Back to Journals » International Journal of Nanomedicine » Volume 15
Potential Therapeutic Usage of Nanomedicine for Glaucoma Treatment
Authors Kwon S, Kim SH, Khang D , Lee JY
Received 26 March 2020
Accepted for publication 5 June 2020
Published 6 August 2020 Volume 2020:15 Pages 5745—5765
DOI https://doi.org/10.2147/IJN.S254792
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 7
Editor who approved publication: Dr Thomas Webster
Song Kwon,1,* Sung Hyun Kim,2,* Dongwoo Khang,1,3,4 Jong Yeon Lee2
1Lee Gil Ya Cancer and Diabetes Institute, Gachon University, Incheon 21999, South Korea; 2Department of Ophthalmology, Gil Medical Center, Gachon University, College of Medicine, Incheon 21565, South Korea; 3Department of Gachon Advanced Institute for Health Science & Technology (GAIHST), Gachon University, Incheon 21999, South Korea; 4Department of Physiology, School of Medicine, Gachon University, Incheon 21999, South Korea
*These authors contributed equally to this work
Correspondence: Jong Yeon Lee
Department of Ophthalmology, College of Medicine, Gachon University, Incheon 21565, South Korea
Email [email protected]
Dongwoo Khang
Department of Physiology, School of Medicine, Gachon University, Incheon 21999, South Korea
Tel/ Fax +82 32 899 1525
Email [email protected]
Abstract: Glaucoma is a group of diseases characterized by progressive degeneration of retinal ganglion cells, leading to irreversible blindness. Currently, intraocular pressure reduction is the only established treatment available for glaucoma. With this treatment, the progression of the disease can only be delayed and there is no recovery. In addition, the commercially available eye drops have the disadvantage of low compliance and short therapeutic time, while glaucoma surgery always has the risk of failure due to wound fibrosis. Nanotechnology can overcome the limitations of the current treatment through the encapsulation and conjugation of drugs used for lowering intraocular pressure and antifibrotic agents using biodegradable or biocompatible nanoparticles for the sustained release of the drugs to protect the damaged ocular cells. Furthermore, using nanotechnology, treatment can be administered in various forms, including eye drops, contact lens, and ocular inserts, according to the convenience of the patients. Despite the promising results of delaying the progression of glaucoma, the regeneration of damaged ocular cells, including trabecular meshwork and retinal ganglion cells, is another critical hurdle to overcome. Bone marrow-derived mesenchymal stem cells and Müller glia cells can secrete neurogenic factors that trigger the regeneration of associated cells, including trabecular meshwork and retinal ganglion cells. In conclusion, this review highlights the potential therapeutic applications of nanotechnology- and stem cell-based methods that can be employed for the protection and regeneration of ocular cells.
Keywords: glaucoma, ocular regeneration, protection, stem cell, nanotechnology
Introduction
Glaucoma is the second leading cause of blindness worldwide. According to World Health Organization, 4.5 million or more than 12% of all cases of blindness globally were the result of glaucoma.1 Glaucoma is a group of optic neuropathies characterized by progressive degeneration of retinal ganglion cells (RGCs) in the inner retina and loss of their axons in the optic nerve.2 The disease progresses slowly without obvious symptoms until it leads to irreversible visual field loss and optic nerve damage.
There are several reasons for blindness from glaucoma. First, it is often diagnosed late because the patients remain unaware of the gradual contraction of their visual fields until finally their visual acuity begins to fail. Second, the disease is improperly controlled by medication and surgery. Currently, intraocular pressure (IOP) reduction is the only proven treatment for glaucoma.3 The effectiveness of glaucoma surgery decreases with time because of the fibrosis of the surgical site.4 In addition, insufficient reduction of IOP by medical treatment is a cause for the progression of disease. In some patients with glaucoma, the visual field loss can continue to progress even with lowered IOP.5 Difficulty in detection of IOP fluctuation and non-IOP factors such as neurodegeneration may contribute to the progression.6 Another reason is the issue of compliance with the treatment. Since glaucoma is a life-long condition, many patients with glaucoma using eye drops experience difficulties in administration of the drops and suffer from ocular discomfort. This difficulty in the use of eye drops can increase the risk of glaucoma progression because of poor compliance.7,8 Trans-corneal drug penetration through topical eye drops is currently the most commonly used route of drug delivery in ophthalmic medical fields. However, preservative or active ingredients of topical eye drops can have an adverse effect on the ocular surface, leading to dry eye symptom.9 Thus, the current ophthalmic drug delivery system needs to be improved for better patient compliance and better efficiency.
Among the various fields in which nanotechnology can be applied, biology and medicine have gained increasing attention recently.10 Nanomedicine is a comprehensive field that combines biology, chemistry, engineering, and medicine to provide more efficient tools for the prevention and treatment of various diseases. It can provide new strategies to clinicians for prevention, diagnosis, and treatment of severe disease such as cancer.11 Additionally, nanotechnology could be used to introduce remarkable improvements in drug delivery systems,12 medical imaging and diagnosis platforms,13 implantable materials,14 and tissue regeneration strategies.15,16 Therefore, the use of nanomedicine could result in development of better diagnostic and therapeutic strategies for the prevention of blindness from glaucoma. In this manuscript, the authors have reviewed and discussed promising strategies involving use of nanotechnology for treatment of patients with glaucoma, in an attempt to overcome the limitations of current treatments for glaucoma.
Glaucoma
Definition and Classification of glaucoma
Clinically, glaucoma is defined as a condition with characteristic appearance of optic disc (optic disc cupping) and corresponding visual field loss.17 The nerve fibers of RGCs pass out of the eye at the optic disc. The neuroretinal rim of the optic disc contains the nerve fibers of RGCs. In glaucoma, the neuroretinal rim of the optic disc becomes progressively thinner, thereby resulting in the enlargement of the optic disc cup (central depression at optic disc).18 This is called as optic disc cupping.19 The loss of the RGC nerve fibers leads to a loss of the connection between the retina and visual pathway of brain, leading to visual field defect.20
Elevated IOP is the main risk factor for glaucoma.21 IOP is determined by the balance between the inflow and outflow of aqueous humor.22 Aqueous humor is a clear fluid found in the anterior and posterior chambers of the eye, which is required to provide nutrients and remove cellular waste products.23 In addition, aqueous humor has a role in maintaining the shape of the eyeball and related refractive properties of the eye. Aqueous humor is actively produced by the ciliary body in the posterior chamber and exits the anterior chamber through two distinct routes, including the trabecular pathway and the uveoscleral pathway.24 Under physiological conditions, the trabecular pathway accounts for about 90% of the total outflow of aqueous humor. In the trabecular pathway, aqueous humor drains through the filter-like region of the trabecular meshwork (TM) and Schlemm’s canal (SC), and finally enters systemic circulation through the episcleral veins.23
Glaucoma can be clinically classified by several factors. First, based on the appearance of the iridocorneal angle, where TM is located, glaucoma is defined as angle-closure glaucoma or open-angle glaucoma. Angle-closure glaucoma develops when the angle between the peripheral iris and the TM closes due to mechanical contraction, which results in decreased aqueous humor outflow and increased IOP. Conversely, in primary open-angle glaucoma, the IOP is elevated due to increased resistance against the outflow of aqueous humor despite open angle. In primary open-angle glaucoma, the major site of resistance to aqueous outflow is believed to be located in the area between the TM and SC.25 Both open-angle and angle-closure glaucoma can be primary or secondary, and can be classified into different developmental categories. Primary open-angle glaucoma can occur with or without IOP; the latter is called normal-tension glaucoma. Secondary glaucoma can develop due to trauma, corticosteroids, inflammation, tumor, and conditions such as pigment dispersion or pseudoexfoliation.17 In secondary glaucoma, the alteration in outflow resistance in the TM and SC is responsible for IOP elevation. Thus, the TM and SC are important therapeutic target tissues for IOP reduction for the treatment of glaucoma.26
Pathogenesis of Glaucoma
There are many clinical types of glaucoma; however, optic nerve damage is common to all. The optic nerve is damaged through a mechanism called apoptosis of the RGC.27 This apoptosis is mediated by two main mechanisms. Mechanical injury, resulting from increased IOP, causes damage to RGCs. Elevated IOP causes stasis of RGC axonal flow at the lamina cribrosa in the optic disc, leading to blockage of neurotrophic proteins (NFPs), which finally results in apoptosis of RGCs.28 The other mechanism is local vascular insufficiency at the optic nerve head. This ischemic damage can lead to decrease in the levels of neurotrophic factors (NFs) in the optic nerve head, which results in RGC death (Table 1 and Figure 1A and B).29,30 Besides, mitochondrial dysfunction, low cerebrospinal fluid pressure-mediated translaminar cribrosa pressure gradient, excitotoxicity, and oxidative stress are also proposed to be involved in glaucomatous optic nerve damage.31,32
 |
Table 1 Limitations of Current Glaucoma Medical Treatments |
Despite multiple factors other than IOP being related to the pathogenesis of glaucoma, current treatments are mainly concentrated on decreasing the IOP. Effective reduction of IOP significantly prevents glaucoma progression in most patients in clinical trials, including patients with normal tension glaucoma.33 There are several methods available for reducing the IOP in patients with glaucoma. The first and most commonly used method for IOP reduction is medical therapy.34 Topical eye drops lower IOP via two mechanisms. Topical beta-blockers, alpha-agonists, and carbonic anhydrase inhibitors reduce the production of aqueous humor in the ciliary body.35 Conversely, topical cholinergic drugs, such as pilocarpine, enhance the outflow of aqueous humor through the TM-SC pathway.28 Prostaglandins can also decrease IOP by increasing the outflow of aqueous humor through the uveoscleral pathway.36,37 When antiglaucoma medication is not enough, the aqueous humor can be drained through an extraocular site via trabeculectomy or drainage devices.38
Limitations of Current Glaucoma Treatment
Despite their effectiveness, there are several disadvantages of using topical eye drops. First, most topical eye drops that are currently used should be administered 1–3 times per day. Ocular discomfort when dropping the eye drops and frequent administration cycles results in poor patient compliance. Low compliance is an especially important issue in the elderly population, as it renders the medical treatment ineffective with the patients having to undergo surgery (Table 2).39 Second, the bioavailability of topical eye drops inside the eye is very low.40 The volume of commercial drop dispensers (25–50 μL) generally exceeds the capacity of the conjunctival sac (10 μL), so that the major portion of the liquid drains out of the eye and onto the eyelids and cheeks, where further absorption may occur. The capacity of the conjunctival sac depends on several factors, such as blink rate, position (applying when standing or lying down), and the means of application. Therefore, bioavailability has to be classified as extremely low and is reported in the literature to be in the order of 5–10%.41,42 The small volume of eye drops that remains in the conjunctival sac is absorbed via two routes. Small lipophilic drugs are absorbed via the transcorneal route.43 Conversely, large hydrophilic drugs are absorbed via the transconjunctival and transscleral routes.43 Prostaglandins have been reported to enter the eyeball through the sclera rather than cornea.44 In the transcorneal route, the drugs first meet the precorneal tear film constituted of a deep mucous layer and superficial aqueous layer.
 |
Table 2 Limitations of Current Glaucoma Treatment |
The ocular system is protected by effective clearing mechanisms, including lacrimal secretion in the precorneal tear film for removal of irritants and blinking reflex. As a result, the half-life of a topical drop in the precorneal tear film is about 1 min, which is the only time available to the drug for penetrating the cornea and accessing the aqueous humor.45 The next barrier, corneal epithelium, which contains multiple desmosomes and tight junctions, prevents molecules larger than 500 Da to penetrate the cornea.46 As a result, 80% of the delivered drug cannot penetrate cornea, and may be absorbed into the blood vessels of the conjunctiva.47 Only less than 10% of the drug is absorbed into the eye and approximately 1% of that reaches the aqueous humor.48 Moreover, in transconjunctival and transscleral routes, over half dosage of the drugs is absorbed into systemic circulation through the vessels of conjunctiva and sclera.49 A few drug molecules that can penetrate cornea are quickly filtered through the TM, where their half-life is less than 2 hours. This makes it difficult for the drug molecules to reach their target tissue.50 Low compliance, maintaining sustained drug levels, and effects at their targets are important problems to be solved in glaucoma medical treatment.
