Back to Journals » Nutrition and Dietary Supplements » Volume 13
Potential Nutraceuticals for COVID-19
Authors Savant S, Srinivasan S, Kruthiventi AK
Received 29 November 2020
Accepted for publication 8 January 2021
Published 18 February 2021 Volume 2021:13 Pages 25—51
DOI https://doi.org/10.2147/NDS.S294231
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Gary Johanning
Sayali Savant, Shraddha Srinivasan, Anil Kumar Kruthiventi
Arna Immuno Ingredients Private Limited, Mumbai, Maharashtra, India
Correspondence: Anil Kumar Kruthiventi
Arna Immuno Ingredients Private Limited, 1st Floor, 41/44, Minoo Desai Marg, Colaba, Mumbai, 400005, India
Tel +91 954 550 0481
Fax +91 22 674 900 001
Email [email protected]
Abstract: SARS-CoV-2 infection has caused, and is continuing to cause, considerable human suffering. Studies on the viral pathogenesis has resulted in convergent findings from several lines of evidence on the entry and spread of the virus in the host. These studies have also revealed a strong association between innocuous inflammation, ageing and metabolic disorders, with SARS-CoV-2 infection and its prognosis. Diet helps modulate inflammation, and nutraceuticals can inhibit viral entry. Hence, we have collated literature on antiviral nutraceuticals effective against other similar coronaviruses. The objective of this study is to comprehensively review available information on the antiviral activity of nutraceuticals and to discuss the implications of these findings in designing a diet that would boost the innate immunity and act as preventive care against COVID-19. This review highlights the fundamental impact of nutraceuticals and diet on inhibition of viral entry and provides a new perspective on the prevention and treatment of COVID-19.
Keywords: SARS-CoV-2, diet, plant protease inhibitors, polyphenols, bioactive peptides
Introduction
SARS-CoV-2 has disrupted global health and economic wellbeing since the beginning of 2020. The regional office of World Health Organization (WHO) in China was first alerted to the virus infection in Wuhan on December 31, 2019 and termed the infection as an epidemic on March 11, 2020. Since then, laboratories across the globe have been collaborating to develop vaccines and therapeutic agents for this novel coronavirus.
The SARS-CoV-2 belongs to a group of viruses called the coronaviruses. These are single-stranded, positive sense, RNA viruses, enveloped in a helical capsid with spike shaped trans-membrane proteins. They can further be classified into four subtypes: alpha, beta, delta, and gamma.1 The coronaviruses are capable of infecting both humans and animals and have the ability to jump the species barrier thereby accelerating the spread of the disease into an epidemic or a pandemic. The severe acute respiratory syndrome coronaviruses (SARS-CoV and SARS-CoV-2) and Middle East respiratory syndrome coronavirus (MERS-CoV) are the three highly pathogenic coronaviruses. The remaining four (HCoV-NL63, HCoV-229E. HCoV-OC43, and HCOV-HKU1) human coronaviruses are less virulent.2
What makes the SARS-CoV-2 threatening is not just the viral infection but the accompanying cytokine storm and other associated comorbidities. Analysis of clinical data from 326 confirmed COVID-19 patients in Shanghai revealed IL-6 and IL-8 to show the most significant changes and the levels of these two interleukins inversely co-related with the lymphocyte count.3 A reduced CD4+/CD8+ ratio is also a manifestation of the disease. A detailed explanation of the host immune response and the viral evasion methods follows in the later part of this paper.
Initial genomic phylogeny testing revealed that the SARS-CoV-2 virus shared about 79% and 50% gene similarity with SARS-CoV and MERS-CoV, respectively.2 Therefore, the initial treatment has included therapeutics known to be effective against SARS-CoV and MERS-CoV for SARS-CoV-2.
In this article we give an overview of the present understanding of the viral lifecycle and host immune responses, which would form the basis for selecting nutraceuticals and natural products that can be potentially explored both as therapeutic and preventive interventions for the SARS-CoV-2 infection.
Virus Replication Cycle and Potential Therapeutic Targets
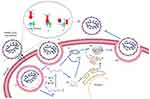
Understanding the cellular basis of SARS-CoV-2 infection could reveal treatments that prevent the development to a severe disease, and thus reduce mortality. Figure 1 represents a simplified illustration of the infection cycle of SARS-CoV-2.
The spike glycoprotein (S) is a 180–200 kDa transmembrane homotrimer which recognizes and binds to the host angiotensin-converting enzyme 2 (ACE2) receptors.4 The S protein can further be subdivided into S1 (the homotrimer head) and the S2 protein (the tail part). Figure 2 gives an enlarged view of the spike protein of the virus. ACE2 is a human receptor largely found in the respiratory and intestinal epithelial cells but it is also present in the kidney, heart, brain, etc. ACE2 has a short C-terminal intracellular domain and a long N-terminal extracellular domain, to which the S2 head of the virus binds.5 The spike protein mediates two essential events: binding to ACE2 by the amino-terminal region and fusion of viral and cellular membranes through the carboxyl terminal region.
The spike protein bound to ACE2 can be cleaved by two enzymes, the transmembrane serine protease 2 (TMPRSS2) at the S2 site and by furin at the S1/S2 connection site.6 TMPRSS2 acts at monobasic cleavage sites, ie it cleaves at a single arginine or lysine residue. Furin, however, cleaves at a polybasic site.6 Infection of lung cells requires host proteolytic activation of spike at a polybasic furin cleavage site. Cleavage by the furin protease, therefore, can expand SARS-CoV-2 cell tropism and may have facilitated transmission from bats to humans.2 SARS-CoV-2 tropism is, therefore, dependent on expression of cellular proteases, as well as ACE2.
However, TMPRSS2 is not expressed in all the cells expressing ACE2 suggesting the existence of alternative pathways for viral entry such as through use of cathepsin L and cathepsin B.7 However, this is a matter of debate since studies have also found that cathepsins are not required for SARS-CoV-2 infection.6 Post-cleavage, the virus can enter the host cells either through membrane fusion or through endocytosis.5
Two-thirds of the viral genome produces two polypeptides, pp1a and pp1ab which are processed by the viral proteases chymotrypsin like cysteine protease (3CLPro) or main protease (MPro) and one or two papain-like proteases into 16 nonstructural proteins (nsps) that play diverse roles in the subsequent viral replication and infection.8 The remaining one-third of the genome encodes for the main viral proteins namely, the spike protein, the nucleocapsid protein, the membrane protein, and the envelope protein. Replication occurs within double membrane vesicles (DMVs) which comprise RNA-dependent RNA polymerase (RdRp) and the nsps. The sub-genomic particles manufactured here then move towards the endoplasmic reticulum golgi complex compartment where they undergo maturation. The virulent particles present in vesicles are further released through the process of exocytosis.1 Thus, the life cycle of the virus with the host consists of five steps: attachment, penetration, biosynthesis, maturation, and release.
Therapeutics, therefore, can target either the viral proteins or the host proteases. Since the host proteases play multiple roles within the human body, targeting the host proteases may also interfere with other physiological activities in the host body and so caution must be exerted in this approach. Hence, viral proteins seem more attractive and safe targets.
