Back to Journals » ClinicoEconomics and Outcomes Research » Volume 8
Potential resource and cost saving analysis of subcutaneous versus intravenous administration for rituximab in non-Hodgkin's lymphoma and for trastuzumab in breast cancer in 17 Italian hospitals based on a systematic survey
Authors Ponzetti C, Canciani M, Farina M, Era S, Walzer S
Received 29 September 2015
Accepted for publication 21 December 2015
Published 23 May 2016 Volume 2016:8 Pages 227—233
DOI https://doi.org/10.2147/CEOR.S97319
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Giorgio Colombo
Clemente Ponzetti,1 Monica Canciani,2 Massimo Farina,2 Sara Era,3 Stefan Walzer4,5
1Group Policlinic Monza, Alessandria, National Scientific Associazione Nazionale dei Medici delle Direzioni Ospedaliere (National Association of Hospital Physicians), Bologna, 2Studio EmmEffe S.r.l, Milan, 3Roche S.p.a., Monza, Italy; 4MArS Market Access and Pricing Strategy GmbH, Weil am Rhein, 5Health Care Management, State University Baden-Wuerttemberg, Loerrach, Germany
Introduction: Subcutaneous versions of different oncology therapies have been available for patients for a few years, yet patient-relevant and hospital benefits have not been assessed in real life.
Methods: In order to analyze the impact of subcutaneous administrations for rituximab or trastuzumab in comparison to the respective intravenous mode a primary research in Italy was executed. The study’s primary objectives were to analyze the resource and cost implications from different perspectives (patient, medical staff) in the real world. The route of administration was discussed and aligned with the participating centers in order to capture all relevant therapy parts. After the successful execution of a pilot study 19 centers in six regions in Italy were recruited to participate.
Results: Significant time savings might be achieved with the subcutaneous mode through significantly lower patient preparation time including less time preparing the actual dosing for each individual patient. The total time difference is 3.3 hours with rituximab in hematology (non-Hodgkin’s lymphoma), which adds up to 23.55 hours for a full course of treatment per patient (overall preparation time: 40.1 hours intravenous [95% confidence interval (CI): ±0.47] vs 16.6 hours subcutaneous [95% CI: ±0.2]). In early breast cancer (trastuzumab), the time saving might be 3.3 hours for the first cycle and the total time saving for patient preparation might be 17.2 hours (overall preparation time: 38.8 hours intravenous [95% CI: ±9.42] vs 21.6 hours subcutaneous [95% CI: ±9.9]). Furthermore, in both settings, the time of medical staff was reduced and could hence be used elsewhere. Finally, in case wastage was experienced with intravenous therapies, there were potential significant reductions in wastage through the subcutaneous administration (93%–100%) with cost savings of €6,057 with rituximab subcutaneous and €28,399 with trastuzumab subcutaneous administration for the full treatment course.
Conclusion: There are significant resource and cost savings due to subcutaneous administration with rituximab and trastuzumab in Italy based on a systematic survey. With the availability of a subcutaneous use of rituximab and trastuzumab, hospitals, patients and payers in general still have the current standard of care therapies available in the approved indications for a more efficient use of time and resources.
Keywords: health economics, cost savings, oncology, intravenous therapy, subcutaneous therapy
Corrigendum for this paper has been published
Introduction
Oncology includes a variety of different diseases and indications and can not be recognized as one disease on its own. Dependent on the therapy advances in most recent years and the severity of the malignancy, the overall survival can range from a couple of months to years in the advanced metastatic setting. In the adjuvant indications, where the tumor is being detected early in its stage of development, a cure could also be achieved, such as the innovation being launched in early breast cancer with the monoclonal antibody trastuzumab.1 However, more patients might be detected in late stages of their disease. The goals of care are to optimize both length and quality of life,2,3 which could be achieved with different treatment options. Another active area with innovative therapy options in the last century are the different hematology indications.2,3 Oncology and hematology medications have historically been administered intravenously. Over the past decade, the emergence of orally administered agents has increased treatment options and changed the way in which many patients are treated. Oral oncology agents are seen as a potential therapeutic advancement by freeing patients of the need to make regular visits to health posts/facilities for lengthy intravenous infusions. However, physicians and caregivers alike sometimes still prefer intravenous therapies in order to improve compliance to the therapy and making sure that the treatments are administered according to guidelines and the product’s label.4 However, some intravenous therapies last a couple of hours in terms of administration to the patient sometimes even including hospital stays and are linked to severe injection reactions or side effects.