Surgical drainage devices are used in glaucoma when IOP-lowering medications fail.51 These devices provide a new route to the aqueous humor from the anterior chamber of the eye to the collection plate beneath the conjunctiva. Currently, the major commonly used surgical drainage devices are Ahmed, Molteno, Krupin, and Baerveldt.52 After implantation of these devices, the major cause of surgical failure is fibrosis around these devices. It decreases the overall surgical success rate to about 40–50%. Intraoperative or postoperative antimetabolite injections like mitomycin C (MMC) or 5-fluorouracil (5-FU) can prevent fibrosis around these devices.53 However, these antimetabolite injections can increase the risk of infection around the bleb. However, if the drainage device is coated with nanomaterial, it may lead to a more successful surgical outcome without side effects.54
Another fundamental limitation is that visual loss from glaucoma has not been shown to be reversible with any current treatment. Furthermore, RGC loss may continue to progress in spite of IOP reduction in some patients with glaucoma.55 In this regard, neuroprotective strategy has been proposed.56 This review is focused on the application of nanotechnology-based strategies to overcome these current limitations in glaucoma treatment.
Nanotechnology Approach in Medicine
Nanomedicine
Nanotechnology, as defined by the National Nanotechnology Initiative, is the “science, engineering, and technology conducted at the nanoscale, which is about 1–100 nm”.57 In 1959, Richard Feynman, known as the father of nanotechnology, proposed the use of nanoscale machines in molecular and atomic modifications.57 Scientists first visualized the nanoscale using a scanning tunneling microscope in 1981.58 Thereafter, our understanding and ability to manipulate matter at molecular and atomic scales became tremendously enhanced. There are two definitions of nanomedicine. The first is the technology providing molecular aid for treatment and diagnostics using already existing knowledge on the human body, further described as using nanostructures with therapeutic effects.59 The second is "the comprehensive monitoring, control, construction, repair, defense, and improvement of human biological systems at the molecular level, using engineered nanodevices and nanostructures that operate massively in parallel at the single-cell level, ultimately to achieve medical benefit".60
The size of nanoparticles is the essential feature of nanotechnology.61 While matter interactions at macroscopic scales can be predicted in classic physics, nanoscale interactions can be predicted through quantum mechanics in nanotechnology.62 Thus, nanomaterials have unique chemical and physical characteristics that differ from materials at the macroscopic scale. As the size of particles becomes smaller, surface-to-volume proportion of the particles becomes larger. A nano-scale material will offer a larger number of locales for synthetic responses than a macro-scale material at per-unit size of a given material.62 Because of these characteristics of nanomaterial, medical network has great interest in nanotechnology. Many nanomaterials provide interesting features, including electrical conductivity, biocompatibility, magnetic properties, and biodegradability.59
Nanomedicine as Novel Drug Delivery System
Glaucoma is a type of ocular disease characterized by gradual degeneration and functional exacerbation of optic nerve, which progressively decreases visual sensitivity, and may lead to blindness. In open-angle glaucoma, the outflow of aqueous humor is blocked internally, resulting in the gradual elevation of intraocular pressure, whereas in closed-angle glaucoma, trabecular meshwork outflow pathway is blocked by iris which leads to rapid elevation of intraocular pressure.2 Unlike open- or closed-angle glaucoma, the eye pressure of normal-tension glaucoma does not exceed the normal range of intraocular pressure. Both secondary open- and closed-angle glaucoma refer to any form of glaucoma with identifiable cause of elevated intraocular pressure. Uveitic glaucoma is associated with uveitis, and childhood glaucoma is early-onset glaucoma which could be attributed heredity.17
The primary treatment for glaucoma is topical medication or eye drops. Several therapeutic classes, including prostaglandin analogs, β-blockers, carbonic anhydrase inhibitors, α-2 agonists, and cholinergic agents, were developed in the form of eye drops to relieve the IOP. Proper administration of eye drops can prevent glaucoma progression through reduction of IOP; however, it requires manual dexterity, which is found to be challenging in the elderly population. Furthermore, poor adherence and short residence time requiring high frequency of dosing can lead to missing of proper dosing time. A new drug delivery system that allows gradual release of the drug for a few months with a single administration needs to be developed.
Numerous methods are available for developing hollow, solid, or porous nanoparticles with various shapes and sizes.57 Molecules like drug compounds, DNA, RNA, or antibodies can be included as components or encapsulated within nanoparticles. Widely used nanodelivery systems are nanoparticles, nanosuspension, nanodiamonds (NDs), nanocrystals, liposomes, niosomes, dendrimers, cyclodextrins, and other devices (Figure 2).63 The advantages and disadvantages of nanomaterials are summarized in Table 3. These nanomaterials can incorporate the drugs in two ways: through encapsulation inside the nanomaterials or conjugation on the surface of nanomaterials. The encapsulated drug is released as the nanomaterial disassembles at the target site, while the nanomaterial-conjugated drug is released after the bond between the nanomaterial and drug is cleaved at the target site.64 These drug delivery strategies of nanomaterials can further enhance and compensate the limitations of conventional treatment for glaucoma. In addition, inorganic nanoparticles can be incorporated into a hydrogel to mimic the mechanical property of contact lens for sustained release.65
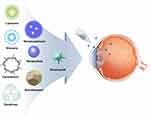 |
Figure 2 Delivery route of nanomedicine. Topical administration of various types of nanomaterials to reduce IOP. |
 |
Table 3 Advantages and Disadvantages of Nanomaterials |
The selection of an appropriate system depends on the drug type (hydrophobicity, size, stability), target tissue, and route of administration. Nanodelivery systems can provide more targeted delivery, sustained release, bioavailability, dose accuracy, minimal tissue irritation, longer shelf life, and better solubility.66,67
Most treatments for glaucoma are designed to lower IOP, and the most common route of administration is topical administration. However, low bioavailability and poor corneal permeability of the administered drug remain as challenges for the improved therapeutic treatment. Nanotechnology-based drug delivery system can provide sustained release and better bioavailability without irritation.68 Furthermore, the surface of nanoparticles can be coated to specifically target the desired site for drug delivery. Nanotechnology-based drug delivery is very similar to that of conventional treatment, but with improved efficacy at the same dosage through appropriate nanotechnology.
Desired drugs are administered through four routes, including topical, subconjunctival, systemic, and intracameral, to reach the anterior segment.69 Administered drugs need to overcome specific barriers depending on the administration route. In general, the drug-loaded nanoparticles are formulated in the form of eye drops for topical administration, so the limitation of topically administered drug will briefly be discussed.70,71 For better efficacy of topically administered drugs, two factors need to be considered: tear film and cornea.69 Drugs are quickly cleared due to blinking reflex and lacrimal secretion in the tear film and the cornea provides a physical barrier to the penetration of drug.
Nanoparticles need to circumvent the existing barriers by increasing their precorneal retention time through surface functionalization.72 Then, most nanoparticles can enter cells through endocytic pathway. Although the uptake pathway of each nanoparticle is unique, the factors affecting the corneal penetration of nanoparticles remain the same and mainly include size, shape, and charge.73 For instance, 35–200 nm sized indomethacin nanoparticles can penetrate corneal epithelium through energy-dependent endocytosis pathway including clathrin- and caveolae-endocytosis, and micropinocytosis.74
Toxicity of nanomaterials also needs to be considered. Many studies have extensively evaluated the toxicity of nanoparticles on main organs, whereas only a few studies have examined the potential toxicity of nanomedicine regarding their exposure time or size on the eye.75 Furthermore, each nanoparticle has unique physiochemical properties including chemical composition, surface area, and charge that can affect its toxicity on the eye.75,76
Various biological models have been utilized for determining potential toxicity of nanoparticles in the eyes. Most biological models (including in vitro and in vivo models) when treated with inorganic nanoparticles exhibited oxidative stress and damaged ocular system.75,77 The toxicity of nanoparticles needs to be considered before using them for safe and effective glaucoma treatment.
Liposomes
Liposomes are artificial lipid bilayers of phospholipids that are biocompatible with the human body.78 Liposomes provide a potential delivery system that can carry both hydrophilic and hydrophobic drugs. They can encapsulate and protect solutes while delivering drug molecules until they reach the target structures.79 Liposomes can be designed to have more bioavailability, bioefficacy, and sustained release of drug molecules.80 Another interesting characteristic of liposomes is their responsiveness to modifiable triggers such as thermosensitivity, electromagnetic waves, and pH environment. Thus, release of the entrapped molecules can be strictly controlled.81 Besides of the above characteristics, liposomes can reduce the elimination of the drug substance from the body and increase drug dosage at the targeted site. This factor may increase patient compliance by decreasing the dosing frequency.82 In an in vitro drug release study, timolol-loaded liposome formulations showed a 1.93-fold increase in permeability coefficients. They also reduced IOP from 30 to 300 min after instillation (minimum IOP = 11.96 ± 0.74 mmHg at 1 hour), and decreased IOP from 30 to 180 min (minimum IOP = 13.61 ± 0.95 mmHg at 2 hours) when used in the form of eye drops in albino rabbit eyes.83
Surface charge of liposomes is an important factor of drug contact time at corneal epithelial surface. The corneal epithelium has negatively charged mucinous membrane, which provides a good binding surface to positively charged liposomes. Because of prolonged contact time at corneal surface, positively charged liposomes demonstrate better corneal permeation than neutral or negatively charged liposomes.46,84 In addition, positively charged liposomes demonstrated higher encapsulation efficiency than neutral and negatively charged liposomes in an in vitro study.46 The entrapment efficiency would increase because of electrostatic attraction between anionic drug and positively charged liposomes. Charge repulsion between drug anion and negatively charged liposomes may induce low entrapment efficiency and may suppress drug-loading efficiency.84 Meanwhile, electrostatic repulsion may occur between drug anion and negatively charged liposomes, resulting in a higher percentage of drug release. After drug release from the liposomal vesicles, drug molecules only rely on passive diffusion to cross the corneal barrier for intraocular absorption. Thus, the longer the contact time at the corneal surface in the encapsulated state, the higher the bioavailability of the drug.85 Positively charged liposomes produce high binding affinity between the negatively charged mucin of corneal epithelium and liposomal vesicles, enhancing the contact time of positively charged liposomes.86
Positively charged acetazolamide (ACZ)-sterylamine liposomes demonstrated more IOP reduction than neutral and negative-charged liposomes in rabbit eyes (−7.80±1.04 vs −5.50 ± 1.65 vs −3.70 ±2.18 3 hours after topical administration). The effect of IOP reduction persists for longer period in positively charged ACZ-sterylamine liposomes than neutral and negatively charged liposomes in rabbit eyes (8 hours vs 6 hours vs 3 hours). Moreover, it showed higher encapsulation efficiency compared with neutral and negative liposomes (48.27 ± 1.01 vs 39.73 ± 0.44 vs 25.55 ± 1.50) in an in vitro study.85
Neutrally charged vesicles of L-a-dipalmitoyl phosphatidylcholine (DPPC):pilocarpine hydrochloride showed a prolonged drug release up to 600 minutes in rabbit eyes, which is greater than that of negatively charged liposomes or free-drug eye drops.80 These prolonged release profiles and high bioavailability of surface charged liposomes indicate the possibility for better patient compliance through reduction of dosing frequency.