In case of the SARS-CoV-2 virus, the following have been identified as the potential therapeutic targets: the spike glycoprotein (S), the envelope glycoprotein (E), the nucleocapsid protein (NP), the membrane protein (M), the papain like protease (PLPro) or the chymotrypsin like cysteine protease (CLPro), (also known as the main protease MPro) and the RNA dependent RNA polymerase (RdRp). In addition to this, certain nsps could also be targeted. The nsp13 helicase is an important component for replication of the virus which is target for many virus inhibitors.9 Nsp10 plays a major role in viral transcription wherein nsp14 3ʹ-5ʹ exoribonuclease and nsp16 2ʹ-O methyltransferase are stimulated by playing a lead role in viral mRNAs cap methylation.10
The following sections give an overview of the different plant-derived ingredients and more specifically nutraceuticals, that can potentially be developed into therapeutic molecules targeting one or more multiple targets discussed above.
Nutraceuticals and Coronavirus Infection
Nutraceuticals is a portmanteau of “nutrition” and “pharmaceuticals”. The structure of the word itself directs us towards the dual use of these molecules: for preventive health care (source of nutrients that will prevent the occurrence of disease) and as therapeutic molecules (like pharmaceuticals taken to cure a disorder or disease). Table 1 mentions the details of a few nutraceuticals that have been tested against the earlier coronaviruses SARS-CoV and MERS-CoV.
 |
Table 1 Experimentally Tested Nutraceuticals Against Different Coronavirus and Host Proteins |
Yi et al11 performed in vitro and animal testing on multiple small molecules and found luteolin to be effective in blocking the S2 protein of the SARS-CoV virus. The SARS-CoV and SARS-CoV-2 S proteins share about 76% amino acid similarity.7 Since both the viruses bind to the same host receptor, there is an increased likelihood that molecules that block or interact with SARS-CoV S protein will most likely be effective against SARS-CoV-2 S protein. The authors also tested the effect of quercetin using a pseudotype virus assay, the rationale being that quercetin shared structural similarity to luteolin. Quercetin is an ingredient of antioxidant and antiallergy medicines that had been approved by the US Food and Drug Administration (FDA; the national drug code numbers of the medicines are 65448–3085, 65448–3005).
This strategy by Yi et al11 can be extended to current search by identifying structures similar to the ones that are showing promise in silico and in vitro, but already have a generally recognized as safe (GRAS) status owing to their documented food or medicinal use. This can drastically reduce the time required for regulatory approvals and give more confidence for clinical trials. In fact, a similar strategy was earlier used by Lin et al12 when they decided to test the efficacy of resveratrol, a stilbene derivative, based on earlier studies that showed antiviral activities on SARS-CoV.
The coronavirus main protease (MPro or 3CLPro) structural backbone and active site conformation are conserved despite sequence variations.13 Hence, nutraceuticals such as epigallocatechin gallate (EGCG), gallocatechin gallate (GCG), hesperetin, quercetin, which were previously shown to be effective against SARS-CoV 3CLPro can be tested for their efficacy in SARS-CoV-2 infection. Nguyen et al14 found that a galloyl moiety present at the 3ʹOH position of a potential inhibitor, belonging to the flavonoid class, is crucial for inhibition of 3CLPro in SARS-CoV. Enzyme kinetics suggested that GCG competitively inhibited 3CLPro with an inhibition constant of 25 µM.
As seen in Table 1, quercetin seems to have multiple targets of action and may prove to be a better candidate molecule for therapeutic development. Administration of quercetin (1000 mg) showed a decrease in the incidence and extent of upper respiratory tract infections (URTIs). Abian et al17 identified quercetin as a reasonably potent inhibitor of SARS-CoV-2 3CLpro protease, with the inhibition constant being –Ki ~ 7 μM. Quercetin also modulates the cellular unfolded protein response (UPR). As coronaviruses can utilize the UPR to complete different stages of the viral life cycle during infection, this is an important finding.18 Early clinical data suggests that quercetin has broad antiviral property and acts at various steps of viral life cycle. As an FDA-approved drug ingredient, with potent antiviral action, quercetin offers great promise as a potential drug candidate in the clinical treatment of SARS.
In silico Studies Reveal Potential Therapeutic Nutraceuticals
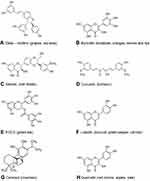
Given the importance of rapid generation of lead compounds, many authors have used in silico docking and modelling studies to generate a library of potential therapeutic lead molecules targeting different stages of the viral infection life cycle, some of which have been collated in Table 2. It is interesting to observe the structural similarities among these molecules—having a catechol/resorcinol moiety—seems to be a common feature in these nutraceuticals (Figure 3).
 |
Table 2 Potential Nutraceutical Therapeutic Targets as Revealed by in silico Studies |
Khan et al19 docked 18 known natural antiviral compounds against seven SARS-CoV-2 proteins and compared them with remdesivir and chloroquine. Their modelling studies showed EGCG and curcumin to affect multiple targets of SARS-CoV-2. EGCG showed binding affinity towards main protease, spike protein, post fusion S2 protein, and nsp15 endoribonuclease. Curcumin, however, showed increased binding affinity towards the main protease and nsp15 endoribonuclease. Curcumin binding to NP and nsp10 was observed by Suravajhala et al.10
Joshi et al13 screened a custom-made library of more than 7000 natural molecules such as flavonoids, glucosinolates, terpenoids, alkaloids, and others against the MPro. MPro does not have a sequence homologous structure in humans and hence targeting this protease would be specific for the virus. Since coughing is a major symptom of COVID-19, Joshi et al also screened traditionally known antitussive medicines against various structural proteins of SARS-CoV-2. They followed this up by screening the hits against RdRp and ACE2 in order to find multi-target inhibitors. Multi-target inhibitors have the added advantage that they could be effective in all stages of the virus life cycle. EGCG is shown to reduce TMPRSS2 secretion. Clerodendrum species have been used as an age-old remedy for respiratory ailments. Hence, phytochemicals from 12 different species were assessed for their potential to bind to the coronavirus proteins, wherein stigmasterol was found to be a promising candidate.24
Silibinin not only acts as a direct inhibitor of viral proteins, but is also found to play a role in modifying the inflammatory response. Similarly, EGCG, has additional fat burning action (increasing fat oxidation), which prevents disease progression.
Hu et al,22 observed that flavonoids in general, showed higher affinity towards MPro compared to alkaloids, terpenes and saponins. They suggest that this may be owing to the phenolic hydroxyl group of the flavonoids which can interact more easily with the heteroatoms of the amino acids of MPro. They found rutin to be especially effective in binding to MPro. Buckwheat, apple and tea contain considerable amount of rutin and can be consumed in higher quantities to protect oneself from the infection. The screening study conducted by Fischer et al,25 revealed rhamnetin, found in Moringa oleifera plant, to show greater affinity for MPro. Rhamnetin is already commercially available. Rhamnetin is also structurally similar to quercetin. Myricitrin is a glycosylated analog of myricetin and is found in black grapes. Glycosylated flavonoids have an increased bioavailability compared to the aglycone forms.25 Myricitrin, myricetin, resveratrol, and δ-viniferin, have been found to be potential anti-COVID-19 therapeutics.
Kar et al,23 evaluated multiple phytochemicals and nutraceuticals for their potential to bind to either S, MPro or RdRp proteins. Post evaluation of in silico binding, these authors have also assessed the adherence of the selected ligands to the Lipinsky’s rule of five. While eugenol was found to adhere to the Lipinsky’s rule of five, mangiferin showed slight deviation. However, these deviations can be corrected through formulation innovations.