Subcutaneous formats of different oncologic therapies have been available since mid-2014.1,5 Subcutaneous therapy should benefit all stakeholders in the health care system. Patients could receive their treatment in a faster and still safe way, whereas physicians and nurses could potentially save time and hence increase the number of patients being treated during their given office hours.6 The purpose of the underlying study was to analyze the benefits of a subcutaneous therapy in comparison to an intravenous therapy in an Italian health care setting with trastuzumab taken in patients with breast cancer and rituximab taken in patients with non-Hodgkin’s lymphoma (NHL); both being examples of monoclonal antibodies. The hypothesis behind the analysis was that available drugs could support the health care system not only through the direct patient impact but also through other benefits when provided in other administration modes.
Methods
In order to analyze the impact of a subcutaneous administration of an existing therapy in comparison to the intravenous mode a primary research in Italy was executed. The primary objectives of the study were to analyze the resource and cost implications from different perspectives (patients, hospital administration, and medical staff). Due to the nature of the study as being a survey, no agreement by an ethics committee was required.
In the first instance, four centers in two regions (Emilia-Romagna and Lombardia) were identified in order to run a pilot study phase analyzing the feasibility. The regions were selected with one breast cancer and one hematologic center per region. The pilot study was successful and has shown trends toward a benefit of the subcutaneous therapy.7 After the successful execution of the pilot study, 19 centers in six regions in Italy were recruited to participate. The two largest regions participating with overall ten centers for the two disease areas were Emilia-Romagna and Lazio contributing more than 50% of participating patients in NHL and four out of 16 centers in breast cancer contributed 50% of participating patients (Table 1). The study was executed before the subcutaneous availability in Italy and included only centers interested in sharing their detailed information on the potential savings and benefits based on a systematic survey.
  | Table 1 Overview of the 19 centers in the six Italian regions participating in the study |
The responsible analysis managers in the clinical centers collected information based on a questionnaire, for five patients per study center, collecting the current information on the administration of trastuzumab in breast cancer and rituximab in NHL and comparing that information against the expected results of the subcutaneous therapy (Figure 1). The rationale for the sample size per center was based on the average patient records per week per center. Base assumptions were as follows: the health care processes are consistent and well-defined between the centers and the sample did not have the purpose of being statistically representative but rather to provide an overview of operating modes consolidated.
The route of administration and its specifics was discussed and aligned with the participating centers in order to capture all relevant parts of the therapy.
Besides the location, the annual number of treated patients could also have an impact on the interpretability of the study. As seen in Table 2, 50% of participating hematology centers treat more than 100 patients annually and were defined as being large centers. The proportion of medium- and small-sized centers is quite evenly distributed with 24% and 29%, respectively. For the oncology centers with a focus on breast cancer patients, there are roughly 41% of small study centers compared to 29% medium size and 24% large centers.
  | Table 2 Size differences in participating centers |
Results
The first cycle of administration of rituximab needs to be done in an intravenous mode due to tolerability reasons.5 However, significant time savings might be achieved with the subcutaneous mode in the cycles two to eight due to significantly fewer patient preparations, which includes less time to prepare the actual dosing for each individual patient. The main reason for that time saving might be the supply of the subcutaneous therapy as a fixed dose, which reduces pharmacy preparation time and overall impact on hospital resources. The overall time difference is 3.3 hours (200 minutes), which corresponds to a 79% faster administration of the subcutaneous mode of delivery (Table 3) in NHL. The overall preparatory time savings for the eight treatment cycles might add up to 23.55 hours and corresponds to a 59% faster preparation time comparing a subcutaneous versus an intravenous administration. Additionally, the time required to assist a patient by a medical nurse was found to be 1.48 hours on the intravenous therapy and 0.68 hours (41 minutes) on the subcutaneous administration. Hence besides the preparation time also the resources supplied by the medical staff were significantly reduced by 54%, which could then be used for more time with the patients or serving more patients in the same time.
Trastuzumab in breast cancer
In breast cancer the premedication for the subcutaneous administration is not required and hence the time savings could be seen from the first cycle of therapy.1 In the first cycle, the preparation time saving might be 73% (3.3 hours or 200 minutes) and the cycles two to eight, the time savings per treatment session could be 41% (0.8 hours or 48 minutes) (Table 3). In breast cancer, the total time saving for patient preparation might be 17.2 hours and relates to a 44% time saving in advantage of the subcutaneous therapy. Also in the breast cancer setting, the medical staffing time required is potentially reduced by 77% (from 1.64 to 0.38 hours), which could then be used for more time with the patients or serving more patients in the same time.
Those resource savings were translated into full time equivalents (FTEs), which is another term for full-time medical staff. One FTE saved would then mean that a nurse could spend more time with patients or other services. The preparation time for eight cycles of therapy in NHL could be reduced from 1.6 to 0.5 hours, which could correspond to a saving of 63%. This saving could be utilized by the medical staff as it relates to a saving of 0.12 FTEs, which could either be saved by the hospitals or be used with other patients and tasks (Table 4). A similar result was detected in the breast cancer setting where the difference was 68% corresponding to 0.27 FTEs saving.
