) trial compared a new anticoagulant, dabigatran, with warfarin in patients with NVAF and at least one risk factor for stroke (Table 1).5 It was shown that 110 mg twice daily of dabigatran was noninferior to warfarin in preventing stroke or systemic embolism (relative risk [RR] 0.91, 95% confidence interval [CI] 0.74–1.11; P<0.001) and the 150 mg twice-daily dose regimen was superior to warfarin (RR 0.66, 95% CI 0.53–0.82; P<0.001). The rate of all-cause death was 4.13%/year in the warfarin-treated patients, whereas it was 3.75%/year in the 110 mg dabigatran group (RR 0.91, 95% CI 0.80–1.03; P=0.13) and 3.64%/year in the 150 mg dabigatran group (RR 0.88, 95% CI 0.77–1.00; P=0.051).
Rivaroxaban, a direct factor Xa inhibitor, was tested against warfarin in the Rivaroxaban Once Daily Oral Direct Factor Xa Inhibition Compared With Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation (ROCKET-AF) trial. Patients included in this study had a mean CHADS2 score of 3.5, higher than the RE-LY population.6 It was shown that 20 mg daily of rivaroxaban (or 15 mg daily in patients with creatinine clearance of 30–49 mL/min) was noninferior to warfarin in preventing stroke and systemic embolism (hazard ratio [HR] 0.79, 95% CI 0.66–0.96; P<0.001). No significant difference was observed in the rates of all-cause death within the two groups (4.5%/year rivaroxaban versus 4.9%/year warfarin, HR 0.92, 95% CI 0.82–1.03; P=0.15).
The Apixaban Versus Acetylsalicylic Acid to Prevent Stroke in Atrial Fibrillation Patients Who Have Failed or Are Unsuitable for Vitamin K Antagonist Treatment (AVERROES) trial compared apixaban with aspirin for AF patients who were considered unsuitable for VKA treatment. This study was terminated prematurely since an overwhelming treatment benefit was shown in favor of apixaban (HR 0.45, 95% CI 0.32–0.62; P<0.001 for the primary outcome of stroke/systemic embolism) at similar rates of major bleeding when compared with aspirin.9 The Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation (ARISTOTLE) trial compared apixaban 5 mg twice daily to dose-adjusted warfarin to achieve INR between 2.0 and 3.0.7 An apixaban dose of 2.5 mg twice daily was given in 4.7% of the patients, in whom two or more of these characteristics were present: age ≥80 years, weight ≤60 kg, or serum creatinine of ≤1.5 mg/dL. Treatment with apixaban was associated with a lower rate of stroke or systemic embolism as compared with warfarin (HR 0.79, 95% CI 0.66–0.95; P=0.01). The rate of hemorrhagic stroke was 0.24%/year with apixaban and 0.47%/year with warfarin (HR 0.51, 95% CI 0.35–0.75; P<0.001). The occurrence of ischemic or uncertain type of stroke was 8% lower with apixaban, as compared with warfarin (HR 0.92, 95% CI 0.74–1.13). A lower rate of all-cause death was also observed in patients using apixaban (HR 0.89, 95% CI 0.80–0.998; P=0.047).
Edoxaban, another factor Xa inhibitor, was studied in the Effective Anticoagulation With Factor Xa Next Generation in Atrial Fibrillation-Thrombolysis in Myocardial Infarction 48 (ENGAGE AF-TIMI 48) trial.8 Both dose regimens of edoxaban (60 mg and 30 mg) were noninferior to warfarin in preventing stroke and systemic embolism in NVAF patients (HR 0.79, 95% CI 0.63–0.99; P<0.001 for high-dose edoxaban and HR 1.07, 95% CI 0.87–1.31; P=0.005 for low-dose edoxaban). However, the low-dose edoxaban regimen was associated with a higher rate of ischemic stroke, as compared with warfarin (HR 1.41, 95% CI 1.19–1.67; P<0.001). The rate of death from cardiovascular causes was lower in both edoxaban groups, in comparison with warfarin (2.74%/year high-dose edoxaban versus 2.71%/year low-dose edoxaban versus 3.17%/year warfarin). The rate of all-cause death was also lower in the low-dose edoxaban group as compared with warfarin (3.8%/year versus 4.35%/year, HR 0.87, 95% CI 0.79–0.96; P=0.006); however, the low-dose regime was not approved in the United States.