The conventional liposomes have some limitations in terms of clinical uses. They tend to aggregateand may cause leakage of entrapped drug, and are susceptible to phagocytosis. The modification of surface characteristics of liposomes has helped to overcome these drawbacks of conventional liposomes. Bioadhesive polymers are used to coat liposomes. Coating with bioadhesive polymers inhibits the aggregation of liposomes and increases their viscosity and hence their corneal residence time.87 Poly L-lysine-modified liposomes can not only enhance the efficacy of eye drop formulations, but also deliver drugs at the retinal site where RGC death occurs.88 Chitosan (mucoadhesive polysaccharide)-modified liposomes increase the residual time of the drug formulation in ocular tissue by enhancing the viscosity of the solution.89 Timolol maleate chitosan-coated liposomes (TMCHL) produced a 3.18-fold increase in the corneal permeability coefficient in an in vitro drug release study. In a study on transcorneal permeation of New Zealand rabbits, TMCHL was found to be more effective in lowering IOP than timolol eye drops (final IOP = 19.67 ± 1.14 mmHg vs 23.80 ± 1.49 mmHg, respectively).90 Latanoprost-loaded Egg-phosphatidylcholine (EggPC) liposomes developed using a thin film hydration technique enhance the stability of phospholipids in liposomes, effectively lowering the IOP in rabbit eyes for at least 90 days; this effect was significantly greater than that of once-daily topical latanoprost controls.91
Microbubbles are contrast agents used for medical ultrasound imaging. They improve the transfection efficiency after ultrasound-induced cavitation.92,93 However, microbubbles are unstable and are too large in size (1–6 μm) to be used in intravascular clinical application.94 The size of liposomes can be controlled easily and they can be used as drug, antigen, and gene carriers, and can be transferred to targeted organ.95 Thus, bubble liposomes are a new and promising strategy for the delivery of genes through ultrasonication, which intensifies the penetration of genes.96
Recently, some clinical studies have explored the effect and safety of drug-loaded nanoliposomes in glaucoma treatment. Dorzolamide (DRZ)-loaded nanoliposome demonstrated better IOP reduction than commercially available DRZ formulation in rabbit.97 IOP lowering observed at 2 weeks was 23.26 ± 9.24%, 9.25 ± 5.76%, 32.60 ± 7.90%, and 17.48 ± 7.62% for the DRZ-loaded nanoliposome group and the commercially available DRZ formulation group.98 Latanoprost/thymoquinone-loaded liposomes and latanoprost-loaded liposomes were able to significantly reduce the IOP in glaucomatous white albino rabbits, which lasted for 8 hours. Conversely, the effect of the free latanoprost did not persist for more than 24 hours.99
For evaluating the safety and efficacy of nanoliposomes in human eye, liposomal latanoprost was injected in subconjunctival space once in six subjects who were diagnosed with either ocular hypertension (OHT) or primary open-angle glaucoma (POAG). Subconjunctival injection of liposomal latanoprost was well tolerated by all the six subjects. From a baseline IOP of 27.55±3.25 mmHg, the mean IOP decreased within 1 h to 14.52±3.31 mmHg (range 10–18 mmHg). This represented a mean decrease of 13.03± 2.88 mmHg (range 9–17 mmHg), or 47.43±10.05% (range 37–63%). A clinically and statistically significant IOP reduction (≥20% IOP reduction, P=0.001 to 0.049) was observed for 3 months after injection.100
Niosomes
Niosomes are spherical, closed bilayer structures of non-ionic amphiphiles. Niosomes provide a potential delivery system that can carry both hydrophilic and hydrophobic drugs simultaneously, which has possible usage in combined drug therapies.101 Niosomes can provide longer time period to enhance bioavailability, and have positive therapeutic responses at low cost. However, the major advantage of niosomes is the non-toxic nature of non-ionic surfactants.102 Timolol maleate-loaded niosomes using chitosan have shown a prolonged effect in reducing IOP by releasing the timolol for longer time intervals.103 In glaucoma rabbits, timolol maleate niosomes coated with chitosan were effective for over 8 hours, and IOP reduction was longer than the conventional dosage form, which lasts up to 2 hours.104 Niosomes of ACZ show better and prolonged drug release in the ocular tissue, which can eliminate the need for oral medication.105 Some types of niosomes, such as multilamellar niosomes, can entrap a higher dose of drug and can release it for more longer period.106
Polymeric Nanoparticles
Polymeric nanoparticles have spherical shapes with sizes in the nanometer range. Because of their nanometer size, nanoparticles easily pass through biological membrane barrier systems, and can easily carry drugs to the target organ.107 The smaller the size of nanoparticles, the larger the surface area, and higher the drug-loading capacity.108 There are three types of nanoparticles. First-generation nanoparticles are simple polymer matrix–type particles. Second-generation nanoparticles are simple nanoparticles with polymer coating. This polymer coating can enhance adhesion of nanoparticles to target site. Third-generation nanoparticles are antibody-attached nanoparticles, which can bind with specific group of cells and release the drug at the particular target site. Nanoparticles of propoxylated glyceryl triacylate (PGT) with timolol in 1:1 ratio can release the timolol for longer duration.109 Additionally, Poly(butyl)-cyano acrylate nanoparticles of pilocarpine can significantly decrease IOP without any side effects.110
ACZ-loaded Eudragit nanoparticles displayed better permeability and flow across corneal tissue than a conventional drug suspension in an in vitro transcorneal permeability study. ACZ loaded Eudragit nanoparticles and ocular insert demonstrated substantial reduction in IOP and improved ocular tolerability when compared to ACZ suspension in an in vivo study.111
Ex vivo transcorneal permeation study displayed higher ACZ permeation at 8 hours with nanoparticle-in situ gel (74.50 ± 2.20 mg/cm2) than with ACZ eye drops (20.08 ± 3.12 mg/cm2) and ACZ suspension (16.03 ± 2.14 mg/cm2). In addition, nanoparticle-in situ gel did not display harmful effects on any corneal layer. Moreover, 1% of PLGA nanoparticle-in situ gel exhibited greater IOP-lowering effect 1 hour after administration, which was sustained for up to 8 hours, while 1% ACZ eye drops sustained their action for approximately 2 hours in normotensive rabbit eyes.112
Dendrimers
Dendrimers are polymeric materials with excessive flexible branching, resulting in a large surface area. Dendrimers can provide enough space for drugs inside the excessively flexible branching.113 Evidently, dendrimers also have also biocompatible and nonimmunogenic characteristics similar to other nanomaterials. Dendrimers with quaternary ammonium group can also diminish the need for benzalkonium chloride (BAK) as a preservative.114 Polyamidoamine (PAMAM) dendrimers showed longer residual time of pilocarpine, for up to 5 hours. In addition, PAMAM dendrimers significantly lower ocular irritation and have greater bioavailability than commercially available Carbopol eye drop formulations.115 In a study, dendrimers of carteolol showed longer residual duration, which can decrease daily eye drop frequency, in glaucoma rabbit eyes.114 Topically administered hybrid dendrimer hydrogel/poly(lactic-co-glycolic acid) nanoparticle showed greater concentration of brimonidine and timolol than saline control in aqueous humor for 7 days.116
In vitro investigation of cytotoxicity and cell viability of ACZ-loaded water-soluble mucoadhesive carbosilane dendrimers revealed that generations 1 and 2 of cationic dendrimers and all 3 generations of anionic dendrimers are well tolerated at 10 μM, with higher than 80% cell survival for all of them, except for G3-C (from the 3rd generation of carbosilane cationic dendrimers). G3 cationic carbosilane dendrimers (5 μM) demonstrated rapid (1 hour post-instillation) and sustained (up to 7 hour) hypotensive effect, reaching a peak 22.6% IOP reduction in normotensive rabbit eyes.117
Nanosuspension
Using high-pressure homogenization or different milling methods, dispersed solid particles in the liquid phase can be incorporated into a colloidal drug delivery system (nanosuspension). This system can be easily integrated with other delivery systems, such as hydrogels, because of non-water soluble characteristics. Nanosuspensions are especially effective in delivery of lipophilic drugs, and can enhance their bioavailability.63 In situ gel-forming nanosuspension of forskolin (coleonol) lowered IOP by 31%, and the drug efficacy lasted for 12 hours, which is significantly longer than that achieved with other conventional delivery systems.118 A pilocarpine-loaded chitosan-poly(acrylic acid) nanosuspension showed zero-order release kinetics of pilocarpine.108
When molecules of drug are transported in the body, they encounter several ions. There can be several electrostatic interactions between drug molecules and ionic medium. This can reduce the stability of drug molecules. Ion Exchange Resins (IERs) are polymers containing appropriately substituted acidic groups, such as carboxylic and sulfonic groups for cation exchangers, or basic groups, such as quaternary ammonium group for anion exchangers. IER protects ionic drugs through shielding and competitive binding effect. It can be synthesized or purchased depending on its application.
Betaxolol-loaded nanoparticles using Ion Exchange Resin (IER) complex suspension have been approved and are commercially available in the US market as Betoptic S for use as ophthalmic drug delivery system. The cationic exchange resin containing 0.25% betaxolol provides microscopic beads of 5 μm diameter and its residence time in the cul-de-sac is increased with the addition of polyacrylic polymer.119 In a multicenter, double-masked parallel study of 352 patients with primary open-angle glaucoma or ocular hypertension, no significant difference was found between 0.5% betaxolol solution and Betoptic S in terms of IOP reduction, whereas prevalence of ocular discomfort after instillation was significantly lower for the Betoptic S.120 As such, nanosuspension will be an attractive drug delivery system for lipophilic antiglaucoma drugs, such as carbonic anhydrase inhibitors, in the future.
Cyclodextrin Complexes
Cyclodextrins are cyclic oligosaccharides in which sugar molecules are arranged in a ring-like pattern. Based on the sugar molecules in the ring structure, cyclodextrins are categorized in three main classes: α–cyclodextrins, β–cyclodextrins, and γ–cyclodextrins containing 6-, 7-, and 8-membered sugar rings in the cyclic structure.121 The drug molecules are enclosed within cage-like ring structure. Primary advantage of these structures is delivery of the drug compounds without changing their molecular structure. Because of hydrophilic surface characteristics of cyclodextrins, lipophilic drug enclosed in cyclodextrins can easily pass through the hydrophilic barrier of the external eye and can safely reach the lipophilic corneal surface. Finally, drug compounds can be released in the aqueous humor.122 The sulfobutyl ether of beta-cyclodextrin (SBE7-beta-CD) complex of pilocarpine prodrug showed effective protective effect on pilocarpine before reaching the target area, and decreased the strong irritation caused by pilocarpine by preventing its rapid absorption.123 The multicomponent complex of hydroxypropyl β–cyclodextrin and triethanamine with ACZ can release ACZ for up to 4 hours and yields a reduction in IOP by approximately 30% without any irritation.124 Dorzolamide with γ cyclodextrin showed prolonged residual time in the aqueous humor, and can be a great potential system for a once-daily formulation.125
BZL-hydropropyl-ß-cyclodextrin (HP-ß-CD) inclusion complex (HP-ß-CD/BZL) into nanoliposomes (“HP-ß-CD/BZL-loaded nanoliposomes”, BCL) demonstrated a 9.36-fold increase in the permeability coefficient compared with commercially available BZL. BCL formulation reduced IOP in less than 1 hour, reached peak efficacy (32.3%) at 2 hours, and showed a sustained release effect for 12 hours. From 2 to 12 hours after instillation, BCL resulted in significantly lower IOP when compared with BZL suspension.126
In vitro analyses of stability and phase solubility of Latanoprost-propylamino-ß cyclodextrin (CD) demonstrated a significant improvement in its solubility and stability. In vivo evaluation of ocular tolerability revealed that ocular irritation was 15.5% with the commercially marketed formulation of latanoprost and 9.5% with the latanoprost-propylamino-ß-CD formulation. Microscopic evaluation of ocular tissues showed that commercially marketed formulation of latanoprost induced higher inflammatory mixed cell infiltrates than latanoprost-propylamino-ß-CD formulation.127
Nanocrystals
Nanocrystals may be defined as pure solid particles with a mean diameter of less than 1 μm and a crystalline structure. Nanocrystals are composed entirely of drug itself with crystalline coating.128 Nanocrystals can provide a large effective surface volume area and have a good bioavailability without need for carriers, such as other nanosubstances. Nanocrystals have fast initial dissolution within 1 hour, indicating better bioavailability.129
Nanocrystals of brinzolamide (BZL) showed higher IOP-lowering efficacy than the commercially available market product (75% vs 49%).130 Pilocarpine hydrochloride loaded nanocomposite formulations (cellulose nanocrystals and triblockpoloxamer copolymer) showed higher sustained drug release and less toxicity than topical formulation of in vitro gel.131 Trimethyl lock BZL prodrug nanoparticles showed similar efficacy as commercial BZL at 1/5th the molar concentration without toxic effects on the cornea in normotensive rats.132
Nanodiamonds
Nanodiamonds are 2–10 nm large carbon nanoparticles with a truncated octahedral structure.133 Nanodiamonds have tailorable surface structure. Many functional groups are covalently or non-covalently conjugated on the surface of nanodiamonds. The surface of nanodiamonds coated with polyethyleneimine (PEI) can be conjugated with enzyme-cleavable N-acetylated chitosan along with timolol maleate to prepare a nanogel in the form of contact lens. Therefore, the sustained release of timolol maleate can be achieved in the presence of lysozyme as the chitosan dissociates. Nanodiamonds also provide the mechanical support of contact lens.