Another component when analyzing differences between subcutaneous and intravenous mode of administrations is the potential savings of drug wastage (Table 5). In hematology, the savings could be as much as 94% or €6,057 per patient annually. Even though the subcutaneous therapy is delivered in a fixed dose format, there might not be a 100% saving as the first cycle of therapy still requires intravenous administration of rituximab due to tolerability questions at first administration. In breast cancer, no initial intravenous administration is required and hence, no wastage is accrued in the subcutaneous scenario. However this result needs to be interpreted with caution, as 12 out of 17 participating centers reported no potential wastage for these therapies.
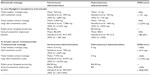  | Table 5 Wastage calculation between intravenous and subcutaneous administration in non-Hodgkin’s lymphoma (rituximab) and in breast cancer (trastuzumab) |
Discussion
Based on the authors’ knowledge, this analysis is the first published for the Italian health care setting analyzing the impact of a new formulation in oncology. The results show a significant impact from a timing and cost perspective on the various aspects. Furthermore, it is in line with similar research, which was carried out in other countries, which analyzed the impact of a switch from an intravenous to a subcutaneous administration of anticancer treatments.6
In the current economic situation hospitals in Europe and especially in Italy are in, these results might have a large relevance in terms of cost savings with respect to wastage and amount of drug utilized. Furthermore, the results show potential ways to more efficiently utilize the existing medical staff in case time savings could be utilized in other areas in need of medical staff, for example, investing more nurse time to patients or having nurses working with more patients at the same time.
However, the underlying analysis might be criticized especially based on the applied method. The analysis was based on the comparison of the actual intravenous available therapies against the theoretical application of a subcutaneous therapy, standardized through questionnaires and answered by the hospital representatives. A clinical study in actual patients comparing intravenous versus subcutaneous therapies could have also added more clarity with respect to an evaluation of the consistency of preparation, observed side effects, and potential evaluation of patient dosing that accompanies the difference in administration. Furthermore, no hospitals from the south of Italy were included, which could also bias the results toward a more efficient use of resources. Finally, the number of centers were acceptable for such research, however, two out of 17 centers contributed more than 50% of patients observed for the analysis in NHL and four out of 16 centers, in breast cancer, contributed 50% of participating patients. This center bias will most likely have an impact on the results. However, when sensitivity analyses were run, the results were consistent across the different regions. Furthermore, the results of the study are consistent with the findings in the Rule et al analysis, which had a more controlled approach.6
Conclusion
Through the availability of the subcutaneous administration mode of oncology and hematology therapies, such as trastuzumab and rituximab, resource and cost savings could be achieved in using this mode of administration more often in comparison to intravenous therapies, as shown in an Italian setting based on a systematic survey. With the availability of a subcutaneous use of rituximab and trastuzumab, hospitals, patients and payers in general still have the current standard of care therapies available in the approved indications for a more efficient use of time and resources.
Disclosure
MC and MF are employees of EmmEffe and received funding from Roche S.p.a. executing the research. SE is an employee of Roche S.p.a. SW received funding from S.p.a. for interpretation, analysis, and writing of the research. The authors report no other conflicts of interest in this work.
References
European Medicine Agency. Trastuzumab (Herceptin). Summary of Product Characteristics. EMA; 2014. | |
Cardoso F, Costa A, Norton L, et al. ESO-ESMO 2nd international consensus guidelines for advanced breast cancer (ABC2). Ann Oncol. 2014;25:1871–1888. | |
Cardoso F, Costa A, Norton L, et al. ESO-ESMO 2nd international consensus guidelines for advanced breast cancer (ABC2). Breast. 2014;23:489–502. Available from: http://dx.doi.org/10.1016/j.breast. 2014.08.009. Accessed February 9, 2016. | |
Mastroianni CM, Viscomi C, Ceniti S, et al. Preferences of patients with advanced colorectal cancer for treatment with oral or intravenous chemotherapy. Patient. 2008;1(3):181–187. | |
European Medicine Agency. Rituximab (Mabthera). Summary of Product Characteristics. EMA; 2015. | |
Rule S, Collins GP, Samanta K. Subcutaneous vs intravenous rituximab in patients with non-Hodgkin lymphoma: a time and motion study in the United Kingdom. J Med Econ. 2014;17(7):459–468. | |
Farina M, Canciani M, Ponzetti C. The role of subcutaneous formulations of biologics in the optimization processes of care in oncology and hematology. Frammenti Educational. 2014; Suppl n. 32 anno 8. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.