Back to Journals » Therapeutics and Clinical Risk Management » Volume 14
Potential risk factors for poor outcome after anterior surgery for patients with cervical ossification of the posterior longitudinal ligament
Authors Li SQ, Zhang P, Gao XD, Miao DC, Gao YL, Shen Y
Received 23 September 2017
Accepted for publication 15 January 2018
Published 20 February 2018 Volume 2018:14 Pages 341—347
DOI https://doi.org/10.2147/TCRM.S152416
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Deyun Wang
Shaoqing Li, Peng Zhang, Xianda Gao, Dechao Miao, Yanlong Gao, Yong Shen
Department of Orthopedic Surgery, The Third Hospital of Hebei Medical University, Shijiazhuang, People’s Republic of China
Objective: Our purpose here was to identify risk factors of poor outcome after anterior operation in patients with cervical ossification of the posterior longitudinal ligament (OPLL).
Methods: This study retrospectively reviewed 98 patients who underwent anterior surgery for improving neurological symptoms. The Japanese Orthopedic Association (JOA) recovery rate <50% was defined as poor surgical outcome. We investigated the relationship between various predictors and outcome by logistic regression analysis and receiver operating characteristic curves. To explore the cause of cerebrospinal fluid (CSF) leakage, we used the Mann–Whitney U-test, χ2 test, or independent t-test.
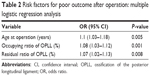
Results: Multivariate logistic regression analysis showed that age (odds ratio [OR] =1.1, 95% confidence interval [CI] =1.03–1.18, P=0.005), occupying ratio of OPLL (OR =1.08, 95% CI =1.03–1.12, P=0.001), and residual ratio of OPLL (OR =1.07, 95% CI =1.02–1.13, P=0.008) were independently associated with poor outcome. The cutoffs of the above risk factors were set at 63.5 years, 39.65%, and 25.165%, respectively. Predictors for CSF leakage were occupying ratio of OPLL, the K-line, and shape of the ossified lesion (P<0.001, P=0.019, and P=0.003).
Conclusion: These findings suggest that advanced age, high occupying ratio of OPLL, and high residual ratio of OPLL were risk factors for postoperative poor outcome in patients with OPLL. In addition, the high occupying ratio of OPLL, the K-line (-), and hill-shape ossification were potential causes of CSF leakage.
Keywords: cervical ossification of the posterior longitudinal ligament, cerebrospinal leakage, occupying ratio, residual ratio, K-line
Introduction
Cervical ossification of the posterior longitudinal ligament (OPLL) is one of the main causes of cervical myelopathy,1 which is classified by structure (continuous-type, segmental-type, mixed-type and circumscribed-type) and shape (plateau-shaped and hill-shaped).2,6 The circumscribed-type OPLL with massive beak-shaped ossification has narrower spinal cord cross-sectional area and worse neurologic symptoms. When nonoperative treatment fails, surgery should be considered. The posterior approach generates an indirect decompression resulting from the posterior shift of the spinal cord, which restricts neurologic recovery. Some patients require revision surgery after initial cervical posterior surgery due to persistent cord compression and late neurological deterioration.7 The anterior cervical discectomy and fusion (ACDF) and the anterior cervical corpectomy and fusion (ACCF) procedures for the treatment of cervical OPLL are theoretically feasible, which directly removes the lesion of the anterior spinal cord. However, it is considered technically demanding and is associated with serious complications, such as intraoperative cerebrospinal fluid (CSF) leakage, graft subsidence, loss of cervical lordosis, and adjacent-segment disease.4,5 In addition, with anterior cervical surgery, it is difficult to completely remove the ossification of lesion due to the narrow operating space and the location of the ossification. Some studies have investigated risk factors of poor outcomes, including age, occupying ratio of OPLL, function of the K-line, preoperative Japanese Orthopedic Association (JOA) score, and increased signal intensity on magnetic resonance imaging (MRI).2,3,8–11 However, few studies have focused on residual ratio of OPLL and the causes of the CSF leakage. We hypothesized that the poor outcome after cervical surgery is associated with the high residual ratio of OPLL and the high occupying ratio of OPLL in terms of neurological function. We designed the present study to examine this relationship between predictors and poor postoperative outcome, particularly the residual ratio of OPLL. This exploration of the causes of intraoperative CSF leakage provides insight into how surgeons cope with unexpected situations.