Octacosanol, cinametic acid, lauric acid, ascorbyl palmitate, and palmidrol are few of the nutraceuticals found to bind to the envelope protein. These can potentially disrupt the viroporin activity, and can provide relief from edema and inflammation, commonly observed in acute respiratory distress syndrome (ARDS).26
Sulforaphane is an isothiocyanate formed upon hydrolysis of glucosinolates found in cruciferous vegetables like broccoli and cabbage and can modulate levels of TMPRSS2.27 It is also shown to upregulate Nrf2 thus enhancing the anti-inflammatory response of the body. Hence, consumption of cruciferous vegetables may be helpful as a preventive strategy against SARS-CoV-2.
Given that these molecules target different viral proteins at different stages of viral replication, and a majority of them are common ingredients of the food chain (eg curcumin from turmeric, carnosol from rosemary, quercetin in apples, etc), it can be assumed that consumption of such foods may positively impact protection against SARS-CoV-2 infection. However, supraphysiological dosing of nutraceuticals is a double-edged sword, with evidence on both sides—of effectiveness and toxicity. For example, the supraphysiological dosing of vitamins and nutrients (mainly flavonoids and omega-3 fatty acids) for treatment of brain injury is now well established with strong evidence backing their use.28 However, high doses of chlorogenic acid are known to increase plasma homocysteine levels.29 This kind of unexpected spinoff is more pronounced in the case of antioxidants, as they function as antioxidants at physiological dose, while occasionally at higher amounts a pro-oxidant activity is observed. Antioxidants by their very nature are “redox” agents, hence they have both antioxidant and pro-oxidant activities inherent in their structure. It is important to note that the physiological concentration of these nutraceuticals determines their function.
Plant Protease Inhibitors as Potential Therapeutic Molecules Against SARS-CoV-2
Viruses make use of proteases (generally host proteases) to gain entry into the host. Synthetic protease inhibitors have been widely used as treatment tools for a variety of viral diseases such as those caused by HIV, HCV, picornaviruses, SARS, rotavirus and others.30 They are also being investigated as potential treatment options for diabetes, cancer and inflammation. Viral protease inhibitors against HIV, HCV, picornaviruses, SARS have also been studied from bacterial and fungal origins.31
TMPRSS2 and furin are host serine proteases that facilitate the entry of SARS-CoV-2 into the host cell. TMPRSS2 is a trypsin like serine protease while furin is a subtilisin type serine protease.32 In addition, PLPro and the MPro which is a chymotrypsin-like cysteine protease, play important roles in viral replication. The epithelial cells of the respiratory tract themselves release proteases/protease inhibitors that help maintain homeostasis required for healthy lung functioning.27 The protease/protease inhibitors balance determines the extent of respiratory viral pathogenesis. A shift towards more proteases can increase viral pathogenesis while the reverse may help protect against it.
Animal studies by Deng et al33 have shown that coronaviruses can gain quick resistance to 3CLPro inhibitors but this resistance comes at the fitness cost of the virus not being able to replicate fast enough thereby reducing infectivity. TMPRSS2 is a host secreted type 2 transmembrane serine protease and is shown to be sensitive to antiproteases in the midst of an infection.22 Therefore, protease inhibitors or antiproteases against these protease enzymes can be explored as potential therapeutic molecules.
The reviews by Hellinger and Gruber34 as well as Srikanth and Chen35 describe a number of plant protease inhibitors, some of which have been collated in Table 3. This could be a good starting point to start assessing these molecules for their effectiveness against the SARS-CoV-2 virus 3CLPro and papain-like proteases.
 |
Table 3 Examples of Plant Protease Inhibitors (PPIs) as Potential Antiviral Agents |
Although the main function of antiproteases is inhibiting or deactivating proteases, newer research is identifying more roles for them in controlling excessive inflammation and microbial infection.27 Hence identifying multifunctional plant protease inhibitors can allow multistep protection in coronavirus infection.
Immunomodulatory Effects and Inflammatory Responses Brought About by the SARS-CoV-2 Virus
The Immune System—Natural Defense Mechanism
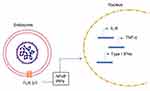
During viral infection, the host cells recognize an invading virus through the pathogen-associated molecular patterns (PAMPs). This recognition further activates a series of cell signaling pathways through the pattern recognition receptors (PRRs). The toll-like receptors (TLRs), particularly TLR3 and TLR7 (endosomal receptors) and TLR8, recognize single-stranded RNA while other TLRs recognize the different viral components and set off the cellular signaling pathways. This leads to the production of pro-inflammatory cytokines (IL-6, TNF-α, type-I interferons (IFNs) etc) (Figure 4) and certain chemokines which further triggers the immune responses by the various immune cells (such as the antigen presenting cells, APCs). The release of the cytokines and chemokines is the starting point for the downstream effects such as inflammatory responses, apoptosis or pyroptosis, and the activation of the adaptive immune response. Type-I IFN pathway plays a central role in mediating innate immune responses to viral infections.
The type-I IFNs activate the JAK-STAT pathway and the phosphorylation of the STAT1and STAT2 causes the transcription of IFN-stimulated genes (ISGs) to further reduce viral replication and thereby reduce the viral load in the system.1 The release of NF-κB transcription factors occurs downstream of TLRs activation and causes the release of pro-inflammatory cytokines.
The mitogen-activated protein kinases (MAPKs) pathway also gets activated during SARS-CoV infection. SARS-CoV also distinctly enhances the NLRP3 inflammasome pathway leading to a profound expression of pro-inflammatory cytokines and ROS.
The adaptive immune system is activated by the phagocytes that present the antigen to the CD4+ T-helper cells which fight against the virus by developing into Th1 cells. The APCs can also activate CD8+T cells which further exhibit their cytotoxic effects along with natural killer (NK) cells. The CD4+ cells also stimulate the CD8+ cells through co-stimulatory signals. The T cells further activate the B cells leading to antibody production (specifically IgM and IgG) (Figure 5).
Immunomodulatory and Inflammatory Responses Triggered by SARS-CoV-2
At the onset of the infection cytokines play an important role in recruiting other phagocytes at the site of infection and eliciting immune response. Increase in viral load and evasion of the innate immune system by the virus causes a cytokine storm viz a marked increase in the levels of pro-inflammatory cytokines through various cellular pathways triggering inflammation, which contributes to morbidity and mortality. It has been reported that the cytokine storm aggravates the disease resulting in ARDS, a characteristic condition exhibited by critically ill patients.
The virus has developed several strategies to evade the human defense system. It avoids the immune system by forming double vesicles that escape detection through PRRs. Furthermore, the viral particles are able to inhibit the STAT family transcription factors and suppress the expression of type-I IFNs.36 The nsps function to alter the structure of the virus to mimic the host cell components and thereby evade detection by the host immune system.1
Lymphopenia has also been cited as a cause for exacerbation of adverse effects of the disease.37 The low count of T cells results are not only due to the viral load, but also due to the cytokine storm. Previously, it was observed in SARS-CoV infected individuals that there was an imbalance in the CD4+ and CD8+ ratio with a significant decrease in the CD4+ count compared to CD8+ cell count.38,39 Ageing, by itself results in similar disruption of CD4+/CD8+ ratios, which in turn may be responsible for the increased susceptibility of the aged to SARS- CoV-2 infection. The cytokine storm and the immune dysregulation renders an individual with other comorbidities more susceptible to the disease. Hence, it is essential to build or enhance immunity not only to prevent infection, but also to prevent the acute stage of the disease once infected.