Safety of NOACs
Bleeding is the major concern related to anticoagulation therapy and is the main reason for why only about 50% of eligible AF patients receive an appropriate treatment with anticoagulants. Treatment with dabigatran 110 mg twice daily was associated with a lower rate of major bleeding as compared with warfarin (RR 0.80, 95% CI 0.69–0.93; P=0.003), whereas the use of 150 mg twice daily had similar rates of major bleeding as warfarin (3.11%/year versus 3.36%/year; P=0.31).5 However, patients using dabigatran 150 mg twice daily were more likely to present gastrointestinal bleeding than when treated with warfarin (RR 1.5, 95% CI 1.19–1.89; P<0.001). The rate of hemorrhagic stroke was lower in both doses of dabigatran (0.12%/year in the 110 mg group and 0.10%/year in the 150 mg group) when compared with warfarin (0.38%/year; P<0.001 for both comparisons). The rates of intracranial hemorrhage were lower among patients using either dose of dabigatran (0.23%/year in the 110 mg group and 0.30%/year in the 150 mg group) as compared with warfarin (0.74%/year; P<0.001 for both comparisons).
In the ROCKET-AF trial, no difference was observed in the rates of major and clinically relevant nonmajor bleeding between rivaroxaban-treated and warfarin-treated patients (14.9%/year versus 14.5%/year; P=0.44).6 Rivaroxaban was also associated with less intracranial bleeding when compared with warfarin (0.5%/year in the rivaroxaban group and 0.7%/year in the warfarin group; P=0.02).
In the AVERROES trial, major bleeding occurred in 1.4%/year in the apixaban group and 1.2%/year in the aspirin group (P=0.57). In addition, no statistically significant difference between the study arms was observed in the rates of intracranial and fatal hemorrhages.9 In the ARISTOTLE trial, major bleeding, the primary safety outcome, occurred significantly less in the apixaban group as compared with warfarin (2.13%/year versus 3.09%/year, HR 0.69, 95% CI 0.60–0.80; P<0.001).7 Also, major bleeding followed by death within 30 days occurred half as often in apixaban patients, in comparison with warfarin (95% CI 0.33–0.74; P<0.001).10 Consistently with the results for the primary safety outcome of major bleeding, a reduction in intracranial bleeding events was observed with apixaban use in comparison with warfarin (0.33%/year versus 0.80%/year, HR 0.42, 95% CI 0.30–0.58; P<0.001).7
Both doses of edoxaban studied in the ENGAGE AF-TIMI 48 trial were also associated with a reduction in the primary safety outcome of major bleeding, when compared with warfarin (2.75%/year high-dose edoxaban, 1.61%/year low-dose edoxaban versus 3.43%/year warfarin; P<0.001 for both comparisons with warfarin).8 Hemorrhagic stroke was less likely to occur with edoxaban, as compared with warfarin (0.26%/year high-dose edoxaban versus 0.16%/year low-dose edoxaban versus 0.47%/year for warfarin; P<0.001 for both comparisons with warfarin). Lower rates of intracranial hemorrhage were shown with both doses of edoxaban (0.39%/year in the high-dose edoxaban group versus 0.26%/year in the low-dose edoxaban group versus 0.85%/year in the warfarin group; P<0.001 for both comparisons with warfarin).
Currently, specific antidotes for NOACs are under investigation and have not been approved for clinical use yet. Thus, in cases of bleeding with NOACs, it is important to access the timing of the last dose and the patient’s renal function in order to estimate duration of drug effect. Vitamin K and fresh frozen plasma are commonly used for reversal of VKA effects. However, it usually takes hours to normalize INR levels and there is no scientific evidence that these agents can improve clinical outcomes after major bleeding on warfarin. Since NOACs’ half-lives are much shorter than that for warfarin, time seems to be a good strategy to manage bleeding events with these new agents.