Materials and methods
Patient population
A total of 98 patients with cervical compressive myelopathy due to OPLL who underwent decompression surgery at the Department of Spinal Surgery, the Third Hospital of Hebei Medical University in China, from January 2010 to March 2014 were reviewed retrospectively. The criteria for inclusion were the following: 1) diagnosis of cervical compressive myelopathy due to OPLL; 2) increased signal intensity of the spinal cord on MRI; 3) one or two levels involved; and 4) anterior surgery for direct removal of OPLL. The exclusion criteria were presence of rheumatoid arthritis, previous cervical spine surgery, vitamin B deficiency, neuromuscular diseases, or presence of other complex concomitant diseases. There were 48 males and 50 females with OPLL enrolled in our study, and their mean age at surgery was 60.3±8.5 years. In the present study, 32 patients were treated by ACCF and 66 patients by ACDF. Patients with OPLL have various symptoms such as sensory abnormality of the trunk or extremities, gait disturbance, and urinary dysfunction. The more removal of OPLL is a prerequisite for good postoperative outcome. We selected 2 patient groups based on the outcome after surgical procedure: good or poor. The number of operated segments and the surgical approach (ACDF or ACCF) were determined by the occupying ratio of OPLL, preoperative JOA score, age at operation, and degree of symptoms. Because 12 months is a typical time period of optimum recovery after the operation, the important predictors of outcome were assessed at 12-month follow-up.
This study was approved by the Institutional Ethics Board of the Third Hospital of Hebei Medical University, and all the clinical data were collected after acquisition of written informed consent from the patients. The methods were carried out in accordance with the Doctor-patient relationship regulations of the Third Hospital of Hebei Medical University.
Neurological assessment
The JOA score was used to evaluate neurological function immediately after the operation and at 12-month follow-up. Postoperative improvement of symptoms was estimated by means of the recovery rate = (postoperative JOA score − preoperative JOA score)/(17 − preoperative JOA score) ×100%. A score ≥75% was designated as excellent, ≥50% but <75% as good, ≥25% but <50% as fair, and <25% as poor. Therefore, we defined recovery rates <50% as a poor postoperative outcome in this study.
Radiologic assessment
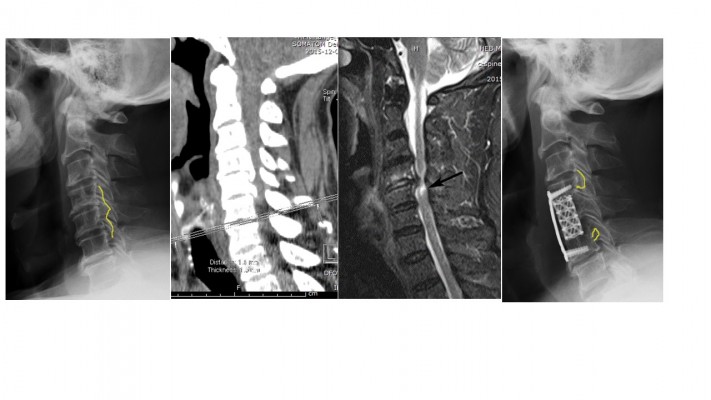
The cervical sagittal alignment was evaluated on lateral radiographs. We measured the range between the inferior border of the C2 vertebral body and the inferior border of the C7 vertebral body to represent the Cobb angle. The range of motion (ROM) was defined as the change in the maximal flexion and extension on lateral radiographs. The K-line was defined as the connected midpoints of the spinal canal at C2 and C7 level. When the OPLL extended beyond this line, the K-line was defined as minus, but when it did not extend beyond this line, the K-line was defined as plus. The occupying ratio of OPLL was calculated as: ossified thickness of the most stenotic area (b)/anteroposterior diameter of the spinal canal (a) ×100%.12 After the operation, we measured the residual thickness of ossification at the most stenotic area (c), with the residual ratio of OPLL calculated as: c/ossified thickness of the most stenotic area (b) ×100% (Figure 1). The ossification of the most stenotic area was measured in the sagittal images obtained by computed tomography. The spinal cord compression was assessed based on high-intensity signal on T2-weighted sagittal MRI (Figure 2). The decrease of intervertebral height ≥2 mm was defined as the cage subsidence.