Interventions to Regulate Immune Response in COVID-19 Patients
Several nutraceuticals and natural compounds are known to have antiviral and immunomodulatory properties. Some of these compounds exhibit their functions by counteracting inflammation and ameliorating the ROS. Some of the strategies used in the past to counteract SARS-CoV infection were downregulation of MAPK pathway, reduction of NLRP3 inflammasome effects and inhibition of NF-κB while targeting a decrease in the pro-inflammatory cytokines, especially IL-6.40,41
Over expression of IL-6 causes enhanced expression of STAT3. Studies suggest that the use of inhibitors of the JAK-STAT pathway can be potential therapeutic strategy. Recent studies have discovered the positive effect of silibinin, a flavolignan obtained from milk thistle and apigenin obtained from dried parsley in inhibiting cytokine storm by preventing STAT3 activation and its nuclear translocation.21 Some of the nutraceuticals that can downregulate these different pathways are mentioned in Table 4. Immunity boosters that restore the adaptive immunity are also potential targets. Jacalin (a lectin from jackfruits) was shown to increase the levels of CD4+cells and thus restore the CD4+ and CD8+ cell ratio in an immunocompromised individual. Thus, it could be used to address the issue of lymphopenia and further enhance immunity.42
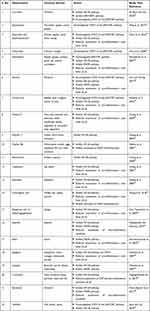 |
Table 4 List of Nutraceuticals Exhibiting Immunomodulatory and Anti-inflammatory Effects |
COVID-19 and Metabolic Diseases
A large number of people suffering from various metabolic diseases, such as hypertension, obesity, cardiovascular diseases, type 2 diabetes, etc are more susceptible to infection from the SARS-CoV-2 virus.60 The immunomodulatory effects due to the infection not only contribute to the severity of the metabolic diseases, but also exacerbate the adverse effects of infection.
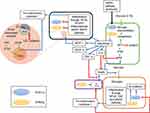
An understanding of the onset and subsequent severity of the viral infection along with other comorbidities will help in identifying suitable nutraceuticals that can act on relevant pathways to ameliorate the conditions. Figure 6 succinctly delineates the cross-talk between the immune and metabolic pathways.
Noncommunicable Diseases
Two major noncommunicable diseases, obesity and type-2 diabetes, are characterized by the dysregulation of PPARγ and IRS-1 expression occurring through the influence of IL-1 and TNF- α.61–63 Downregulation of ppargc1a further leads to pulmonary damage of the alveolar macrophages and epithelial cells, a characteristic symptom in the critically-ill COVID-19 patients. Furthermore, in addition to the alveolar epithelial cells, adipose and pancreatic tissues too express ACE2 and are thus prone to SARS-CoV-2 attack.64 Diabetic patients are often treated with ACE inhibitors, which results in higher expression of ACE2.65 Impaired glucose metabolism in diabetic patients results in dysregulated T cell activation and causes oxidative stress, further compromising the immune system of the infected individual.66
Dyslipidemia
A disruption in lipid homeostasis leads to dyslipidemia, characterized by elevated total cholesterol, triglycerides and low-density lipoprotein (LDL) with low levels of high-density lipoprotein (HDL).67 IL-6 leads to vascular cell damage by enhancing the permeability of oxidized LDL, resulting in lung injury. During the SARS-CoV-2 infection, the cytokine storm increases LDL oxidation and reduces the levels of HDL-c and PON1, leading to dyslipidemia and exacerbation of other metabolic diseases.68
In accordance with these observations, the lipid profile of the epithelial cells in patients suffering from SARS-CoV showed high concentrations of fatty acids and phospholipids and this condition has been considered to be conducive for viral replication.64 Furthermore, SARS-CoV-2 entry results in higher levels of angiotensin II which can lead to upregulation of IL-6, CAMs and several other cytokines and chemokines via the NF-κB pathway causing vascular diseases.69
Suitable Interventions to Address the Metabolic Diseases
In order to reduce this influence of the metabolic diseases and COVID-19 on one another, the target nodes in focus could be, (a) AMPK pathway (the upregulation of AMPK pathway downregulates inflammation and exhibits hypolipidemic effects), (b) specialized pro-resolving mediators, SPMs (agonists of PPARγ as their activation would target lipid metabolism as well as work as potential SPMs to restore homeostasis in a COVID-19 patient), (c) lipid oxidation (fatty acid oxidation ensures minimization of lipotoxicity as well as reduces damages caused to cells by dyslipidemia).
Nutraceuticals used in the management of metabolic disorders are suitable interventions to alleviate the comorbidities in a COVID-19 patient. These are listed in Tables 5 and 6.
 |
Table 5 Natural Food Sources Exhibiting Anti-obesity, Antidiabetic and Hypolipidemic Effects |
 |  | 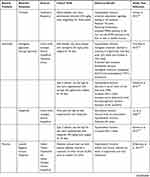 | 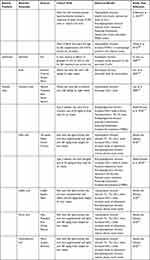 |
Table 6 Natural Products and Their Bioactive Molecules Exhibiting Anti-obesity, Antidiabetic and Hypolipidemic Effects |
Probiotics and Prebiotics
Probiotics or the gut microbiota play an important role in curbing inflammatory response and the associated metabolic diseases. Strains of Bifidobacterium and Lactobacillus species are the common probiotics found naturally as well as available commercially. The brain–gut axis facilitates the expression of anti-inflammatory cytokines through inhibition of TLRs.113 The intestine has a high expression of ACE2 and is thus susceptible to SARS-CoV attack, evident from the GI symptoms exhibited in a few patients.114 Therefore, supplementing a patient with probiotics is a therapeutic approach to control the spread of the infection and can also act as a prophylactic treatment. The presence of microorganisms in the lungs has also been reported, with Bacteroidetes, Firmicutes, and Proteobacteria being the three main phyla characterized.114 Similar to the brain–gut axis, there exists the gut–lung axis—a link between the gut microbiota and the lungs acting through blood vessels, whereby the metabolites from the gut has an influence on the functioning of the lungs, acting via Nod-2 and GM-CSF signaling, and in turn a respiratory ailment could lead to dysbiosis.113
Several studies reported alleviation of symptoms of the common cold and a reduction in its duration by supplementation with Lactobacillus plantarum HEAL9 (DSM 15312) and Lactobacillus paracasei 8700:2 (DSM 13434) at a concentration of 1 ⨰ 109cfu/day115 and Lactobacillus gasseri PA 16/8, Bifidobacterium longum SP 07/3, Bifidobacterium bifidum MF 20/5 at a concentration of 5 × 107cfu/day.116 When these probiotics were administered along with the influenza vaccine, an increase in the antibody titer was observed.117 An ongoing clinical trial is noteworthy in this regard.118,119
Prebiotics too have a beneficial role in conferring anti-inflammatory activity by inducing the production of IL-10 and IL-17.120 The short chain fatty acids (SCFA) like acetate and butyrate activate FFA receptors, leading to a downregulation of NFκB and MAPK pathways.121 The levels of certain LPS-induced pro-inflammatory cytokines and chemokines, like TNF-α, CXCL8, CCL20 show downregulation during butyrate administration to the system.120 SCFAs also exhibit immunomodulatory functions by regulating the function of T cells.120 Oat bran and psyllium husk fiber act as natural prebiotic by undergoing fermentation by the gut microbiota to yield SCFA. These can also bind to PPARs and facilitate homeostasis.122
Bioactive Peptides and Other Natural Molecules and Their Immunomodulatory Actions
Some of the bioactive peptides that have shown beneficial effects in reducing inflammation and functioning as antioxidants are summarized in Table 7.