In summary, large clinical trials experience has shown that NOACs are at least as safe as warfarin with regards to bleeding events (Table 1). More importantly, the rates of intracranial hemorrhage, a feared consequence of anticoagulation, are substantially lower than warfarin with all four NOACs. When a major bleeding event occurred with a NOAC, the associated mortality rates were lower than that with warfarin, as illustrated by Hylek et al.6–8,10–12 For intracranial bleeding, in general, the case fatality rates were not statistically different between NOACs and warfarin.13–15 It is important to mention, however, that in the ENGAGE AF-TIMI 48, the rates of fatal intracranial bleeding were significantly lower in both low-dose and high-dose of edoxaban groups, in comparison with warfarin (0.08%/year low-dose edoxaban, 0.15%/year high-dose edoxaban, 0.27%/year warfarin).16 These findings are important advantages of these new agents over warfarin.
Results in vitamin K antagonist oral anticoagulants-experienced patients
Approximately half of the participants included in the RE-LY trial were VKA-experienced patients (more than 62 days of lifetime VKA exposure).17 A significantly lower rate of the primary outcome was shown with dabigatran in comparison with warfarin, regardless of prior warfarin exposure (interaction P=0.72 for dabigatran 110 mg and interaction P=0.84 for dabigatran 150 mg).
In the ROCKET-AF trial, 55.4% of the patients were defined as VKA-experienced (use of a VKA for at least 6 weeks). A subgroup analysis has shown that rivaroxaban treatment effect in preventing stroke and systemic embolism was consistent regardless of prior warfarin use (interaction P=0.36).18
A subanalysis of the ARISTOTLE trial also aimed to investigate whether there was a difference in apixaban treatment effect between patients who were VKA-naive and VKA-experienced.19 Naivety was defined as prior VKA use for less than 30 consecutive days at any time. A consistency of treatment effect was observed between subgroups for the primary outcome of stroke or systemic embolism (interaction P=0.39). Thus, these results show that NOACs are effective in patients who have been exposed to a VKA in the past.
Among the 21,105 patients included in the ENGAGE AF-TIMI 48 trial, approximately 59% had been previously exposed to VKA for ≥60 days at any time prior to enrolment.8 Overall, the low-dose edoxaban regimen seems to be less efficacious than warfarin among warfarin-experienced patients, as a higher rate of the primary outcome was observed in this subgroup (HR 1.31, 95% CI 1.08–1.60; interaction P=0.019) when compared with warfarin-naive patients (HR 0.92, 95% CI 0.73–1.15). A similar efficacy effect was seen between high-dose of edoxaban and warfarin among warfarin-experienced patients, while a greater benefit on efficacy outcomes was observed among patients receiving the high-dose edoxaban regimen versus warfarin among the warfarin-naive patients (interaction P=0.028).20
Results according to center time in therapeutic range
A stable INR is important to establish warfarin efficacy and one of the biggest limitations of using VKAs is to maintain an INR within a therapeutic range. The mean TTR among warfarin-treated patients in the RE-LY trial was 64%. A subanalysis of this trial divided sites into quartiles of center mean time in therapeutic range (cTTR) to evaluate the efficacy and safety of dabigatran in relation to quality of INR control.21 It was shown that the efficacy of dabigatran in preventing stroke and systemic embolism was consistent among quartiles of cTTR (interaction P=0.89 and 0.20 for 110 mg dabigatran and 150 mg dabigatran versus warfarin, respectively). Importantly, the rates of intracranial bleeding were lower in both dabigatran groups as compared with warfarin, irrespective of cTTR (interaction P=0.71 and 0.89 for 110 mg dabigatran and 150 mg versus warfarin, respectively).
The mean TTR in the ROCKET-AF trial was 55%. A prespecified subanalysis showed the efficacy of rivaroxaban did not vary across cTTR quartiles (interaction P=0.71).22 Even though bleeding events were more likely to occur within increasing cTTR quartiles (HR 0.80, 95% CI 0, 66–0.98 in the lowest quartile, and HR 1.25, 95% CI 1.10–1.41 in the highest quartile; interaction P=0.001), the reduction in the hazards of intracranial hemorrhage was consistent within cTTR quartiles.