Ophthalmic Devices Using Nanotechnology
Contact Lenses
The use of nanoparticles as drug carriers can pave the way for the sustained release of a drug. Nanotechnology can be applied to contact lenses for slow and controlled release of drugs for a long duration (Figure 3A).134 Using solvent impregnation method, both hydrophilic and hydrophobic drugs can be easily incorporated in the lenses without the issue of solubility, which is a major drawback of many currently used drug delivery systems.135 The drug embedded in contact lenses through nanotechnology can result in high drug-loading capacity and controlled release of the drug over a long time, which would result in better patient compliance.136 Various studies have explored the efficacy of nanoparticles embedded in contact lens for potential glaucoma therapy. Silicone contact lenses, ACUVUE® TruEyeTM containing timolol significantly reduced IOP.137 With contact lenses, only 20% of the dose is required to produce a similar therapeutic effect compared with the full dose needed in currently used eye drops.137 Contact lenses with timolol containing PGT nanoparticles released timolol for 1 month in Beagle dogs. In these type of lenses, the ratio of timolol to PGT can be modified.109 Timolol, when loaded onto ethylene glycol dimethacrylate and PGT-based nanoparticles, not only exhibits prolonged drug release time of up to 4 weeks but also remains well encapsulated under refrigerated conditions for up to 5 months.138 Another study has found that hyaluronic acid and chitosan-based polymeric nanoparticles loaded with timolol showed a significant reduction in IOP compared to conventional timolol solution.139 Many promising results from studies have gained interest, which has lead to the development of various nanoparticle-drug formulations for glaucoma treatment. However, most developed drugs are currently in the preclinical stage.140
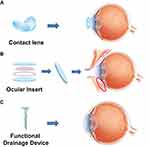 |
Figure 3 The application of nanotechnology to ophthalmic devices. The sustained release of drug through (A) contact lens and (B) ocular insert. (C) The coating of functional drainage device. |
The traditional IOP measurement can only be performed at the hospitals, and, thus, patients cannot check their IOP on a daily basis.141 Because the fluctuation of IOP may be a risk factor for glaucoma progression, daily monitoring of IOP could be clinically useful for some patients in whom glaucoma progress despite stable IOPs in the clinic. Nanotechnology has been used to develop contact lens for continuous monitoring of IOP.142 These contact lenses are composed of a platinum-titanium gauge sensor, which is 170 nm thick and is embedded in two layers, a gold antenna, microprocessor, and an integrated circuit for turning on IOP monitor. If the stress in the gauge is increased, other mechanical forces are compressed, which alters the electrical forces present in the gauge. These contact lenses showed reproducible intraocular pressure in the range 15–30 mm Hg in enucleated pig eyes.143
Based on the development of nano or microelectromechanical system, silicone-based Triggerfish® was developed with reliable IOP measurements and was approved by FDA.144 In addition, a permanent implant Eyemate® for continuous IOP monitoring is under human clinical trials for commercial use.145 As of today, iCare® home tonometer is commercially available as self IOP measurement device but it cannot provide data continuously for 24 hours.146 In this aspect, nanotechnology supported the development of various fields including IOP monitoring, surgical devices, drug delivery systems for glaucoma treatment.
Hydrogel
Hydrogels are three-dimensional networks of hydrophilic polymers, either synthetic or natural, and can be applied to various fields including drug delivery system, regenerative medicine, and cell encapsulation. Hydrogels can be used for treatment of ocular diseases including glaucoma, corneal abrasion, and cataracts.147 Hydrogel is mainly used as a sealant after incision. The mechanical property of hydrogel can be manipulated to meet the requirements of a sealant, and PEG-based hydrogel, known as ReSure® has been approved by the FDA.148 Today, hydrogel is widely used as a sealant in ocular incision due to better leakage prevention than the conventional suture.149 Therefore, many studies have extensively explored the application of PEG-based hydrogel in corneal attachment and retinal attachment.150
Nanoparticles can be incorporated into a type of hydrogel, known as a nanogel, for drug delivery system. In glaucoma treatment, conventional medication including timolol maleate, pilocarpine, and xalatan shows low bioavailability and short retention time; hence incorporating these drugs into hydrogel can increase their retention time through improved bioavailability.109 For instance, corneal penetration of timolol maleate loaded chitosan-alginate nanoparticle was twice than that of conventional timolol maleate, indicating the extended retention time. The use of hydrogel or nanogel as a sealant and drug carrier can accelerate the healing process and limit bacterial infections after incision by incorporating growth factors or antibiotics.
Ocular Inserts
Ocular inserts are thin drug-impregnated devices of solid or semisolid consistency. Using ocular inserts, a drug can be released for a long period and in a controlled manner (Figure 3B). Ocular inserts are usually placed in the conjunctival cul-de-sac.48 Ocular inserts provide the most accurate dosing at a constant rate for a long time, thus they can increase the bioavailability of drugs with better pharmacotherapeutic effect. In addition, through mucoadhesive polymers, ocular inserts can slowly release the drug in the ocular compartment and can reduce systemic drug absorption, thus prevent systemic side effects.122 Ocular inserts of levobunolol HCl were showed to release levobunolol hydrochloride for longer time in a controlled manner, thus providing more effective glaucoma treatment.151
The OTX-TP (Ocular Therapeutix) consisting of PEG-based hydrogel with embedded travoprost-loaded PLA microspheres delivers travoprost to the ocular surface via an intracanalicular punctal plug for up to 3 months, as well as resorbs and drains through the nasolacrimal system. These microspheres slowly degrade and show a sustained drug release over a period of 30 days. Ten days after implantation, IOP was reduced by 5.4–7.5 mmHg with 100% retention rate. It also demonstrated enhanced therapeutic benefit for 90 days with a consistent 90% retention rate.152 The Phase II trial did not find any serious adverse effects and showed only a slightly less hypotensive effect as compared with timolol.
Latanoprost Punctal Plug Delivery System (L-PPDS) is another punctal plug drug delivery system. In a study on L-PPDS in patients with open-angle glaucoma, IOP was reduced by 5.7 mmHg. After implantation of L-PPDS, 60% of subjects in the study showed IOP reduction by at least 5 mmHg or more and 47% of the subjects showed IOP reduction of at least 6 mmHg. A statistically significant mean change in IOP (22.3%) was recorded in the subjects treated with L-PPDS when compared with controls.153
Surgical Nanodevices
Studies have been reported on the improvement of glaucoma drainage devices using nanotechnology. A two-layer film of polylactic-co-glycolic acid (PLGA) loaded with 5-FU and MMC showed continuous drug release at a constant rate in vitro. This film showed continuous release of 5-FU until day 28, with a delay in the first 3 to 5 days.54 Currently, commonly used surgical devices have other postoperative complications. The devices are implanted in the post equator area of the eye and occupy large spaces. This can effect the function of levator muscle of the eyelid, and may result in ptosis.154 Implantable nanodevices may solve these issues. Nanodrainage implant known as ANDI, composed of a polymeric shaft and a nanoporous membrane, has been invented. Microelectromechanical systems and nanofabrication technologies were used for developing ANDI. Major problem of ANDI was over-efficient drainage, which can lead to hypotony (Figure 3C).155
In addition, glaucoma devices with ferrofluid nanoparticles were invented. Because of its supramagnetic characteristics, ferrofluid plays a role like that of a valve. Valves are opened only when liquid flow pressure is more than the magnetic pressure of ferrofluid. After implantation of these ferrofluid drainage device in rabbit eyes, IOP was more reduced in the valves compared with contralateral control eye.156 A biodegradable plug filter, with polylactic acid (PLA) or PLGA, was invented to prevent postoperative hypotony. Plugs are approximately 500 μm long and a hole with diameter of 44 μm is laser-drilled in the center of the plug. These plugs could prevent abrupt IOP reduction and stabilize gradual IOP reduction. However, this plug is only effective for about 15 days because of its biodegradable characteristics. It showed effective IOP reduction for 15 days in rabbit eyes.157 Using microelectromechanical systems, an antifouling glaucoma drainage device was invented. This device was designed as an array of parallel microchannels composed of PEG-214 and PEG-4000. This polymer provides stability against swelling and therefore provides resistance to channel clogging due to biofouling. In addition, in vitro studies indicated that the polymer has lower nonspecific protein adsorption than glass, polypropylene, polydimethylsiloxane, and polymethyl-methacrylate. Finally, the design was proven to be resistant to intrusion by Escherichia coli in a week-long experiment. However, additional studies with most common microbes and in vivo animal studies are yet to be completed to validate the efficiency of the device.158
Trabecular Meshwork Regeneration and Neuroprotection Using Mesenchymal Stem Cells
Although reduction of IOP can delay disease progression, the completely damaged optic nerve system cannot be recovered. Therefore, a new neuroprotective agent is required to improve the patients’ quality of life. Due to their potential to differentiate into multiple lineages and secretion of paracrine factors, mesenchymal stem cells can be a breakthrough in the regeneration of TM and damaged RGCs. TM is located near the cornea and is associated with IOP.159 Increased outflow resistance of aqueous humor at TM gradually increases IOP,160 and therefore treatment of the TM can temporarily relieve IOP. The regeneration of TM can, therefore, reduce IOP.161 Bone marrow-derived mesenchymal stem cells (BMMSCs) and paracrine factors secreted by BMMSCs after they are released in the TM through laser therapy can regenerate ocular tissue. In addition to ocular regeneration, although MSCs cannot differentiate into RGCs, they can secrete factors that can help the dysfunctional RGCs to survive.162
Research on treatment of corneal wound healing and regeneration using stem cells is underway.163 This cell-based approach is being tried as another potential therapeutic strategy for glaucoma treatment because decreased cellularity in the TM increases the outflow resistance.164,165
If successful, it could be a new method of increasing the physiological aqueous outflow by targeting the TM. Du et al demonstrated that after injection of human TM stem cells into mouse anterior chamber, they homed to the TM and differentiated into functional TM cells.166 In the first week, they expressed TM marker protein CHI3L1 and maintained the TM for 4 months. Snider et al reported improved stem cell delivery to the TM using magnetic nanoparticles.167 MSCs were labeled using magnetic nanoparticles. The prussian blue nanocubes labeled MSCs could be delivered to the entire circumference of the TM, which was not achieved without magnetic steering.
Dillinger et al reported hyaluronan coated nanoparticles could deliver small interfering RNA against connective tissue growth factor (CTGF), which is a mediator of pathologic effects in TM and SC, by binding to those cells via CD44. Gene silencing in human TM cells lead to a significant reduction of CTGF expression. Thus, HA-coated nanoparticles combined with RNA interference may be a novel, causative therapeutic modality for glaucoma.168 Studies on nanotechnology and tissue engineering have been conducted to provide in vitro disease model for glaucoma. The biomimetic SC inner wall has been reported to be engineered with microfabrication technique.169,170 More recently, bioengineered glaucomatous 3D human TM has also been developed as an in vitro disease model.171,172 Thus, the models will offer a new platform for studying aqueous outflow physiology and drug screening.