 |
Table 7 Bioactive Peptides and Their Functional Properties in Ameliorating Metabolic Diseases |
Vitamin C
Vitamin C (ascorbic acid) possesses antioxidant and immune-modulatory property. It helps in mitigating the injury caused by oxidative stress during viral infections.135 Patients suffering from ARDS show reduced levels of vitamin C (Vit C) and external administration of Vit C reduced the extent of pulmonary inflammation in ARDS.136 With inflammation being a major cause of severity of COVID-19, supplementation with Vit C is hypothesized to aid in counteracting the actions of pro-inflammatory cytokines, especially IL-6. Treatment with Vit C (500 mg twice a day)137 and its intravenous administration (doses of 6 to 12 g/day and 24 g/day for seven days) (NCT04264533) reduces the levels of IL-6 and other inflammatory cytokines (ferritin and D-dimer).138 Vit C as an adjunct treatment could help in reducing the pneumonia-like symptoms and significantly reduce the mortality rates.139
Clinical trials are being carried out with a combinatorial treatment of Vit C along with other substances such as quercetin, vitamin D and zinc in different formats (IV or oral dosages) to treat COVID-19.137 Co-administering quercetin with Vit C and vitamin Vit B3 in mice with stress-induced H1N1 infection showed a delay in the time-to-death and reduced the mortality, as opposed to administration of single vitamins.140 A similar co-administration of Vit C along with quercetin, vitamins D and B3 could help to ameliorate the symptoms of severe COVID-19 cases. Vit C is known to increase the efficacy of quercetin139 and a combinatorial therapy of quercetin (500 mg)—Vit C (500 mg)—bromelain (50 mg) as a prophylactic treatment on health-care workers was found to be effective over a three-month study.141
Vitamin D
Studies have indicated the role of vitamin D (Vit D) in immune modulation. The review by Grant et al,142 mentions the different mechanisms by which Vit D may exert its antiviral effectiveness. It has been observed that Vit D downregulates the ACE2 receptors by negatively regulating the renin-angiotensin system (RAS).143 A recent study observed an association between COVID-19 severity and the high latitude countries which receive less sunlight leading to Vit D deficiency in people. However, this observation needs to be taken with the understanding that every population would show a mix of people suffering/not suffering from Vit D deficiency and hence generalizations as such may be difficult.144
Ghavideldarestani et al145 suggested the role of Vit D in preventing local lung inflammation in COVID-19 patients. Angiotensin II through binding to the receptor AT1 can increase vasoconstriction, oxidative stress and inflammation. This angiotensin II can be cleaved by ACE2 into angiotensin1-7 which counteracts the harmful effects of angiotensin II. However, in patients with COVID-19, the ACE2 is hijacked by the virus for its entry and hence less of it is available for this regular physiological role. Vit D deficiency is shown to increase renin production thereby increasing ACE and angiotensin II production. This can lead to inflammation in the lungs that is observed in severe cases of COVID-19.
Glinsky16 took a gene-first approach to investigate potential therapeutic targets for COVID-19. They first identified the human genes responsible for the expression and function of ACE2 and FURIN, the attachment molecules of the virus. Next, they studied the expression profile of these genes when infected by the coronavirus. This was followed by searching the literature for molecules that may downregulate the concerned gene expression which led to the discovery of three potential therapeutic molecules—Vit D, quercetin, and estradiol. Vit D was found to alter the expression of 84 of 332 (25%) genes encoding human proteins that serve as target for the SARS-CoV-2. The author suggested a two-ingredient (quercetin-Vit D) or a three-ingredient (quercetin-Vit D-estradiol) formulation as an adjunct therapy for coronavirus patients. In fact, two clinical studies involving Vit D are already registered.
Given the potent antiviral effects of quercetin, especially in the crucial stages of viral entry and its replication, and the ability of ascorbic acid and Vit D to efficiently reduce the oxidative stress induced by inflammation and activate the RAS pathway, respectively, adjunct therapy with these molecules is an approach that warrants further research for its potential benefits.
Lactoferrin
Lactoferrin, experimentally, has been found to inhibit viral entry in murine coronavirus, and human coronaviruses hCOV-NL63 and pseudotype SARS-CoV. Besides preventing viral entry, lactoferrin can also suppress virus replication after the viral entry. Lactoferrin was shown to chelate ferritin thereby downregulating IL-6 and TNF-alpha. There is a clinical trial currently underway to study the effect of lactoferrin as an adjunct therapy for COVID-19.146
Conclusion
Studies have hitherto revealed many details about the SARS-COV-2 virus infection. Although we have gained insights into the SARS-CoV-2 viral pathogenesis, a definitive treatment course is still distant. In such times, the intelligent way is to maintain the best possible health by taking suitable adjuvants to avoid falling ill.
It is evident that there are many ingredients that can be included in daily diet to possibly gain immunity or protection against coronavirus. Polyphenols, leguminous seeds containing plant protease inhibitors, as well as proteins, such as whey protein or jackfruit seed protein, could be incorporated in the daily diet. Other bioactive polyphenols, such as EGCG, GCG are common constituents of green tea, quercetin is found abundantly in apples while hesperetin is present in citric foods. These foods can also become components of daily diet. Spices such as turmeric, thyme, rosemary, garlic have anti-inflammatory properties and can be used in daily cuisine. In conclusion, the current review highlights the potential benefits of a range of nutraceuticals in the management of COVID-19. Further work aimed at clarifying the mechanisms of actions and potential therapeutic utility requires further investigation.
Abbreviations
WHO, World Health Organization; SARS-CoV, severe acute respiratory syndrome coronavirus; MERS-CoV, Middle East respiratory syndrome coronavirus; ACE2, angiotensin converting enzyme 2; TMPRSS2, transmembrane serine protease 2; 3CLPro, chymotrypsin like cysteine protease; MPro, main protease; nsps, nonstructural proteins; DMVs, double membrane vesicles; RdRp, RNA dependent RNA polymerase; PLPro, papain-like protease; FDA, Food and Drug Administration; GRAS, generally regarded as safe; EGCG, epigallocatechin gallate; GCG, gallocatechin gallate; URTIs, upper respiratory tract infections; UPR, unfolded protein response; ARDS, acute respiratory distress syndrome; PAMPs, pathogen-associated molecular pattern; PRRs, pattern recognition receptors; TLRs, toll-like receptors; IFNs, interferons; APCs, antigen presenting cells; MAPKs, mitogen-activated protein kinases; JNK, c-Janus kinase; LDL, low-density lipoprotein; HDL, high-density lipoprotein; SPMs, specialized proresolving mediators; SCFA, short chain fatty acids; RAS, renin-angiotensin system; ADAM17, ADAM metallopeptidase domain 17; ER, endoplasmic reticulum; RBD, receptor binding domain; CTD, C-terminal domain; NTD, N-terminal domain.
Acknowledgments
The authors are thankful to AIPL Director Mr Cyrus P Mistry for his unstinting support and motivation.
Disclosure
The authors are employees of Arna Immuno Ingredients Private Limited and report no other potential conflicts of interest in this work. This article/research received no external funding.