In the ARISTOTLE trial, the median TTR was 66% (interquartile range [IQR] 52.4–76.5). The efficacy of apixaban in reducing the rate of the primary outcome was also consistent among different levels of predicted cTTR (interaction P=0.078), and major bleeding was also significantly lower with apixaban treatment as compared with warfarin across all cTTR quartiles (interaction P=0.095).23
The median TTR in the ENGAGE AF-TIMI 48 trial was 68.4% (interquartile range 56.5–77.4) and the mean TTR was 64.9%, slightly higher than in other NOACs trials.8 No interaction was found among cTTR and treatment effect (interaction P=0.24 for low-dose edoxaban and 0.57 for high-dose edoxaban). These findings indicate that NOACs were associated with consistent efficacy and safety benefits, regardless of quality of INR control in different sites of the world.
Results according to age
Older patients are at higher risk for both cardiovascular and bleeding events.24,25 The efficacy of NOACs in preventing stroke and systemic embolism was consistent with the main trials’ results with no significant interaction among age subgroups in the RE-LY trial (interaction P=0.81 for dabigatran 110 mg and 150 mg), in the ROCKET-AF trial (interaction P=0.3131), in the ARISTOTLE trial (interaction P=0.11), and in the ENGAGE AF-TIMI 48 trial (interaction P=0.59 for high-dose edoxaban and 0.87 for low-dose edoxaban).8,24–26
An analysis of the RE-LY trial has shown that the risk of extracranial bleeding among older patients (age ≥75 years) was higher when using dabigatran than warfarin (interaction P=0.001 for dabigatran 110 mg and <0.001 for dabigatran 150 mg).26 Importantly, the risk of intracranial bleeding was lower with both doses of dabigatran when compared with warfarin, regardless of age. Rivaroxaban has been shown to be as safe as warfarin, regardless of age.24 Apixaban and edoxaban were shown to be safer than warfarin across all age categories.8,25
Main reasons to switch from warfarin to a NOAC
- NOACs are at least as effective as warfarin (some are superior to warfarin) for stroke prevention and as safe as warfarin (some are safer than warfarin). The most important and common finding among the NOACs is the lower rates of intracranial bleeding when compared with warfarin, a drastic and potentially fatal consequence of anticoagulation (Table 2).
- Near half of the patients included in the main trials that investigated the efficacy of NOACs were on VKAs before enrolment, which demonstrates the efficacy and safety when considering switching from a VKA.
- The main trials’ results were consistent regardless of the time in therapeutic range.
- Approximately 2/3 of intracranial bleeding events occur with INR within therapeutic range, which means that even well-controlled patients are at risk for intracranial hemorrhage.27
  | Table 2 Main advantages of NOACs over vitamin K antagonists |
Who should be switched?
NOACs should be considered for almost all NVAF patients, considering their efficacy and safety profile. However, there are some specific populations that might benefit more from switching from VKAs to NOACs. Labile INR has been identified as a risk factor for bleeding events and this variable is included in the HAS-BLED bleeding risk score,28 although the relative efficacy and safety of NOACs compared with warfarin was consistent across INR time in therapeutic strata. Thus, patients with poor INR control are at higher priority to be switched. In addition, NOACs should be considered for subjects who do not desire to have their INR measured in a routine manner.
Patients who had experienced previous stroke or intracranial bleeding are at higher risk of presenting recurrent episodes in the future.29–31 The rates of stroke or systemic embolism were higher among patients with previous stroke or transient ischemic attack (TIA) in comparison to subjects without this history in the RE-LY trial (2.38%/year versus 1.22%/year; P<0.0001), in the ROCKET-AF trial (2.87%/year versus 1.66%/year; P<0.0001), and in the ARISTOTLE trial (2.85%/year versus 1.12%/year).29–31 In addition, in the ARISTOTLE trial, patients with previous stroke/TIA (19% of the total) were more likely to present episodes of major bleeding (HR 1.37, 95% CI 1.17–1.62) and intracranial hemorrhage (HR 2.15, 95% CI 1.57–2.96).31 The efficacy of NOACs in reducing the risk of the primary outcome was consistent with the main trials’ results regardless of prior stroke/TIA (interaction P=0.62 for dabigatran 110 mg, 0.34 for dabigatran 150 mg, 0.23 for rivaroxaban, 0.71 for apixaban, 0.86 for high-dose edoxaban, and 0.84 for low-dose edoxaban). Also, no interaction was found related to the main safety outcomes in these trials. These findings reinforce that NOACs should be chosen for patients with previous stroke or TIA, instead of warfarin.