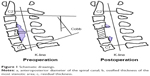  | Figure 1 Schematic drawings. |
  | Figure 2 Measuring ossified thickness and high-intensity signal of spinal cord. |
Surgical procedure
All patients were placed on a surgical bed in supine position with the neck extended slightly for general anesthesia. A right transverse skin incision was used at the level of OPLL. The trachea and esophagus were medially retracted using a thyroid retractor, reaching the vertebral anterior edge of the diseased level. Patients with involvement of the spinal cord with single-segment compression were treated by ACDF. When there were 2 operated segments, we selected single-level ACCF or a 2-level ACDF. We expanded both sides of intervertebral space and removed part of the end plate at ACDF, which would remove the circumscribed-type OPLL for the decompression of spinal cord. It is worth noting that the rupture of venous plexus will lead to increased intraoperative bleeding. After surgery, all the patients were restricted to bed only on the day of surgery and were told to use a cervical collar for 3 weeks.
Statistical analysis
All data analyses were performed using SPSS version 21.0 for Windows (SPSS, Inc., Chicago, IL, USA). Data are presented as the number of subjects in each group or means ± SD. Comparisons of CSF leakage and the other variables were done using the independent t-test, χ2 test, or Mann–Whitney U-test, as appropriate. Multivariate logistic regression analysis was used to predict the risk factors, and P-value <0.05 was set for univariate analyses. P-values of respective predictors are given on the basis of adjusted odds ratios (ORs) with 95% confidence intervals (CIs). The analysis of receiver operating characteristic (ROC) curves was protracted to evaluate the cutoff values for the continuous variables. The relationship between poor surgical outcome and the number of risk factors was examined by logistic regression analysis. A value of P<0.05 was considered to represent a statistically significant difference.
Results
The average preoperative JOA score for all patients was 8.82±2.04 and 13.5±1.86 at 12 months postoperatively. Compared with the preoperative JOA score, there was a statistically significant improvement after surgery (P<0.001). Based on the recovery rate of JOA, there were 66 patients with good postoperative outcomes (recovery rate ≥50%) and 32 patients with poor postoperative outcomes (recovery rates <50%).
The mean ages of the good group and poor group were 58.3±7.9 years and 64.5±8.2 years, respectively. Among all patients, there were 25 cases with the occupying ratio of OPLL ≥50%, and the range of residual ratio of OPLL was 7.28%–51.02% after surgery. Compared with the group with good outcomes, the poor outcome group had a significantly higher age (P<0.001) and occupying ratio of OPLL (P<0.001) and residual ratio of OPLL (P=0.007), whereas sex, body mass index, CSF leakage, preoperative JOA score, preoperative C2-7 Cobb angle, cage subsidence, and C2-7 ROM were not significant variables (Table 1). Based on multivariate logistic regression analysis, age (OR =1.1, 95% CI =1.03–1.18, P=0.005), occupying ratio of OPLL (OR =1.08, 95% CI =1.03–1.12, P=0.001), and residual ratio of OPLL (OR =1.07, 95% CI =1.02–1.13, P=0.008) were independently associated with poor postoperative outcome (Table 2).
Table 3 and Figure 3 summarize the relationship for predicting poor outcome by the specificity, sensitivity, area under the curve, and cutoff of risk factors and the ROC curves. The age at operation, occupying ratio of OPLL, and residual ratio of OPLL predicted poor outcome as area under the curve 0.725, 0.769, and 0.671, respectively, with P<0.001, P<0.001, P=0.006, respectively. The maximum of Youden index was defined as the cutoff, which was the best compromise between sensitivity and specificity. The cutoff values were 63.5 years, 39.65%, and 25.165% in age, occupying ratio of OPLL, and residual ratio of OPLL, respectively. Differences in the incidence of poor postoperative outcome were significant between <2 factors (0 or 1) and ≥2 factors (OR =19.3, 95% CI =5.3–70.5, P<0.001) (Table 4).