References
1. Astuti I, Ysrafil Y. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2): an overview of viral structure and host response. Diabetes Metab Syndr Clin Res Rev. 2020;14(4):407–412. doi:10.1016/j.dsx.2020.04.020
2. Rabaan AA, Al-Ahmed SH, Haque S, et al. SARS-CoV-2, SARS-CoV, and MERS-CoV: a comparative overview. Le Infez Med. 2020;2:174–184. doi:10.3366/edinburgh/9780748632909.003.0011
3. Zhang X, Tan Y, Ling Y, et al. Viral and host factors related to the clinical outcome of COVID-19. Nature. 2020;583(7816):437–440. doi:10.1038/s41586-020-2355-0
4. Huang Y, Yang C, Xu X. Structural and functional properties of SARS-CoV-2 spike protein: potential antivirus drug development for COVID-19. Acta Pharmacol Sin. 2020;41(9):1141–1149. doi:10.1038/s41401-020-0485-4
5. Xiao L, Sakagami H, Miwa N. ACE2: the key molecule for understanding the pathophysiology of severe and critical conditions of COVID-19: demon or angel? Viruses. 2020;12(5):2002–2003. doi:10.3390/v12050491
6. Bestle D, Heindl MR, Limburg H, et al. TMPRSS2 and furin are both essential for proteolytic activation and spread of SARS-CoV-2 in human airway epithelial cells and provide promising drug targets. bioRxiv. 2020. doi:10.1101/2020.04.15.042085
7. Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280.e8. doi:10.1016/j.cell.2020.02.052
8. Chen Y, Liu Q, Guo D. Emerging coronaviruses: genome structure, replication, and pathogenesis. J Med Virol. 2020;92(4):418–423. doi:10.1002/jmv.25681
9. Farshi P, Kaya EC, Hashempour-Baltork F, Khosravi-Darani K. A comprehensive review on the effect of plant metabolites on coronaviruses: focusing on their molecular docking score and IC50 values. Preprints. 2020. doi:10.20944/preprints202005.0295.v1
10. Suravajhala R, Parashar A, Malik B, et al. Comparative docking studies on curcumin with COVID-19 proteins. Preprints. 2020;19:1–37. doi:10.20944/preprints202005.0439.v3
11. Yi L, Li Z, Yuan K, et al. Small molecules blocking the entry of Severe Acute Respiratory Syndrome Coronavirus into host cells. J Virol. 2004;78(20):11334–11339. doi:10.1128/jvi.78.20.11334-11339.2004
12. Lin SC, Ho CT, Chuo WH, Li S, Wang TT, Lin CC. Effective inhibition of MERS-CoV infection by resveratrol. BMC Infect Dis. 2017;17(1):1–10. doi:10.1186/s12879-017-2253-8
13. Joshi RS, Jagdale SS, Bansode SB, et al. Discovery of potential multi-target-directed ligands by targeting host-specific SARS-CoV-2 structurally conserved main protease. J Biomol Struct Dyn. 2020;(May). doi:10.1080/07391102.2020.1760137
14. Nguyen TTH, Woo HJ, Kang HK, et al. Flavonoid-mediated inhibition of SARS coronavirus 3C-like protease expressed in Pichia pastoris. Biotechnol Lett. 2012;34(5):831–838. doi:10.1007/s10529-011-0845-8
15. Lin CW, Tsai FJ, Tsai CH, et al. Anti-SARS coronavirus 3C-like protease effects of Isatis indigotica root and plant-derived phenolic compounds. Antiviral Res. 2005;68(1):36–42. doi:10.1016/j.antiviral.2005.07.002
16. Glinsky GV. Tripartite combination of potential pandemic mitigation agents: vitamin D, Quercetin, and Estradiol manifest properties of candidate medicinal agents for mitigation of the severity of pandemic COVID-19 defined by genomics-guided tracing of SARS-CoV-2 targ. Biomedicines. 2020;8(5):129.
17. Abian O, Ortega-Alarcon D, Jimenez-Alesanco A, et al. Structural stability of SARS-CoV-2 3CLpro and identification of quercetin as an inhibitor by experimental screening. Int J Biol Macromol. 2020;164. doi:10.1016/j.ijbiomac.2020.07.235
18. Nabirotchkin S, Peluffo AE, Bouaziz J, Cohen D. Focusing on the unfolded protein response and autophagy related pathways to reposition common approved drugs against COVID-19. Preprints. 2020.
19. Khan MF, Khan MA, Khan ZA, Ahamad T, Ansari WA. Identification of dietary molecules as therapeutic agents to combat COVID-19 using molecular docking studies. Research Square. 2020. doi:10.21203/rs.3.rs-19560/v1
20. Umesh U, Kundu D, Selvaraj C, Singh SK, Dubey VK. Identification of new anti-nCoV drug chemical compounds from indian spices exploiting SARS-CoV-2 main protease as target. J Biomol Struct Dyn. 2020. doi:10.1080/07391102.2020.1763202
21. Bosch-Barrera J, Martin-Castillo B, Buxó M, Brunet J, Encinar JA, Menendez JA. Silibinin and SARS-CoV-2: dual targeting of host cytokine storm and virus replication machinery for clinical management of COVID-19 patients. J Clin Med. 2020;9(6):1770. doi:10.3390/jcm9061770
22. Hu X, Cai X, Song X, et al. Possible SARS-coronavirus 2 inhibitor revealed by simulated molecular docking to viral main protease and host toll-like receptor. Future Virol. 2020;15(6):359–368. doi:10.2217/fvl-2020-0099
23. Kar P, Kumar V, Vellingiri B, et al. Anisotine and amarogentin as promising inhibitory candidates against SARS-CoV-2 proteins: a computational investigation. J Biomol Struct Dyn. 2020. doi:10.1080/07391102.2020.01860133
24. Kar P, Sharma NR, Singh B, Sen A, Roy A. Natural compounds from Clerodendrum spp. as possible therapeutic candidates against SARS-CoV-2: an in silico investigation. J Biomol Struct Dyn. 2020;1–12. doi:10.1080/07391102.2020.1780947
25. Fischer A, Sellner M, Neranjan S, Smieško M, Lill MA. Potential inhibitors for novel coronavirus protease identified by virtual screening of 606 million compounds. Int J Mol Sci. 2020;21(10):1–17. doi:10.3390/ijms21103626
26. Das G, Das T, Chowdhury N, Chatterjee D, Bagchi A, Ghosh Z. Repurposed drugs and nutraceuticals targeting envelope protein: A possible therapeutic strategy against COVID-19. Genomics. 2020. doi:10.1016/j.ygeno.2020.11.009
27. Meyer M, Jaspers I. Respiratory protease/antiprotease balance determines susceptibility to viral infection and can be modified by nutritional antioxidants. fischer. 2015;308(12):1189–1201. doi:10.1152/ajplung.00028.2015
28. Vonder Haar C, Peterson TC, Martens KM, Hoane MR. Vitamins and nutrients as primary treatments in experimental brain injury: clinical implications for nutraceutical therapies. Brain Res. 2016;1640:114–129. doi:10.1016/j.brainres.2015.12.030
29. Olthof MR, Hollman PC, Zock PL, Katan MB. Consumption of high doses of chlorogenic acid, present in coffee, or of black tea increases plasma total homocysteine concentrations in humans. Am J Clin Nutr. 2001;73:3. doi:10.1093/ajcn/73.3.532
30. Fear G, Komarnytsky S, Raskin I. Protease inhibitors and their peptidomimetic derivatives as potential drugs. Pharmacol Ther. 2007;113(2):354–368. doi:10.1016/j.pharmthera.2006.09.001