Patients at higher risk for bleeding are good candidates to be switched from warfarin to apixaban and dabigatran.32,33 Additionally, individuals with renal dysfunction might benefit from switching from warfarin to rivaroxaban, apixaban, or edoxaban, following the appropriate dose adjustments that are recommended for each agent (Table 3).8,34 Importantly, given that patients with creatinine clearance <30 mL/min were excluded from RE-LY, ROCKET-AF, and ENGAGE AF-TIMI 48, and with creatinine clearance of <25 mL/min or a creatinine >2.5 mg/dL were excluded from ARISTOTLE, these new agents should not be used in those patients. Finally, dabigatran 150 mg twice daily seems an attractive agent for patients at high risk for ischemic stroke and low risk for bleeding.5
How to switch?
Defining the correct timing for switching is extremely important. It is recommended to evaluate INR level before switching from VKAs to NOACs in order to avoid both excess bleeding risk due to combined anticoagulation effects, and thromboembolic risk, when the patient is not under adequate anticoagulation. Different strategies for switching were established for each NOAC based on each trial protocol and drug profile. While for dabigatran and apixaban it is recommended to stop VKA and wait until INR is below 2.0 to start a NOAC, for rivaroxaban, the recommendation is to wait until INR is below 3.0. If edoxaban is the drug chosen, the INR level considered adequate for initiating edoxaban is 2.5 (Figure 1).
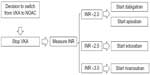  | Figure 1 Timing for switching from a VKA to a NOAC. |
Who should not be switched?
VKAs are still the most appropriate choice for some patients (Table 4). It is important to consider that patients with end-stage renal disease were not included in NOACs trials; therefore, renal function should be evaluated and switching from VKAs to NOACs is not recommended when creatinine clearance is less than 30 mL/min (dabigatran, rivaroxaban, and edoxaban) or less than 25 mL/min for apixaban. Also, it is recommended to monitor renal function during treatment in order to detect renal impairment early and, in some cases, change the dose of the NOAC or even stop it.35 Edoxaban should not be used for patients with creatinine clearance greater than 95 mL/min, since edoxaban plasma levels are lower in this group of individuals and a subanalysis of the ENGAGE AF-TIMI 48 trial has shown higher rates of ischemic stroke in this population when compared with warfarin.8
  | Table 4 Who should not be switched from a vitamin K antagonist to a non-vitamin K antagonist oral anticoagulant |
The main clinical trials that studied NOACs also did not include patients with mechanical heart valves and moderate/severe mitral stenosis. Thus, there is no evidence supporting the use of these new drugs for these groups of patients and therefore VKAs should be maintained. Additionally, dabigatran was associated with a higher risk of stroke, myocardial infarction, and prosthesis thrombosis in patients with mechanical heart valves, and therefore is contraindicated in this population.36 Finally, cost is another issue to be considered before switching from VKAs to NOACs since the latter are more expensive than VKAs.
Conclusion
A new era for anticoagulation therapy in AF has begun. NOACs have a clear benefit in reducing intracranial bleeding and offer a more convenient therapy for patients and health care providers that may help ensuring that more eligible AF patients receive an appropriate anticoagulant therapy based on their stroke and bleeding risks. Strategies for switching vary within each drug and INR values should be checked before transition. Despite that, VKAs are still the preferred anticoagulants for some cases. Importantly, physicians should be looking at reasons to use NOACs, instead of looking at reasons not to use them, so a higher quality of anticoagulation therapy in AF patients can be achieved, improving patient outcomes and safety.
Disclosure
POG reports no conflicts of interest in this work. SK reports receiving speaker honoraria from Boehringer Ingelheim, Bristol-Myers Squibb/Pfizer, CSL Behring, Janssen, and Daiichi Sankyo; and consultancy fees from Janssen, Daiichi Sankyo, Bristol-Myers Squibb/Pfizer, and Boehringer Ingelheim. RDL reports institutional research grants from Bristol-Myers Squibb and Glaxo Smith Kline; and consultancy fees from Bayer, Boehringer-Inglelheim, Bristol-Myers Squibb, Merck, Pfizer, and Portola. The authors report no other conflicts of interest in this work.