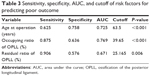  | Table 3 Sensitivity, specificity, AUC, and cutoff of risk factors for predicting poor outcome |
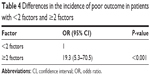  | Table 4 Differences in the incidence of poor outcome in patients with <2 factors and ≥2 factors |
Table 5 shows that there were 18 cases with K-line (−) and 80 cases with K-line (+). Patients were further divided into a group with CSF leakage (n=15) and a group with no CSF leakage (n=83). Compared with the group with no-CSF leakage, the other group was significantly associated with the occupying ratio of OPLL, the K-line, and shape of ossified lesion (P<0.001, P=0.019, and P=0.003, respectively).
Discussion
Patients with cervical compressive myelopathy only due to OPLL are relatively rarer than other patients with cervical degenerative disease. However, recognition of the best approach for patients with OPLL is an equally important clinical issue. Surgical strategies for removing cervical OPLL include 2 approaches: anterior and posterior. Patients with multilevel severe OPLL commonly undergo the posterior approach. Previous studies have proposed that the anterior approach yields better results in long-term follow-up than does cervical laminoplasty.13,14 However, in our study, patients with poor surgical outcome tended to be older, have higher occupying ratio of OPLL, and have higher residual ratio of OPLL, which was demonstrated by a logistic regression model. Therefore, this study provides some helpful information for identifying prognostic factors which could determine neurologic improvement after anterior surgery.
Previous investigations reported that the spinal cord with chronic compression is vulnerable and has low potential for functional reversible capacity in elderly patients.9,15 Motor neurons and myelinated fibers can degenerate with age. In the present study, the patients in the good-outcome group were significantly younger than those in the poor outcome group (P<0.001). Multivariate logistic analysis showed that age was a risk factor for poor postoperative outcome. The cutoffs showed that patients aged over 63.5 years were significantly associated with the poor outcome. Underlying diseases of elderly people may have negatively influenced surgical outcomes. Our study shows that elderly patients with OPLL tend to experience poor neurological recovery. We excluded the patients with complex concomitant diseases on postoperative outcomes. On the other hand, the duration of compressed spinal cord produces different symptoms, including pain, numbness, and weakness of muscles. However, contrast with the good-group, the poor-group had no significant difference in the duration of symptoms was which due to the compression of spinal cord by OPLL.
Some studies showed that the surgical approach was selected on the basis of the occupying ratio of OPLL and the relation between the occupying and poor outcome.2,16,17 However, previous investigations did not provide detailed analysis on the threshold value of occupying ratio of OPLL. In the present study, the cutoffs were set at 39.65% of occupying ratio of OPLL by the ROC curve. We found that the causes of residual ossification during cervical surgery include narrow space of operation, location of ossification, type of ossification, and adhesion of surrounding tissue. The increased residual ossification affects CSF circulation, which is detrimental to spinal cord recovery. We found that the high residual ratio of OPLL was significantly associated with poor surgical outcome, as indicated by univariate analysis. When the residual ratio was higher than 25.165%, a poor outcome was likely (P=0.006). Therefore, our study shows surgeons that when the original thickness of the OPLL was more than 75%, it needs to be removed to prevent poor outcomes.
Numerous factors led to intraoperative CSF leakage in cases with OPLL, such as long-standing OPLL, geometry of the ossified lesion, occupying ratio of OPLL, and technical proficiency of the surgeon. However, some studies reported that outcomes were not predicted by the shape of the ossified lesion.9,19 In the present study, patients with the hill-shaped OPLL may have contributed to the increased number of intraoperative CSF leaks during shaving of the ossified lesions (P=0.003). In addition, the results showed intraoperative CSF leaks were significantly associated with a high occupying ratio of OPLL and K-line (−) (P<0.001 and P=0.019, respectively). Min et al18 reported that spinal cord herniation through the defective dura mater might also have caused neurological sequelae. Therefore, we would halt the decompression and further remove the ossified lesions, when encountering CSF leakage in our study.