Since the deprivation of NFs is a critical mechanism in pathophysiology of glaucoma, the induction of various NFs has been investigated as a modality for the protection of optic nerve damage in glaucoma. Previous studies have showed that injection of microsphere containing glial cell-derived neurotrophic factor (GDNF) into vitreous increased RGC survival in vitro and in vivo.173–176 Ciliary neurotrophic factor (CNTF) also has been shown to have neuroprotective effect on central nervous system. For sustained delivery of CNTF, encapsulated CNTF nanospheres provided neural stem cell differentiation without loss of potency compared to the unencapsulated growth factor.177
Additionally, damaged RGCs are not capable of self-repairing, but specific cell types, including Müller glia cells, could constitute neurogenic progenitors under pathological conditions. Müller glia cells are found in the retina of all species, and are characterized as retina-derived stem cells in cold-blood vertebrates.178 In mammals, Müller glia cells can undergo reactive gliosis through morphological changes, dedifferentiation, and upregulation of markers. However, these cells can re-enter the cell cycle to regenerate rod photoreceptors.179 Furthermore, these cells can express mature retinal neuronal markers under specific culture conditions (Figure 4).180 Thus, manipulation of Müller glia cells could lead to the development of a new regenerative treatment for ocular tissues damaged by glaucoma.
Discussion and Perspectives
Despite being the second leading cause of blindness, glaucoma remains an incurable disease with effective therapeutic treatment. The current treatment of glaucoma is mainly focused on delaying the disease progression. Furthermore, regardless of the technological success in the medicinal field, low compliance and short therapeutic time remain as challenges for pharmacologic treatment of glaucoma. Patients need to be dexterous with both hands and be aware of dosing times for the proper and effective administration of the drug, which is challenging in the elderly population. Thus, the development of a new platform with improved therapeutic time is required.
The advances in nanotechnology can overcome the limitations of current glaucoma treatments. Efficacies of topically administered drug for glaucoma treatment are limited since only a small proportion of administered drug can reach the target sites, whereas most of the drug is washed away. Nanotechnology can be utilized to enable the sustained release of the conventional drug by increasing its bioavailability. Various nanoparticles including liposomes, polymers, and nanocrystals are currently under clinical trials for their efficacies. Effective glaucoma treatment can be achieved through the encapsulation or conjugation of desired drugs to appropriate nanoparticles to maximize the possibility of reaching the desired sites and to prolong the residual time. The surface of nanoparticles can be coated such that the particles can endure the physiological conditions of precorneal tear film. Therefore, nanoparticles, regardless of type, shape, or size, allow the sustained release of drugs unlike free drugs, resulting in the reduction of IOP. These results can be translated into decreased drug administration frequency and, thus improved compliance.
Nanoparticles can also be applied in the form of ophthalmic devices to treat glaucoma. Nanoparticles can be easily embedded in the polymer of contact lenses or ocular inserts for sustained release without major drawbacks, and the encapsulated drug in the contact lenses can remain stable under refrigerated conditions, similar to topical administration of nanoparticles. Furthermore, the mechanical property of contact lens can be manipulated with nanoparticles to prevent potential damage after insertion in the eye. In addition, the encapsulated drug in the contact lens can be released under physiological cues, such as lysozyme, for controlled release. Controlled release of drug can reduce the dosing frequency. Furthermore, nanotechnology applications are varied, including the coating of a surgical device and IOP diagnosis. Recently, an IOP monitoring device has been developed in the form of contact lens to measure IOP of patients for 24 hours. Despite these merits, nanoparticles may induce toxicity upon administration; hence, appropriate dosage needs to be cautiously determined before the initiation of treatment to minimize the side effects. While the use of nanoparticles has improved the glaucoma treatment when compared to the traditional treatment method, stem cells can be used to protect and possibly regenerate the TM and damaged RGCs.
Stem cells are widely known for their multi-lineage differentiation and secretion of NFs. These cells can differentiate into TM to relieve IOP and secrete NPFs to protect damaged ocular tissues from apoptosis. For ocular regeneration, based on the replacement of damaged RGCs, Müller cells could be used because they can express mature retinal markers under certain conditions.
Co-treatment with appropriate stem cells and nanoparticles can synergistically help to protect and regenerate the damaged RGCs. In addition, the targeting ability of stem cells can be enhanced through the usage of nanoparticles, which not only provide a prolonged retention time in physiological conditions, but also reduce the side effects. Despite these beneficial effects of co-treatment, caution is required when using nanoparticles since each type of nanoparticle possesses unique mechanical and physiochemical properties. This review highlighted the usage of drug-loaded nanoparticles and stem cells to effectively halt the disease progression and possibly regenerate the damaged tissue.
Conclusion
In recent years, conventional glaucoma treatment has been equipped with stem cells and nanoparticles due to the advances in nanotechnology and understanding of stem cells. Nanoparticles can help in reaching the desired target sites and provide sustained release to delay the progression of glaucoma. Then, stem cells can assist in glaucoma treatment by regenerating trabecular meshwork for lowering IOP and potentially regenerate damaged retinal ganglion cells. However, the potential adverse effects of nanomedicine need to be determined before using them in the treatment.
Acknowledgments
This research was funded by grants from the National Research Foundation of Korea (2019M3A9H1103690).
Disclosure
The authors declare that they have no conflicts of interest.
References
1. Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90(3):262–267. doi:10.1136/bjo.2005.081224
2. Weinreb RN, Aung T, Medeiros FAJJ. The pathophysiology and treatment of glaucoma: a review. Jama. 2014;311(18):1901–1911.
3. Richter GM, Coleman AL. Minimally invasive glaucoma surgery: current status and future prospects. Clin Ophthalmol. 2016;10:189–206. doi:10.2147/OPTH.S80490
4. Schlunck G, Meyer-ter-Vehn T, Klink T, Grehn F. Conjunctival fibrosis following filtering glaucoma surgery. Exp Eye Res. 2016;142:76–82. doi:10.1016/j.exer.2015.03.021
5. Bengtsson B, Heijl A. Lack of visual field improvement after initiation of intraocular pressure reducing treatment in the early manifest glaucoma trial. Invest Ophthalmol Vis Sci. 2016;57(13):5611–5615. doi:10.1167/iovs.16-19389
6. Song YK, Lee CK, Kim J, Hong S, Kim CY, Seong GJ. Instability of 24-hour intraocular pressure fluctuation in healthy young subjects: a prospective, cross-sectional study. BMC Ophthalmol. 2014;14(1):127. doi:10.1186/1471-2415-14-127
7. Grant WM, Burke JF
8. Susanna R, De Moraes CG, Cioffi GA. Ritch RJTvs, technology. Why do people (still) go blind from glaucoma? Trans vision sci technol. 2015;4(2):1.
9. Asiedu K, Dzasimatu SK, Kyei SJCL, Eye A. Clinical subtypes of dry eye in youthful clinical sample in Ghana. Contact Lens Anterior Eye. 2019;42(2):206–211.
10. Smith DM, Simon JK, Baker Jr JR
11. Chow EK, Ho D. Cancer nanomedicine: from drug delivery to imaging. Sci Transl Med. 2013;5(216):216rv214. doi:10.1126/scitranslmed.3005872
12. Butcher NJ, Mortimer GM, Minchin RF. Drug delivery: unravelling the stealth effect. Nat Nanotechnol. 2016;11(4):310–311. doi:10.1038/nnano.2016.6
13. Bayford R, Rademacher T, Roitt I, Wang SX. Emerging applications of nanotechnology for diagnosis and therapy of disease: a review. Physiol Meas. 2017;38(8):R183–R203. doi:10.1088/1361-6579/aa7182
14. Johari B, Kadivar M, Lak S, et al. Osteoblast-seeded bioglass/gelatin nanocomposite: a promising bone substitute in critical-size calvarial defect repair in rat. Int J Artif Organs. 2016;39(10):524–533. doi:10.5301/ijao.5000533
15. Samadikuchaksaraei A, Gholipourmalekabadi M, Erfani Ezadyar E, et al. Fabrication and in vivo evaluation of an osteoblast-conditioned nano-hydroxyapatite/gelatin composite scaffold for bone tissue regeneration. J Biomed Mater Res A. 2016;104(8):2001–2010. doi:10.1002/jbm.a.35731
16. Kargozar S, Mozafari M, Hashemian SJ, et al. Osteogenic potential of stem cells-seeded bioactive nanocomposite scaffolds: A comparative study between human mesenchymal stem cells derived from bone, umbilical cord Wharton’s jelly, and adipose tissue. J Biomed Mater Res B Appl Biomater. 2018;106(1):61–72. doi:10.1002/jbm.b.33814
17. Weinreb RN, Leung CK, Crowston JG, et al. Primary open-angle glaucoma. Nat Rev Dis Primers. 2016;2(1):16067. doi:10.1038/nrdp.2016.67
18. Lee EJ, Han JC, Park DY, Kee C. A neuroglia-based interpretation of glaucomatous neuroretinal rim thinning in the optic nerve head. Prog Retin Eye Res. 2020;100840. doi:10.1016/j.preteyeres.2020.100840
19. Walsh JT, Robbins SL, Savino PJ. Visual Field Loss in a Patient With Optic Disc Cupping. JAMA Ophthalmol. 2019;137(7):844–845. doi:10.1001/jamaophthalmol.2019.1173
20. Levkovitch-Verbin H. Optic nerve cupping represents neuronal loss. Ophthalmology. 2020;127(4S):S43–S44. doi:10.1016/j.ophtha.2020.01.024
21. Chen SJ, Lu P, Zhang WF, Lu JH. High myopia as a risk factor in primary open angle glaucoma. Int J Ophthalmol. 2012;5(6):750–753. doi:10.3980/j.issn.2222-3959.2012.06.18
22. Choquet H, Thai KK, Yin J, et al. A large multi-ethnic genome-wide association study identifies novel genetic loci for intraocular pressure. Nat Commun. 2017;8(1):2108. doi:10.1038/s41467-017-01913-6
23. Johnson M, McLaren JW, Overby DR. Unconventional aqueous humor outflow: A review. Exp Eye Res. 2017;158:94–111. doi:10.1016/j.exer.2016.01.017
24. Ho LTY, Osterwald A, Ruf I, et al. Role of the autotaxin-lysophosphatidic acid axis in glaucoma, aqueous humor drainage and fibrogenic activity. Biochim Biophys Acta Mol Basis Dis. 2020;1866(1):165560. doi:10.1016/j.bbadis.2019.165560
25. Goel M, Picciani RG, Lee RK, Bhattacharya SK. Aqueous humor dynamics: a review. Open Ophthalmol J. 2010;4(1):52–59. doi:10.2174/1874364101004010052
26. Yan X, Li M, Song Y, et al. Influence of exercise on intraocular pressure, schlemm’s canal, and the trabecular meshwork. Invest Ophthalmol Vis Sci. 2016;57(11):4733–4739. doi:10.1167/iovs.16-19475
27. Vrabec JP, Levin LA. The neurobiology of cell death in glaucoma. Eye (Lond). 2007;21(Suppl 1):S11–S14. doi:10.1038/sj.eye.6702880
28. Weinreb RN, Khaw PT. Primary open-angle glaucoma. Lancet. 2004;363(9422):1711–1720. doi:10.1016/S0140-6736(04)16257-0
29. Bessero AC, Clarke PG. Neuroprotection for optic nerve disorders. Curr Opin Neurol. 2010;23(1):10–15. doi:10.1097/WCO.0b013e3283344461
30. Fang JH, Wang XH, Xu ZR, Jiang FG. Neuroprotective effects of bis(7)-tacrine against glutamate-induced retinal ganglion cells damage. BMC Neurosci. 2010;11(1):31. doi:10.1186/1471-2202-11-31
31. Tezel G. Oxidative stress in glaucomatous neurodegeneration: mechanisms and consequences. Prog Retin Eye Res. 2006;25(5):490–513. doi:10.1016/j.preteyeres.2006.07.003
32. Kong GY, Van Bergen NJ, Trounce IA, Crowston JG. Mitochondrial dysfunction and glaucoma. J Glaucoma. 2009;18(2):93–100. doi:10.1097/IJG.0b013e318181284f
33. Group, C.N.T.G.S. Comparison of glaucomatous progression between untreated patients with normal-tension glaucoma and patients with therapeutically reduced intraocular pressures. Collaborative Normal-Tension Glaucoma Study Group. Am J Ophthalmol. 1998;126(4):487–497. doi:10.1016/S0002-9394(98)00223-2
34. Fedorchak MV, Conner IP, Schuman JS, Cugini A, Little SR. Long term glaucoma drug delivery using a topically retained gel/microsphere eye drop. Sci Rep. 2017;7(1):8639. doi:10.1038/s41598-017-09379-8
35. Sambhara D, Aref AA. Glaucoma management: relative value and place in therapy of available drug treatments. Ther Adv Chronic Dis. 2014;5(1):30–43. doi:10.1177/2040622313511286
36. Hejkal TW, Camras CB. Prostaglandin analogs in the treatment of glaucoma. Semin Ophthalmol. 1999;14(3):114–123. doi:10.3109/08820539909061464
37. Camras C, Toris C. Advances in glaucoma management: risk factors, diagnostic tools, therapies and the role of prostaglandin analogs. Foreword. Surv Ophthalmol. 2008;53(Suppl1):S1–S2. doi:10.1016/j.survophthal.2008.08.013
38. Alizadeh R, Akil H, Tan J, Law SK, Caprioli J. Trabeculectomy outcomes after glaucoma drainage device surgery. J Glaucoma. 2018;27(2):133–139. doi:10.1097/IJG.0000000000000849
39. Gurwitz JH, Glynn RJ, Monane M, et al. Treatment for glaucoma: adherence by the elderly. Am J Public Health. 1993;83(5):711–716. doi:10.2105/AJPH.83.5.711
40. Loch C, Zakelj S, Kristl A, et al. Determination of permeability coefficients of ophthalmic drugs through different layers of porcine, rabbit and bovine eyes. Eur J Pharm Sci. 2012;47(1):131–138. doi:10.1016/j.ejps.2012.05.007
41. Scruggs J, Wallace T, Hanna C. Route of absorption of drug and ointment after application to the eye. Ann Ophthalmol. 1978;10(3):267–271.