References
January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64(21):e1–e76. | |
Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007;146(12):857–867. | |
Baker WL, Cios DA, Sander SD, Coleman CI. Meta-analysis to assess the quality of warfarin control in atrial fibrillation patients in the United States. J Manag Care Pharm. 2009;15(3):244–252. | |
Hylek EM, Evans-Molina C, Shea C, Henault LE, Regan S. Major hemorrhage and tolerability of warfarin in the first year of therapy among elderly patients with atrial fibrillation. Circulation. 2007;115(21):2689–2696. | |
Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009; 361(12):1139–1151. | |
Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365(10):883–891. | |
Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011; 365(11):981–992. | |
Giugliano RP, Ruff CT, Braunwald E, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369(22):2093–2104. | |
Connolly SJ, Eikelboom J, Joyner C, et al. Apixaban in patients with atrial fibrillation. N Engl J Med. 2011;364(9):806–817. | |
Hylek EM, Held C, Alexander JH, et al. Major bleeding in patients with atrial fibrillation receiving apixaban or warfarin: The ARISTOTLE Trial (Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation): Predictors, Characteristics, and Clinical Outcomes. J Am Coll Cardiol. 2014;63(20):2141–2147. | |
Beyer-Westendorf J, Förster K, Pannach S, et al. Rates, management, and outcome of rivaroxaban bleeding in daily care: results from the Dresden NOAC registry. Blood. 2014;124(6):955–962. | |
Majeed A, Hwang HG, Connolly SJ, et al. Management and outcomes of major bleeding during treatment with dabigatran or warfarin. Circulation. 2013;128(21):2325–2332. | |
Hankey GJ, Stevens SR, Piccini JP, et al. Intracranial hemorrhage among patients with atrial fibrillation anticoagulated with warfarin or rivaroxaban: the rivaroxaban once daily, oral, direct factor Xa inhibition compared with vitamin K antagonism for prevention of stroke and embolism trial in atrial fibrillation. Stroke. 2014;45(5):1304–1312. | |
Hart RG, Diener HC, Yang S, et al. Intracranial hemorrhage in atrial fibrillation patients during anticoagulation with warfarin or dabigatran: the RE-LY trial. Stroke. 2012;43(6):1511–1517. | |
Held C, Hylek EM, Alexander JH, et al. Clinical outcomes and management associated with major bleeding in patients with atrial fibrillation treated with apixaban or warfarin: insights from the ARISTOTLE trial. Eur Heart J. 2015;36(20):1264–1272. | |
Giugliano RP, Ruff CT, Rost NS, et al. Cerebrovascular events in 21 105 patients with atrial fibrillation randomized to edoxaban versus warfarin: Effective Anticoagulation with Factor Xa Next Generation in Atrial Fibrillation-Thrombolysis in Myocardial Infarction 48. Stroke. 2014;45(8):2372–2378. | |
Ezekowitz MD, Wallentin L, Connolly SJ, et al. Dabigatran and warfarin in vitamin K antagonist-naive and -experienced cohorts with atrial fibrillation. Circulation. 2010;122(22):2246–2253. | |
Mahaffey KW, Wojdyla D, Hankey GJ, et al. Clinical outcomes with rivaroxaban in patients transitioned from vitamin K antagonist therapy: a subgroup analysis of a randomized trial. Ann Intern Med. 2013; 158(12):861–868. | |
Garcia DA, Wallentin L, Lopes RD, et al. Apixaban versus warfarin in patients with atrial fibrillation according to prior warfarin use: results from the Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation trial. Am Heart J. 2013;166(3):549–558. | |
O’Donoghue ML, Ruff CT, Giugliano RP, et al. Edoxaban vs warfarin in vitamin K antagonist experienced and naive patients with atrial fibrillation†. Eur Heart J. 2015;36(23):1470–1477. | |
Wallentin L, Yusuf S, Ezekowitz MD, et al. Efficacy and safety of dabigatran compared with warfarin at different levels of international normalised ratio control for stroke prevention in atrial fibrillation: an analysis of the RE-LY trial. Lancet. 2010;376(9745):975–983. | |