In the present study, sex, body mass index, CSF leakage, preoperative JOA score, Cobb angle of the C2-7, C2-7 ROM, cage subsidence, the number of involved segments, and surgical approach were not correlated with postoperative poor outcome. The K-line relatively moved forward and the spinal cord cross-sectional area became narrower during flexion at the disc level.20 We found that the occupying ratio of OPLL was more accurate for identifying predictive factors of postoperative prognosis. Therefore, we did not select K-line as the predictors of poor postoperative outcome. In addition, Chiba et al21 showed that postoperative kyphotic changes in cervical alignment were uncommon after anterior surgery. Therefore, we did not consider the changes of the C2-7 Cobb after surgery. Another finding was that patients with ≥2 of the risk factors (age, occupying ratio of OPLL, and residual ratio of OPLL) were more significantly associated with poor surgical outcome. It is possible that the different studies used different statistical tests, which led to our findings being inconsistent with the results of previous studies.
In the present study, several limitations need to be considered. First, there were a limited number of eligible patients, as this was a single-center study. Second, we did not further confirm the relationship between degree of spinal cord injury and poor surgical outcome after removing OPLL. Third, the relationship between adjacent-segment degeneration and clinical outcome could not be clearly evaluated, because the term of our follow-up was limited. Four, the JOA score with patient reports was not considered, which led to the deviation of outcomes. Our study supports that cervical OPLL was directly removed by anterior surgery, but we cannot definitively conclude which is the best surgical procedure for multistage compression of the spinal cord.
Conclusion
Advanced age, high occupying ratio of OPLL, and high residual ratio of OPLL are negative predictors for outcome of surgery in patients with OPLL. Intraoperative CSF leakage is one of the important reasons why OPLL could not be completely removed. The high occupying ratio of OPLL, the K-line (−), and hill-shaped ossification were potential causes of CSF leakage. Therefore, the understanding about the importance of predictive factors can help surgeons cope with unexpected situations and lead to better curative effect.
Acknowledgment
We thank all patients who took part in this study and provided the information to the authors.
Disclosure
All authors have read and contributed to the submitted manuscript. The authors report no conflicts of interest in this work.
References
Matsunaga S, Sakou T. Ossification of the posterior longitudinal ligament of the cervical spine: etiology and natural history. Spine (Phila Pa 1976). 2012;37(5):E309–E314. | ||
Iwasaki M, Okuda S, Miyauchi A, et al. Surgical strategy for cervical myelopathy due to ossification of the posterior longitudinal ligament: Part 1: Clinical results and limitations of laminoplasty. Spine (Phila Pa 1976). 2007;32(6):647–653. | ||
Iwasaki M, Okuda S, Miyauchi A, et al. Surgical strategy for cervical myelopathy due to ossification of the posterior longitudinal ligament: Part 2: Advantages of anterior decompression and fusion over laminoplasty. Spine (Phila Pa 1976). 2007;32(6):654–660. | ||
Wada E, Suzuki S, Kanazawa A, Matsuoka T, Miyamoto S, Yonenobu K. Subtotal corpectomy versus laminoplasty for multilevel cervical spondylotic myelopathy: a long-term follow-up study over 10 years. Spine (Phila Pa 1976). 2001;26(13):1443–1447; discussion 1448. | ||
Park Y, Maeda T, Cho W, Riew KD. Comparison of anterior cervical fusion after two-level discectomy or single-level corpectomy: sagittal alignment, cervical lordosis, graft collapse, and adjacent-level ossification. Spine J. 2010;10(3):193–199. | ||