31. Sabotič J, Kos J. Microbial and fungal protease inhibitors—current and potential applications. Appl Microbiol Biotechnol. 2012;93:1351–1375. doi:10.1007/s00253-011-3834-x
32. van de Ven WJM, Voorberg J, Fontijn R, et al. Furin is a subtilisin-like proprotein processing enzyme in higher eukaryotes. Mol Biol Rep. 1990;14(4):265–275. doi:10.1007/BF00429896
33. Deng X, StJohn SE, Osswald HL, et al. Coronaviruses resistant to a 3C-Like protease inhibitor are attenuated for replication and pathogenesis, revealing a low genetic barrier but high fitness cost of resistance. J Virol. 2014;88(20):11886–11898. doi:10.1128/jvi.01528-14
34. Hellinger R, Gruber CW. Peptide-based protease inhibitors from plants. Drug Discov Today. 2019;24(9):1877–1889. doi:10.1016/j.drudis.2019.05.026
35. Srikanth S, Chen Z. Plant protease inhibitors in therapeutics-focus on cancer therapy. Front Pharmacol. 2016;7:470. doi:10.3389/fphar.2016.00470
36. Lega S, Naviglio S, Volpi S, Tommasini A. Recent insight into SARS-COV2 immunopathology and rationale for potential treatment and preventive strategies in COVID-19. Vaccines. 2020;8(2):1–30. doi:10.3390/vaccines8020224
37. Azkur AK, Akdis M, Azkur D, et al. Immune response to SARS-CoV-2 and mechanisms of immunopathological changes in COVID-19. Allergy Eur J Allergy Clin Immunol. 2020;75(7):1564–1581. doi:10.1111/all.14364
38. Oh HLJ, Gan SKE, Bertoletti A, Tan YJ. Understanding the T cell immune response in SARS coronavirus infection. Emerg Microbes Infect. 2012;1:23. doi:10.1038/emi.2012.26
39. Pedersen SF, Ho YC. SARS-CoV-2: A storm is raging. J Clin Invest. 2020;130(5):2202–2205. doi:10.1172/JCI137647
40. Yuen KS, Ye ZW, Fung SY, Chan CP, Jin DY. SARS-CoV-2 and COVID-19: the most important research questions. Cell Biosci. 2020;10(1):1–5. doi:10.1186/s13578-020-00404-4
41. Mizutani T. Signal transduction in SARS-CoV-infected cells. Ann N Y Acad Sci. 2007;1102:86–95. doi:10.1196/annals.1408.006
42. Pineau N, Aucouturier P, Brugier JC, Preud’homme JL. Jacalin: A lectin mitogenic for human CD4 T lymphocytes. Clin Exp Immunol. 1990;80(3):420–425. doi:10.1111/j.1365-2249.1990.tb03304.x
43. Al Mijan M, Lim BO. Diets, functional foods, and nutraceuticals as alternative therapies for inflammatory bowel disease: present status and future trends. World J Gastroenterol. 2018;24(25):2673–2685. doi:10.3748/wjg.v24.i25.2673
44. Yang DJ, Liu SC, Chen YC, Hsu SH, Chang YP, Lin JT. Three pathways assess anti-inflammatory response of epicatechin with lipopolysaccharide-mediated macrophage RAW264.7 Cells. J Food Biochem. 2015;39(3):334–343. doi:10.1111/jfbc.12134
45. Khan F, Niaz K, Maqbool F, et al. Molecular targets underlying the anticancer effects of quercetin: an update. Nutrients. 2016;8(9):1–19. doi:10.3390/nu8090529
46. Choi JS, Choi YJ, Shin SY, et al. Dietary flavonoids differentially reduce oxidized LDL-induced apoptosis in human endothelial cells: role of MAPK- and JAK/STAT-signaling. J Nutr. 2008;138(6):983–990. doi:10.1093/jn/138.6.983
47. Hämäläinen M, Nieminen R, Vuorela P, Heinonen M, Moilanen E. Anti-inflammatory effects of flavonoids: genistein, kaempferol, quercetin, and daidzein inhibit STAT-1 and NF-κB activations, whereas flavone, isorhamnetin, naringenin, and pelargonidin inhibit only NF-κB activation along with their inhibitory effect on iNOS expression and NO production in activated macrophages. Mediators Inflamm. 2007;2007:1–10.
48. Hsu SC, Chung JG. Anticancer potential of emodin. Biomed. 2012;2(3):108–116. doi:10.1016/j.biomed.2012.03.003
49. Kashyap D, Tuli HS, Sharma AK. Ursolic acid (UA): A metabolite with promising therapeutic potential. Life Sci. 2016;146:201–213. doi:10.1016/j.lfs.2016.01.017
50. Huang MT, Ghai G, Ho CT. Inflammatory Process and Molecular Targets for Anti- inflammatory Nutraceuticals. Compr Rev Food Sci Food Saf. 2004;3(1):127–139.
51. Yanaka N, Koyama TA, Komatsu SI, Nakamura E, Kanda M, Kato N. Vitamin B6 suppresses NF-kappaB activation in LPS-stimulated mouse macrophages. Int J Mol Med. 2005;16(6):1071–1075. doi:10.3892/ijmm.16.6.1071
52. Zhang L, Fan Y, Su H, et al. Chlorogenic acid methyl ester exerts strong anti-inflammatory effects: via inhibiting the COX-2/NLRP3/NF-κB pathway. Food Funct. 2018;9(12):6155–6164. doi:10.1039/c8fo01281d
53. Dos Tramontin N, Luciano TF, de OMarques S, de Souza CT, Muller AP. Ginger and avocado as nutraceuticals for obesity and its comorbidities. Phytother Res. 2020;34(6):1282–1290. doi:10.1002/ptr.6619
54. Majdalawieh AF, Mansour ZR. Sesamol, a major lignan in sesame seeds (Sesamum indicum): anti-cancer properties and mechanisms of action. Eur J Pharmacol. 2019;855:75–89. doi:10.1016/j.ejphar.2019.05.008
55. Sánchez‐sánchez MA, Zepeda‐morales ASM, Carrera‐quintanar L, et al. Alliin, an Allium sativum nutraceutical, reduces metaflammation markers in DIO mice. Nutrients. 2020;12(3). doi:10.3390/nu12030624
56. Nicholas C, Batra S, Vargo MA, et al. Apigenin blocks lipopolysaccharide-induced lethality in vivo and proinflammatory cytokines expression by inactivating NF-κB through the suppression of p65 phosphorylation. J Immunol. 2007;179(10):7121–7127. doi:10.4049/jimmunol.179.10.7121
57. Haghighatdoost F, Jabbari M, Hariri M. The effect of L-carnitine on inflammatory mediators: a systematic review and meta-analysis of randomized clinical trials. Eur J Clin Pharmacol. 2019. doi:10.1024/0300-9831/a000619
58. Marín-Aguilar F, Pavillard LE, Giampieri F, Bullón P, Cordero MD. Adenosine monophosphate (AMP)-activated protein kinase: A new target for nutraceutical compounds. Int J Mol Sci. 2017;18:2. doi:10.3390/ijms18020288
59. Sunil C, Xu B. An insight into the health-promoting effects of taxifolin (dihydroquercetin). Phytochemistry. 2019;166(July):112066. doi:10.1016/j.phytochem.2019.112066
60. Zaki N, Alashwal H, Ibrahim S. Association of hypertension, diabetes, stroke, cancer, kidney disease, and high-cholesterol with COVID-19 disease severity and fatality: A systematic review. Diabetes Metab Syndr Clin Res Rev. 2020;14(5):1133–1142. doi:10.1016/j.dsx.2020.07.005
61. Kim MS, Lee MS, Kown DY. Inflammation-mediated obesity and insulin resistance as targets for nutraceuticals. Ann N Y Acad Sci. 2011;1229(1):140–146. doi:10.1111/j.1749-6632.2011.06098.x
62. Kahn BB, Flier JS, Kahn BB, Flier JS. Obesity and insulin resistance find the latest version: obesity and insulin resistance. J Clin Invest. 2000;106(4):473–481.