42. Prausnitz MR, Noonan JS. Permeability of cornea, sclera, and conjunctiva: a literature analysis for drug delivery to the eye. J Pharm Sci. 1998;87(12):1479–1488. doi:10.1021/js9802594
43. Urtti A. Challenges and obstacles of ocular pharmacokinetics and drug delivery. Adv Drug Deliv Rev. 2006;58(11):1131–1135. doi:10.1016/j.addr.2006.07.027
44. Bito LZ, Baroody RA. The penetration of exogenous prostaglandin and arachidonic acid into, and their distribution within, the mammalian eye. Curr Eye Res. 1981;1(11):659–669. doi:10.3109/02713688109001870
45. Wei G, Xu H, Ding PT, SM L, Zheng JM. Thermosetting gels with modulated gelation temperature for ophthalmic use: the rheological and gamma scintigraphic studies. J Control Release. 2002;83(1):65–74. doi:10.1016/S0168-3659(02)00175-X
46. Hamalainen KM, Kananen K, Auriola S, Kontturi K, Urtti A. Characterization of paracellular and aqueous penetration routes in cornea, conjunctiva, and sclera. Invest Ophthalmol Vis Sci. 1997;38(3):627–634.
47. Lavik E, Kuehn MH, Kwon YH. Novel drug delivery systems for glaucoma. Eye (Lond). 2011;25(5):578–586. doi:10.1038/eye.2011.82
48. Patel A, Cholkar K, Agrahari V, Mitra AK. Ocular drug delivery systems: an overview. World J Pharmacol. 2013;2(2):47–64. doi:10.5497/wjp.v2.i2.47
49. Gaudana R, Ananthula HK, Parenky A, Mitra AK. Ocular drug delivery. AAPS J. 2010;12(3):348–360. doi:10.1208/s12248-010-9183-3
50. Wei G, Ding PT, Zheng JM, Lu WY. Pharmacokinetics of timolol in aqueous humor sampled by microdialysis after topical administration of thermosetting gels. Biomed Chromatogr. 2006;20(1):67–71. doi:10.1002/bmc.529
51. Cardigos J, Ferreira Q, Crisostomo S, et al. Nanotechnology-ocular devices for glaucoma treatment: a literature review. Curr Eye Res. 2019;44(2):111–117. doi:10.1080/02713683.2018.1536218
52. Patel S, Pasquale LR. Glaucoma drainage devices: a review of the past, present, and future. Semin Ophthalmol. 2010;25(5–6):265–270. doi:10.3109/08820538.2010.518840
53. Alvarado JA, Hollander DA, Juster RP, Lee LC. Ahmed valve implantation with adjunctive mitomycin C and 5-fluorouracil: long-term outcomes. Am J Ophthalmol. 2008;146(2):276–284. doi:10.1016/j.ajo.2008.04.008
54. Ponnusamy T, Yu H, John VT, Ayyala RS, Blake DA. A novel antiproliferative drug coating for glaucoma drainage devices. J Glaucoma. 2014;23(8):526–534. doi:10.1097/IJG.0b013e318294869b
55. Sommer A. Collaborative normal-tension glaucoma study. Am J Ophthalmol. 1999;128(6):776–777. doi:10.1016/S0002-9394(99)00369-4
56. Krupin T, Liebmann JM, Greenfield DS, Ritch R, Gardiner S. Low-pressure glaucoma study g. a randomized trial of brimonidine versus timolol in preserving visual function: results from the low-pressure glaucoma treatment study. Am J Ophthalmol. 2011;151(4):671–681. doi:10.1016/j.ajo.2010.09.026
57. Schnyder A, Huwyler J. Drug transport to brain with targeted liposomes. NeuroRx. 2005;2(1):99–107. doi:10.1602/neurorx.2.1.99
58. Li X, Hu D, Dang Y, et al. Nano Mapper: an Internet knowledge mapping system for nanotechnology development. J Nanopart Res. 2009;11(3):529–552. doi:10.1007/s11051-008-9491-z
59. Wagner V, Dullaart A, Bock AK, Zweck A. The emerging nanomedicine landscape. Nat Biotechnol. 2006;24(10):1211–1217. doi:10.1038/nbt1006-1211
60. Zarbin MA, Montemagno C, Leary JF, Ritch R. Nanotechnology in ophthalmology. Can J Ophthalmol. 2010;45(5):457–476. doi:10.3129/i10-090
61. Bucolo C, Drago F, Salomone S. Ocular drug delivery: a clue from nanotechnology. Front Pharmacol. 2012;3:188. doi:10.3389/fphar.2012.00188
62. Zarbin MA, Montemagno C, Leary JF, Ritch R. Nanomedicine in ophthalmology: the new frontier. Am J Ophthalmol. 2010;150(2):144–162. (). doi:10.1016/j.ajo.2010.03.019
63. Sahoo SK, Dilnawaz F, Krishnakumar S. Nanotechnology in ocular drug delivery. Drug Discov Today. 2008;13(3–4):144–151. doi:10.1016/j.drudis.2007.10.021
64. Lu H, Wang J, Wang T, Zhong J, Bao Y, Hao H. Recent Progress on nanostructures for drug delivery applications. J Nanomater. 2016;2016:5762431. doi:10.1155/2016/5762431
65. Kim HJ, Zhang K, Moore L, Ho D. Diamond nanogel-embedded contact lenses mediate lysozyme-dependent therapeutic release. ACS Nano. 2014;8(3):2998–3005. doi:10.1021/nn5002968
66. Goyal G, Garg T, Rath G, Goyal AK. Current nanotechnological strategies for treating glaucoma. Crit Rev Ther Drug Carrier Syst. 2014;31(5):365–405. doi:10.1615/CritRevTherDrugCarrierSyst.2014010123
67. Pita-Thomas DW, Goldberg JL. Nanotechnology and glaucoma: little particles for a big disease. Curr Opin Ophthalmol. 2013;24(2):130–135. doi:10.1097/ICU.0b013e32835cfe92
68. Gaafar PM, Abdallah OY, Farid RM, Abdelkader H. Preparation, characterization and evaluation of novel elastic nano-sized niosomes (ethoniosomes) for ocular delivery of prednisolone. J Liposome Res. 2014;24(3):204–215. doi:10.3109/08982104.2014.881850
69. Janagam DR, Wu L, Lowe TL. Nanoparticles for drug delivery to the anterior segment of the eye. Adv Drug Deliv Rev. 2017;122:31–64. doi:10.1016/j.addr.2017.04.001
70. Puglia C, Offerta A, Tirendi GG, et al. Design of solid lipid nanoparticles for caffeine topical administration. Drug Deliv. 2016;23(1):36–40. doi:10.3109/10717544.2014.903011
71. Kim M, Park JH, Jeong H, et al. An Evaluation of the in vivo Safety of Nonporous Silica Nanoparticles: ocular Topical Administration versus Oral Administration. Sci Rep. 2017;7(1):8238. doi:10.1038/s41598-017-08843-9
72. Natarajan JV, Darwitan A, Barathi VA, et al. Sustained drug release in nanomedicine: a long-acting nanocarrier-based formulation for glaucoma. ACS Nano. 2014;8(1):419–429. doi:10.1021/nn4046024
73. Salatin S, Yari Khosroushahi A. Overviews on the cellular uptake mechanism of polysaccharide colloidal nanoparticles. J Cell Mol Med. 2017;21(9):1668–1686. doi:10.1111/jcmm.13110
74. Nagai N, Ogata F, Otake H, Nakazawa Y, Kawasaki N. Energy-dependent endocytosis is responsible for drug transcorneal penetration following the instillation of ophthalmic formulations containing indomethacin nanoparticles. Int J Nanomedicine. 2019;14:1213–1227. doi:10.2147/IJN.S196681
75. Zhu S, Gong L, Li Y, Xu H, Gu Z, Zhao Y. Safety Assessment of Nanomaterials to Eyes: an Important but Neglected Issue. Adv Sci. 2019;6(16):1802289. doi:10.1002/advs.201802289
76. Prow TW. Toxicity of nanomaterials to the eye. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2010;2(4):317–333. doi:10.1002/wnan.65
77. Kim KT, Zaikova T, Hutchison JE, Tanguay RL. Gold nanoparticles disrupt zebrafish eye development and pigmentation. Toxicol Sci. 2013;133(2):275–288. doi:10.1093/toxsci/kft081
78. Immordino ML, Dosio F, Cattel L. Stealth liposomes: review of the basic science, rationale, and clinical applications, existing and potential. Int J Nanomedicine. 2006;1(3):297–315.