Iwasaki M, Yonenobu K. Ossification of the posterior longitudinal ligament. In: Herkowitz HN, Rothman RH, Simeone FA, editors. The Spine, 5th ed. Philadelphia, PA: Elsevier; 2006:896–912. | ||
Odate S, Shikata J, Soeda T, Yamamura S, Kawaguchi S. Surgical results and complications of anterior decompression and fusion as a revision surgery after initial posterior surgery for cervical myelopathy due to ossification of the posterior longitudinal ligament. J Neurosurg Spine. 2017;26(4):466–473. | ||
Fujiyoshi T, Yamazaki M, Kawabe J, et al. A new concept for making decisions regarding the surgical approach for cervical ossification of the posterior longitudinal ligament: the K-line. Spine (Phila Pa 1976). 2008;33(26):E990–E993. | ||
Kim B, Yoon DH, Shin HC, et al. Surgical outcome and prognostic factors of anterior decompression and fusion for cervical compressive myelopathy due to ossification of the posterior longitudinal ligament. Spine J. 2015;15(5):875–884. | ||
Li H, Jiang LS, Dai LY. A review of prognostic factors for surgical outcome of ossification of the posterior longitudinal ligament of cervical spine. Eur Spine J. 2008;17(10):1277–1288. | ||
Uchida K, Nakajima H, Takeura N, et al. Prognostic value of changes in spinal cord signal intensity on magnetic resonance imaging in patients with cervical compressive myelopathy. Spine J. 2014;14(8):1601–1610. | ||
Masaki Y, Yamazaki M, Okawa A, et al. An analysis of factors causing poor surgical outcome in patients with cervical myelopathy due to ossification of the posterior longitudinal ligament: anterior decompression with spinal fusion versus laminoplasty. J Spinal Disord Tech. 2007;20(1):7–13. | ||
Ikenaga M, Shikata J, Tanaka C. Long-term results over 10 years of anterior corpectomy and fusion for multilevel cervical myelopathy. Spine (Phila Pa 1976). 2006;31(14):1568–1574; discussion 1575. | ||
Sakai K, Okawa A, Takahashi M, et al. Five-year follow-up evaluation of surgical treatment for cervical myelopathy caused by ossification of the posterior longitudinal ligament: a prospective comparative study of anterior decompression and fusion with floating method versus laminoplasty. Spine (Phila Pa 1976). 2012;37(5):367–376. | ||
Terao S, Sobue G, Hashizume Y, Li M, Inagaki T, Mitsuma T. Age-related changes in human spinal ventral horn cells with special reference to the loss of small neurons in the intermediate zone: a quantitative analysis. Acta Neuropathol. 1996;92(2):109–114. | ||
Wang X, Chen D, Yuan W, Zhang Y, Xiao J, Zhao J. Anterior surgery in selective patients with massive ossification of posterior longitudinal ligament of cervical spine: technical note. Eur Spine J. 2012;21(2):314–321. | ||
Tani T, Ushida T, Ishida K, Iai H, Noguchi T, Yamamoto H. Relative safety of anterior microsurgical decompression versus laminoplasty for cervical myelopathy with a massive ossified posterior longitudinal ligament. Spine (Phila Pa 1976). 2002;27(22):2491–2498. | ||
Min JH, Jung BJ, Jang JS, Kim SK, Jung DJ, Lee SH. Spinal cord herniation after multilevel anterior cervical corpectomy and fusion for ossification of the posterior longitudinal ligament of the cervical spine. J Neurosurg Spine. 2009;10(3):240–243. | ||
Matsuoka T, Yamaura I, Kurosa Y, Nakai O, Shindo S, Shinomiya K. Long-term results of the anterior floating method for cervical myelopathy caused by ossification of the posterior longitudinal ligament. Spine (Phila Pa 1976). 2001;26(3):241–248. | ||
Ito K, Yukawa Y, Ito K, et al. Dynamic changes in the spinal cord cross-sectional area in patients with myelopathy due to cervical ossification of posterior longitudinal ligament. Spine J. 2015;15(3):461–466. | ||
Chiba K, Ogawa Y, Ishii K, et al. Long-term results of expansive open-door laminoplasty for cervical myelopathy – average 14-year follow-up study. Spine (Phila Pa 1976). 2006;31(26):2998–3005. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.