Piccini JP, Hellkamp AS, Lokhnygina Y, et al. Relationship between time in therapeutic range and comparative treatment effect of rivaroxaban and warfarin: results from the ROCKET AF trial. J Am Heart Assoc. 2014;3(2):e000521. | |
Wallentin L, Lopes RD, Hanna M, et al. Efficacy and safety of apixaban compared with warfarin at different levels of predicted international normalized ratio control for stroke prevention in atrial fibrillation. Circulation. 2013;127(22):2166–2176. | |
Halperin JL, Hankey GJ, Wojdyla DM, et al. Efficacy and safety of rivaroxaban compared with warfarin among elderly patients with nonvalvular atrial fibrillation in the Rivaroxaban Once Daily, Oral, Direct Factor Xa Inhibition Compared With Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation (ROCKET AF). Circulation. 2014;130(2):138–146. | |
Halvorsen S, Atar D, Yang H, et al. Efficacy and safety of apixaban compared with warfarin according to age for stroke prevention in atrial fibrillation: observations from the ARISTOTLE trial. Eur Heart J. 2014;35(28):1864–1872. | |
Eikelboom JW, Wallentin L, Connolly SJ, et al. Risk of bleeding with 2 doses of dabigatran compared with warfarin in older and younger patients with atrial fibrillation: an analysis of the randomized evaluation of long-term anticoagulant therapy (RE-LY) trial. Circulation. 2011;123(21):2363–2372. | |
Rosand J, Eckman MH, Knudsen KA, Singer DE, Greenberg SM. The effect of warfarin and intensity of anticoagulation on outcome of intracerebral hemorrhage. Arch Intern Med. 2004;164(8):880–884. | |
Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. 2010;138(5):1093–1100. | |
Diener HC, Connolly SJ, Ezekowitz MD, et al. Dabigatran compared with warfarin in patients with atrial fibrillation and previous transient ischaemic attack or stroke: a subgroup analysis of the RE-LY trial. Lancet Neurol. 2010;9(12):1157–1163. | |
Hankey GJ, Patel MR, Stevens SR, et al. Rivaroxaban compared with warfarin in patients with atrial fibrillation and previous stroke or transient ischaemic attack: a subgroup analysis of ROCKET AF. Lancet Neurol. 2012;11(4):315–322. | |
Easton JD, Lopes RD, Bahit MC, et al. Apixaban compared with warfarin in patients with atrial fibrillation and previous stroke or transient ischaemic attack: a subgroup analysis of the ARISTOTLE trial. Lancet Neurol. 2012;11(6):503–511. | |
Oldgren J, Alings M, Darius H, et al. Risks for stroke, bleeding, and death in patients with atrial fibrillation receiving dabigatran or warfarin in relation to the CHADS2 score: a subgroup analysis of the RE-LY trial. Ann Intern Med. 2011;155(10):660–667, W204. | |
Lopes RD, Al-Khatib SM, Wallentin L, et al. Efficacy and safety of apixaban compared with warfarin according to patient risk of stroke and of bleeding in atrial fibrillation: a secondary analysis of a randomised controlled trial. Lancet. 2012;380(9855):1749–1758. | |
Fox KA, Piccini JP, Wojdyla D, et al. Prevention of stroke and systemic embolism with rivaroxaban compared with warfarin in patients with non-valvular atrial fibrillation and moderate renal impairment. Eur Heart J. 2011;32(19):2387–2394. | |
Camm AJ, Lip GY, De Caterina R, et al. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J. 2012;33(21):2719–2747. | |
Eikelboom JW, Connolly SJ, Brueckmann M, et al. Dabigatran versus warfarin in patients with mechanical heart valves. N Engl J Med. 2013;369(13):1206–1214. | |
Dabigatran: Pradaxa® (dabigatran) [package insert]. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals; 2010. | |
Rivaroxaban: Xarelto® (rivaroxaban) [package insert]. Titusville, NJ: Janssen Pharmaceuticals; 2011. | |
Apixaban: Eliquis® (apixaban) [package insert]. Princeton, NJ, and New York, NY: Bristol-Myers Squibb, and Pfizer; 2012. | |
Edoxaban: Savaysa™ (edoxaban) [package insert]. Parsippany, NJ: Daiichi Sankyo; 2015. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