79. Mastrobattista E, Koning GA, van Bloois L, Filipe AC, Jiskoot W, Storm G. Functional characterization of an endosome-disruptive peptide and its application in cytosolic delivery of immunoliposome-entrapped proteins. J Biol Chem. 2002;277(30):27135–27143. doi:10.1074/jbc.M200429200
80. Monem AS, Ali FM, Ismail MW. Prolonged effect of liposomes encapsulating pilocarpine HCl in normal and glaucomatous rabbits. Int J Pharm. 2000;198(1):29–38. doi:10.1016/S0378-5173(99)00348-8
81. Puri A, Loomis K, Smith B, et al. Lipid-based nanoparticles as pharmaceutical drug carriers: from concepts to clinic. Crit Rev Ther Drug Carrier Syst. 2009;26(6):523–580. doi:10.1615/CritRevTherDrugCarrierSyst.v26.i6.10
82. Jain RL, Shastri JP. Study of ocular drug delivery system using drug-loaded liposomes. Int J Pharm Investig. 2011;1(1):35–41. doi:10.4103/2230-973X.76727
83. Yu S, Wang QM, Wang X, et al. Liposome incorporated ion sensitive in situ gels for opthalmic delivery of timolol maleate. Int J Pharm. 2015;480(1–2):128–136. doi:10.1016/j.ijpharm.2015.01.032
84. Law SL, Huang KJ, Chiang CH. Acyclovir-containing liposomes for potential ocular delivery. Corneal penetration and absorption. J Control Release. 2000;63(1–2):135–140. doi:10.1016/S0168-3659(99)00192-3
85. Hathout RM, Mansour S, Mortada ND, Guinedi AS. Liposomes as an ocular delivery system for acetazolamide: in vitro and in vivo studies. AAPS PharmSciTech. 2007;8(1):1. doi:10.1208/pt0801001
86. Velpandian T, Gupta SK, Gupta YK, Biswas NR, Agarwal HC. Ocular drug targeting by liposomes and their corneal interactions. J Microencapsul. 1999;16(2):243–250. doi:10.1080/026520499289211
87. Mehanna MM, Elmaradny HA, Samaha MW. Mucoadhesive liposomes as ocular delivery system: physical, microbiological, and in vivo assessment. Drug Dev Ind Pharm. 2010;36(1):108–118. doi:10.3109/03639040903099751
88. Sasaki H, Karasawa K, Hironaka K, Tahara K, Tozuka Y, Takeuchi H. Retinal drug delivery using eyedrop preparations of poly-L-lysine-modified liposomes. Eur J Pharm Biopharm. 2013;83(3):364–369. doi:10.1016/j.ejpb.2012.10.014
89. Ruel-Gariepy E, Leclair G, Hildgen P, Gupta A, Leroux JC. Thermosensitive chitosan-based hydrogel containing liposomes for the delivery of hydrophilic molecules. J Control Release. 2002;82(2–3):373–383. doi:10.1016/S0168-3659(02)00146-3
90. Tan G, Yu S, Pan H, et al. Bioadhesive chitosan-loaded liposomes: A more efficient and higher permeable ocular delivery platform for timolol maleate. Int J Biol Macromol. 2017;94(Pt A):355–363. doi:10.1016/j.ijbiomac.2016.10.035
91. Natarajan JV, Ang M, Darwitan A, Chattopadhyay S, Wong TT, Venkatraman SS. Nanomedicine for glaucoma: liposomes provide sustained release of latanoprost in the eye. Int J Nanomedicine. 2012;7:123–131. doi:10.2147/IJN.S25468
92. Li T, Tachibana K, Kuroki M, Kuroki M. Gene transfer with echo-enhanced contrast agents: comparison between Albunex, Optison, and Levovist in mice–initial results. Radiology. 2003;229(2):423–428. doi:10.1148/radiol.2292020500
93. Taniyama Y, Tachibana K, Hiraoka K, et al. Development of safe and efficient novel nonviral gene transfer using ultrasound: enhancement of transfection efficiency of naked plasmid DNA in skeletal muscle. Gene Ther. 2002;9(6):372–380. doi:10.1038/sj.gt.3301678
94. Lindner JR. Microbubbles in medical imaging: current applications and future directions. Nat Rev Drug Discov. 2004;3(6):527–532. doi:10.1038/nrd1417
95. Mizoue T, Horibe T, Maruyama K, et al. Targetability and intracellular delivery of anti-BCG antibody-modified, pH-sensitive fusogenic immunoliposomes to tumor cells. Int J Pharm. 2002;237(1–2):129–137. doi:10.1016/S0378-5173(02)00044-3
96. Sugano M, Negishi Y, Endo-Takahashi Y, et al. Gene delivery system involving Bubble liposomes and ultrasound for the efficient in vivo delivery of genes into mouse tongue tissue. Int J Pharm. 2012;422(1–2):332–337. doi:10.1016/j.ijpharm.2011.11.001
97. Kouchak M, Bahmandar R, Bavarsad N, Farrahi F. Ocular Dorzolamide Nanoliposomes for Prolonged IOP Reduction: in-Vitro and in-vivo Evaluation in Rabbits. Iran J Pharm Res. 2016;15(1):205–212.
98. Kouchak M, Malekahmadi M, Bavarsad N, Saki Malehi A, Andishmand L. Dorzolamide nanoliposome as a long action ophthalmic delivery system in open angle glaucoma and ocular hypertension patients. Drug Dev Ind Pharm. 2018;44(8):1239–1242. doi:10.1080/03639045.2017.1386196
99. Fahmy HM, Saad E, Sabra NM, El-Gohary AA, Mohamed FF, Gaber MH. Treatment merits of Latanoprost/Thymoquinone - Encapsulated liposome for glaucomatus rabbits. Int J Pharm. 2018;548(1):597–608. doi:10.1016/j.ijpharm.2018.07.012
100. Wong TT, Novack GD, Natarajan JV, Ho CL, Htoon HM, Venkatraman SS. Nanomedicine for glaucoma: sustained release latanoprost offers a new therapeutic option with substantial benefits over eyedrops. Drug Deliv Transl Res. 2014;4(4):303–309. doi:10.1007/s13346-014-0196-9
101. Mahale NB, Thakkar PD, Mali RG, Walunj DR, Chaudhari SR. Niosomes: novel sustained release nonionic stable vesicular systems–an overview. Adv Colloid Interface Sci. 2012;183–184:46–54. doi:10.1016/j.cis.2012.08.002
102. Kaur IP, Kanwar M. Ocular preparations: the formulation approach. Drug Dev Ind Pharm. 2002;28(5):473–493. doi:10.1081/DDC-120003445
103. Kaur IP, Aggarwal D, Singh H, Kakkar S. Improved ocular absorption kinetics of timolol maleate loaded into a bioadhesive niosomal delivery system. Graefes Arch Clin Exp Ophthalmol. 2010;248(10):1467–1472. doi:10.1007/s00417-010-1383-0
104. Aggarwal D, Kaur IP. Improved pharmacodynamics of timolol maleate from a mucoadhesive niosomal ophthalmic drug delivery system. Int J Pharm. 2005;290(1–2):155–159. doi:10.1016/j.ijpharm.2004.10.026
105. Aggarwal D, Pal D, Mitra AK, Kaur IP. Study of the extent of ocular absorption of acetazolamide from a developed niosomal formulation, by microdialysis sampling of aqueous humor. Int J Pharm. 2007;338(1–2):21–26. doi:10.1016/j.ijpharm.2007.01.019
106. Kaur IP, Garg A, Singla AK, Aggarwal D. Vesicular systems in ocular drug delivery: an overview. Int J Pharm. 2004;269(1):1–14. doi:10.1016/j.ijpharm.2003.09.016
107. Liu G, Molas M, Grossmann GA, et al. Biological properties of poly-L-lysine-DNA complexes generated by cooperative binding of the polycation. J Biol Chem. 2001;276(37):34379–34387. doi:10.1074/jbc.M105250200
108. Nagarwal RC, Kant S, Singh PN, Maiti P, Pandit JK. Polymeric nanoparticulate system: a potential approach for ocular drug delivery. J Control Release. 2009;136(1):2–13. doi:10.1016/j.jconrel.2008.12.018
109. Jung HJ, Abou-Jaoude M, Carbia BE, Plummer C, Chauhan A. Glaucoma therapy by extended release of timolol from nanoparticle loaded silicone-hydrogel contact lenses. J Control Release. 2013;165(1):82–89. doi:10.1016/j.jconrel.2012.10.010
110. Harmia T, Speiser P, Kreuter J. A solid colloidal drug delivery system for the eye: encapsulation of pilocarpin in nanoparticles. J Microencapsul. 1986;3(1):3–12. doi:10.3109/02652048609049580
111. Rathod LV, Kapadia R, Sawant KK. A novel nanoparticles impregnated ocular insert for enhanced bioavailability to posterior segment of eye: in vitro, in vivo and stability studies. Mater Sci Eng C Mater Biol Appl. 2017;71:529–540. doi:10.1016/j.msec.2016.10.017
112. Singh J, Chhabra G, Pathak K. Development of acetazolamide-loaded, pH-triggered polymeric nanoparticulate in situ gel for sustained ocular delivery: in vitro. Ex vivo evaluation and pharmacodynamic study. Drug Dev Ind Pharm. 2014;40(9):1223–1232. doi:10.3109/03639045.2013.814061
113. Nanjwade BK, Bechra HM, Derkar GK, Manvi FV, Nanjwade VK. Dendrimers: emerging polymers for drug-delivery systems. Eur J Pharm Sci. 2009;38(3):185–196. doi:10.1016/j.ejps.2009.07.008
114. Spataro G, Malecaze F, Turrin CO, et al. Designing dendrimers for ocular drug delivery. Eur J Med Chem. 2010;45(1):326–334. doi:10.1016/j.ejmech.2009.10.017
115. Vandamme TF, Brobeck L. Poly(amidoamine) dendrimers as ophthalmic vehicles for ocular delivery of pilocarpine nitrate and tropicamide. J Control Release. 2005;102(1):23–38. doi:10.1016/j.jconrel.2004.09.015
116. Yang H, Leffler CT. Hybrid dendrimer hydrogel/poly(lactic-co-glycolic acid) nanoparticle platform: an advanced vehicle for topical delivery of antiglaucoma drugs and a likely solution to improving compliance and adherence in glaucoma management. J Ocul Pharmacol Ther. 2013;29(2):166–172. doi:10.1089/jop.2012.0197
117. Bravo-Osuna I, Vicario-de-la-Torre M, Andres-Guerrero V, et al. Novel Water-Soluble Mucoadhesive Carbosilane Dendrimers for Ocular Administration. Mol Pharm. 2016;13(9):2966–2976. doi:10.1021/acs.molpharmaceut.6b00182
118. Chen MC, Gupta M, Cheung KC. Alginate-based microfluidic system for tumor spheroid formation and anticancer agent screening. Biomed Microdevices. 2010;12(4):647–654. doi:10.1007/s10544-010-9417-2
119. Weinreb RN, Jani R. A novel formulation of an ophthalmic beta-adrenoceptor antagonist. J Parenter Sci Technol. 1992;46(2):51–53.
120. Weinreb RN, Caldwell DR, Goode SM, et al. A double-masked three-month comparison between 0.25% betaxolol suspension and 0.5% betaxolol ophthalmic solution. Am J Ophthalmol. 1990;110(2):189–192. doi:10.1016/S0002-9394(14)76990-9
121. Ranpise NS, Kulkarni NS, Mair PD, Ranade AN. Improvement of water solubility and in vitro dissolution rate of aceclofenac by complexation with beta-cyclodextrin and hydroxypropyl-beta-cyclodextrin. Pharm Dev Technol. 2010;15(1):64–70. doi:10.3109/10837450903002165
122. Ludwig A. The use of mucoadhesive polymers in ocular drug delivery. Adv Drug Deliv Rev. 2005;57(11):1595–1639. doi:10.1016/j.addr.2005.07.005
123. Laza-Knoerr AL, Gref R, Couvreur P. Cyclodextrins for drug delivery. J Drug Target. 2010;18(9):645–656. doi:10.3109/10611861003622552
124. Granero GE, Longhi MR. Promising complexes of acetazolamide for topical ocular administration. Expert Opin Drug Deliv. 2010;7(8):943–953. doi:10.1517/17425247.2010.497536
125. Jansook P, Stefansson E, Thorsteinsdottir M, et al. Cyclodextrin solubilization of carbonic anhydrase inhibitor drugs: formulation of dorzolamide eye drop microparticle suspension. Eur J Pharm Biopharm. 2010;76(2):208–214. doi:10.1016/j.ejpb.2010.07.005
126. Wang F, Bao X, Fang A, et al. Nanoliposome-Encapsulated Brinzolamide-hydropropyl-beta-cyclodextrin Inclusion Complex: A Potential Therapeutic Ocular Drug-Delivery System. Front Pharmacol. 2018;9:91. doi:10.3389/fphar.2018.00091
127. Rodriguez-Aller M, Guinchard S, Guillarme D, et al. New prostaglandin analog formulation for glaucoma treatment containing cyclodextrins for improved stability, solubility and ocular tolerance. Eur J Pharm Biopharm. 2015;95(Pt B):203–214. doi:10.1016/j.ejpb.2015.04.032
128. Junghanns JU, Muller RH. Nanocrystal technology, drug delivery and clinical applications. Int J Nanomedicine. 2008;3(3):295–309. doi:10.2147/ijn.s595
129. Wu W, Li J, Wu L, et al. Ophthalmic delivery of brinzolamide by liquid crystalline nanoparticles: in vitro and in vivo evaluation. AAPS PharmSciTech. 2013;14(3):1063–1071. doi:10.1208/s12249-013-9997-2
130. Tuomela A, Liu P, Puranen J, et al. Brinzolamide nanocrystal formulations for ophthalmic delivery: reduction of elevated intraocular pressure in vivo. Int J Pharm. 2014;467(1–2):34–41. doi:10.1016/j.ijpharm.2014.03.048
131. Orasugh JT, Sarkar G, Saha NR, et al. Effect of cellulose nanocrystals on the performance of drug loaded in situ gelling thermo-responsive ophthalmic formulations. Int J Biol Macromol. 2019;124:235–245. doi:10.1016/j.ijbiomac.2018.11.217
132. Ikuta Y, Aoyagi S, Tanaka Y, et al. Creation of nano eye-drops and effective drug delivery to the interior of the eye. Sci Rep. 2017;7(1):44229. doi:10.1038/srep44229
133. Mochalin VN, Shenderova O, Ho D, Gogotsi Y. The properties and applications of nanodiamonds. Nat Nanotechnol. 2011;7(1):11–23. doi:10.1038/nnano.2011.209
134. Lloyd AW, Faragher RG, Denyer SP. Ocular biomaterials and implants. Biomaterials. 2001;22(8):769–785. doi:10.1016/S0142-9612(00)00237-4
135. Cocero MJMÁ, Mattea F, Varona S, Varona S. Encapsulation and co-precipitation processes with supercritical fluids: fundamentals and applications. J Supercrit Fluid. 2009;47(3):546–555. doi:10.1016/j.supflu.2008.08.015
136. Choi SW, Kim J. Therapeutic Contact Lenses with Polymeric Vehicles for Ocular Drug Delivery: A Review. Materials (Basel). 2018;11(7):1125. doi:10.3390/ma11071125
137. Peng CC, Burke MT, Carbia BE, Plummer C, Chauhan A. Extended drug delivery by contact lenses for glaucoma therapy. J Control Release. 2012;162(1):152–158. doi:10.1016/j.jconrel.2012.06.017
138. Jung HJ, Chauhan A. Temperature sensitive contact lenses for triggered ophthalmic drug delivery. Biomaterials. 2012;33(7):2289–2300. doi:10.1016/j.biomaterials.2011.10.076
139. Wadhwa S, Paliwal R, Paliwal SR, Vyas SP. Hyaluronic acid modified chitosan nanoparticles for effective management of glaucoma: development, characterization, and evaluation. J Drug Target. 2010;18(4):292–302. doi:10.3109/10611860903450023
140. Zhang K, Zhang L, Weinreb RN. Ophthalmic drug discovery: novel targets and mechanisms for retinal diseases and glaucoma. Nat Rev Drug Discov. 2012;11(7):541–559. doi:10.1038/nrd3745
141. Liang SY, Lee GA, Shields D. Self-tonometry in glaucoma management–past, present and future. Surv Ophthalmol. 2009;54(4):450–462. doi:10.1016/j.survophthal.2009.04.006
142. Kim J, Kim M, Lee MS, et al. Wearable smart sensor systems integrated on soft contact lenses for wireless ocular diagnostics. Nat Commun. 2017;8(1):14997. doi:10.1038/ncomms14997
143. Leonardi M, Pitchon EM, Bertsch A, Renaud P, Mermoud A. Wireless contact lens sensor for intraocular pressure monitoring: assessment on enucleated pig eyes. Acta Ophthalmol. 2009;87(4):433–437. doi:10.1111/j.1755-3768.2008.01404.x
144. Dunbar GE, Shen BY, Aref AA. The Sensimed Triggerfish contact lens sensor: efficacy, safety, and patient perspectives. Clin Ophthalmol. 2017;11:875–882. doi:10.2147/OPTH.S109708
145. Enders P, Cursiefen C. Device profile of the EYEMATE-IO system for intraocular pressure monitoring: overview of its safety and efficacy. Expert Rev Med Devices. 2020;1–7. doi:10.1080/17434440.2020.1761788
146. Salim S, Du H, Wan J. Comparison of intraocular pressure measurements and assessment of intraobserver and interobserver reproducibility with the portable ICare rebound tonometer and Goldmann applanation tonometer in glaucoma patients. J Glaucoma. 2013;22(4):325–329. doi:10.1097/IJG.0b013e318237caa2
147. Peng HT, Shek PN. Novel wound sealants: biomaterials and applications. Expert Rev Med Devices. 2010;7(5):639–659. doi:10.1586/erd.10.40
148. Spierer O, O’Brien TP. Endothelial keratoplasty combined with cataract surgery or alone using polyethylene glycol hydrogel sealant for closure of corneal incisions. J Cataract Refract Surg. 2015;41(3):492–496. doi:10.1016/j.jcrs.2015.02.002
149. Masket S, Hovanesian JA, Levenson J, et al. Hydrogel sealant versus sutures to prevent fluid egress after cataract surgery. J Cataract Refract Surg. 2014;40(12):2057–2066. doi:10.1016/j.jcrs.2014.03.034
150. Barliya T, Sandalon S, Ofri R, Livnat T, Weinberger D. Transcleral approach for closing retinal tears using DuraSeal hydrogel sealant. Indian J Ophthalmol. 2018;66(2):238–243. doi:10.4103/ijo.IJO_758_17
151. Preethi G, Prashanth K. Design and evaluation of controlled-release ocular inserts of brimonidine-tartrate and timolol maleate. Int J Pharm Pharm Sci. 2017;9(1):79–82.
152. Perera SA, Ting DS, Nongpiur ME, et al. Feasibility study of sustained-release travoprost punctum plug for intraocular pressure reduction in an Asian population. Clin Ophthalmol. 2016;10:757–764. doi:10.2147/OPTH.S102181
153. A Phase 2 study evaluating safety and efficacy of the latanoprost punctal plug delivery system (L-PPDS) in subjects with ocular hypertension (OH) or open-angle glaucoma (OAG). 2019. https://iovs.arvojournals.org/article.aspx?articleid=2358808.
154. Park AJ, Eliassi-Rad B, Desai MA. Ptosis after glaucoma surgery. Clin Ophthalmol. 2017;11:1483–1489. doi:10.2147/OPTH.S134562
155. Pan T, Brown JD, Ziaie B. An artificial nano-drainage implant (ANDI) for glaucoma treatment. Conf Proc IEEE Eng Med Biol Soc. 2006;1:3174–3177.
156. Paschalis EI, Chodosh J, Sperling RA, Salvador-Culla B, Dohlman C. A novel implantable glaucoma valve using ferrofluid. PLoS One. 2013;8(6):e67404. doi:10.1371/journal.pone.0067404
157. Maleki T, Chitnis G, Park JH, Cantor LB, Ziaie B. Biodegradable microfabricated plug-filters for glaucoma drainage devices. IEEE Trans Biomed Eng. 2012;59(6):1507–1513. doi:10.1109/TBME.2011.2179031
158. Harake RS, Ding Y, Brown JD, Design PT. Fabrication, and In Vitro Testing of an Anti-biofouling Glaucoma Micro-shunt. Ann Biomed Eng. 2015;43(10):2394–2405. doi:10.1007/s10439-015-1309-4
159. Abu-Hassan DW, Acott TS, Kelley MJ. The Trabecular Meshwork: A Basic Review of Form and Function. J Ocul Biol. 2014;2(1).
160. Gong H, Swain D. The histopathological changes in the trabecular outflow pathway and their possible effects on aqueous outflow in eyes with primary open-angle glaucoma. Glaucoma res clin adv. 2016;17–40.
161. Castro A, Du Y. Trabecular Meshwork Regeneration - A Potential Treatment for Glaucoma. Curr Ophthalmol Rep. 2019;7(2):80–88. doi:10.1007/s40135-019-00203-2
162. Hu Y, Tan HB, Wang XM, Rong H, Cui HP, Cui H. Bone marrow mesenchymal stem cells protect against retinal ganglion cell loss in aged rats with glaucoma. Clin Interv Aging. 2013;8:1467–1470. doi:10.2147/CIA.S47350
163. Mobaraki M, Abbasi R, Omidian Vandchali S, Ghaffari M, Moztarzadeh F, Mozafari M. Corneal Repair and Regeneration: current Concepts and Future Directions. Front Bioeng Biotechnol. 2019;7:135. doi:10.3389/fbioe.2019.00135
164. Alvarado J, Murphy C, Polansky J, Juster R. Age-related changes in trabecular meshwork cellularity. Invest Ophthalmol Vis Sci. 1981;21(5):714–727.
165. Grierson I, Howes RC. Age-related depletion of the cell population in the human trabecular meshwork. Eye (Lond). 1987;1(Pt 2):204–210.
166. Du Y, Yun H, Yang E, Schuman J. Science v. stem cells from trabecular meshwork home to TM tissue in vivo. Investophthalmol visual sci. 2013;54(2):1450–1459.
167. Snider EJ, Kubelick KP, Tweed K, et al. Improving stem cell delivery to the trabecular meshwork using magnetic nanoparticles. Sci Rep. 2018;8(1):12251. doi:10.1038/s41598-018-30834-7
168. Dillinger AE, Guter M, Froemel F, et al. Intracameral delivery of layer‐by‐layer coated siRNA nanoparticles for glaucoma therapy.Small. 2018;14(50):1803239.
169. Dautriche CN, Szymanski D, Kerr M, et al. A biomimetic Schlemm’s canal inner wall: A model to study outflow physiology, glaucoma pathology and high-throughput drug screening. Biomaterials. 2015;65:86–92.
170. Dautriche CN, Xie Y, Sharfstein S. Walking through trabecular meshwork biology: toward engineering design of outflow physiology. Biotechnol adv. 2014;32(5):971–983.
171. Torrejon KY, Papke EL, Halman JR, et al. Bioengineered glaucomatous 3D human trabecular meshwork as an in vitro disease model. Biotechnol bioeng. 2016;113(6):1357–1368.
172. Waduthanthri KD, He Y, Montemagno C, Cetinel S. An injectable peptide hydrogel for reconstruction of the human trabecular meshwork. Acta biomater. 2019;100:244–254.
173. Ward M, Khoobehi A, Lavik E, Langer R, Young M. Neuroprotection of retinal ganglion cells in DBA/2J mice with GDNF-loaded biodegradable microspheres. J pharm sci. 2007;96(3):558–568.
174. Jiang C, Moore MJ, Zhang X, Klassen H, Langer R, Young M. Intravitreal injections of GDNF-loaded biodegradable microspheres are neuroprotective in a rat model of glaucoma. 2007.
175. Checa-Casalengua P, Jiang C, Bravo-Osuna I, et al. Retinal ganglion cells survival in a glaucoma model by GDNF/Vit E PLGA microspheres prepared according to a novel microencapsulation procedure. J Control Rel. 2011;156(1):92–100.
176. García-Caballero C, Prieto-Calvo E, Checa-Casalengua P, et al. Six month delivery of GDNF from PLGA/vitamin E biodegradable microspheres after intravitreal injection in rabbits0. Eur J Pharm Sci. 2017;103:19–26.
177. Nkansah MK, Tzeng SY, Holdt AM, Lavik EBJB. bioengineering. Poly (lactic‐co‐glycolic acid) nanospheres and microspheres for short‐and long‐term delivery of bioactive ciliary neurotrophic factor. Biotechnol bioeng. 2008;100(5):1010–1019.
178. Eastlake K, Wang W, Jayaram H, et al. Phenotypic and functional characterization of muller glia isolated from induced pluripotent stem cell-derived retinal organoids: improvement of retinal ganglion cell function upon transplantation. Stem Cells Transl Med. 2019;8(8):775–784. doi:10.1002/sctm.18-0263
179. Ooto S, Akagi T, Kageyama R, et al. Potential for neural regeneration after neurotoxic injury in the adult mammalian retina. Proc Natl Acad Sci U S A. 2004;101(37):13654–13659. doi:10.1073/pnas.0402129101
180. Lawrence JM, Singhal S, Bhatia B, et al. MIO-M1 cells and similar muller glial cell lines derived from adult human retina exhibit neural stem cell characteristics. Stem Cells. 2007;25(8):2033–2043. doi:10.1634/stemcells.2006-0724
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.