63. Aggarwal BB. Targeting inflammation-induced obesity and metabolic diseases by curcumin and other nutraceuticals. Annu Rev Nutr. 2010;30:173–199. doi:10.1146/annurev.nutr.012809.104755
64. Ayres JS. A metabolic handbook for the COVID-19 pandemic. Nat Metab. 2020;2(7):572–585. doi:10.1038/s42255-020-0237-2
65. Singh AK, Gupta R, Ghosh A, Misra A. Diabetes in COVID-19: prevalence, pathophysiology, prognosis and practical considerations. Diabetes Metab Syndr Clin Res Rev. 2020;14(4):303–310. doi:10.1016/j.dsx.2020.04.004
66. Rebello CJ, Kirwan JP, Greenway FL. Obesity, the most common comorbidity in SARS-CoV-2: is leptin the link? Int J Obes. 2020;44(9):1810–1817. doi:10.1038/s41366-020-0640-5
67. Chen G, Wang H, Zhang X, Yang ST. Nutraceuticals and functional foods in the management of hyperlipidemia. Crit Rev Food Sci Nutr. 2014;54(9):1180–1201. doi:10.1080/10408398.2011.629354
68. Sorokin AV, Karathanasis SK, Yang ZH, Freeman L, Kotani K, Remaley AT. COVID-19—Associated dyslipidemia: implications for mechanism of impaired resolution and novel therapeutic approaches. FASEB J. 2020;34(8):9843–9853. doi:10.1096/fj.202001451
69. Houston M. The role of nutrition and nutraceutical supplements in the treatment of hypertension. World J Cardiol. 2014;6(2):38. doi:10.4330/wjc.v6.i2.38
70. Chao CY, Yin MC, Huang CJ. Wild bitter gourd extract up-regulates mRNA expression of PPARα, PPARγ and their target genes in C57BL/6J mice. J Ethnopharmacol. 2011;135(1):156–161. doi:10.1016/j.jep.2011.03.001
71. Sridhar MG, Vinayagamoorthi R, Arul Suyambunathan V, Bobby Z, Selvaraj N. Bitter gourd (Momordica charantia) improves insulin sensitivity by increasing skeletal muscle insulin-stimulated IRS-1 tyrosine phosphorylation in high-fat-fed rats. Br J Nutr. 2008;99(4):806–812. doi:10.1017/S000711450783176X
72. Misawa K, Hashizume K, Yamamoto M, Minegishi Y, Hase T, Shimotoyodome A. Ginger extract prevents high-fat diet-induced obesity in mice via activation of the peroxisome proliferator-activated receptor δ pathway. J Nutr Biochem. 2015;26(10):1058–1067. doi:10.1016/j.jnutbio.2015.04.014
73. Maharlouei N, Tabrizi R, Lankarani KB, et al. The effects of ginger intake on weight loss and metabolic profiles among overweight and obese subjects: A systematic review and meta-analysis of randomized controlled trials. Crit Rev Food Sci Nutr. 2019;59(11):1753–1766. doi:10.1080/10408398.2018.1427044
74. Shao W, Yu Z, Chiang Y, et al. Curcumin prevents high fat diet induced insulin resistance and obesity via attenuating lipogenesis in liver and inflammatory pathway in adipocytes. PLoS One. 2012;7(1):1–13. doi:10.1371/journal.pone.0028784
75. Panahi Y, Khalili N, Sahebi E, et al. Curcuminoids modify lipid profile in type 2 diabetes mellitus: A randomized controlled trial. Complement Ther Med. 2017;33:1–5. doi:10.1016/j.ctim.2017.05.006
76. Lone J, Choi JH, Kim SW, Yun JW. Curcumin induces brown fat-like phenotype in 3T3-L1 and primary white adipocytes. J Nutr Biochem. 2016;27:193–202. doi:10.1016/j.jnutbio.2015.09.006
77. Yousaf S, Butt MS, Suleria HAR, Iqbal MJ. The role of green tea extract and powder in mitigating metabolic syndromes with special reference to hyperglycemia and hypercholesterolemia. Food Funct. 2014;5(3):545–556. doi:10.1039/c3fo60203f
78. Hsu SP, Wu MS, Yang CC, et al. Chronic green tea extract supplementation reduces hemodialysisenhanced production of hydrogen peroxide and hypochlorous acid, atherosclerotic factors, and proinflammatory cytokines. Am J Clin Nutr. 2007;86(5):1539–1547. doi:10.1093/ajcn/86.5.1539
79. Kang JH, Kim CS, Han IS, Kawada T, Yu R. Capsaicin, a spicy component of hot peppers, modulates adipokine gene expression and protein release from obese-mouse adipose tissues and isolated adipocytes, and suppresses the inflammatory responses of adipose tissue macrophages. FEBS Lett. 2007;581(23):4389–4396. doi:10.1016/j.febslet.2007.07.082
80. Jain PG, Patil SD, Haswani NG, Girase MV, Surana SJ. Atividade hipolipidemica de Moringa oleifera Lam., Moringaceae, na hiperlipidemia induzida por dieta rica em gordura em ratos albinos (Hypolipidemic activity of Moringa oleifera Lam., Moringaceae, on high fat diet induced hyperlipidemia in albino rats). Brazilian J Pharmacogn. 2010;20(6):969–973. doi:10.1590/S0102-695X2010005000038
81. Kumari DJ. Hypoglycaemic effect of Moringa oleifera and Azadirachta indica in type 2 Diabetes mellitus. The Bioscan. 2010;5(2):211–214.
82. Pipe EA, Gobert CP, Capes SE, Darlington GA, Lampe JW, Duncan AM. Soy protein reduces serum LDL cholesterol and the LDL cholesterol: HDL cholesterol and apolipoprotein B: apolipoproteinA-I ratios in adults with type 2 diabetes. J Nutr. 2009;139(9):1700–1706. doi:10.3945/jn.109.109595
83. Labban L, Mustafa UE-S, Ibrahim YM. The effects of Rosemary (Rosmarinus officinalis) leaves powder on glucose level, lipid profile and lipid perodoxation. Int J Clin Med. 2014;05(06):297–304. doi:10.4236/ijcm.2014.56044
84. Esmaillzadeh A, Tahbaz F, Gaieni I, Alavi-Majd H, Azadbakht L. Cholesterol-lowering effect of concentrated pomegranate juice consumption in type II diabetic patients with hyperlipidemia. Int J Vitam Nutr Res. 2006;76(3):147–151. doi:10.1024/0300-9831.76.3.147
85. Rock W, Rosenblat M, Miller-Lotan R, Levy AP, Elias M, Aviram M. Consumption of Wonderful variety pomegranate juice and extract by diabetic patients increases paraoxonase 1 association with high-density lipoprotein and stimulates its catalytic activities. J Agric Food Chem. 2008;56(18):8704–8713. doi:10.1021/jf801756x
86. Burton-Freeman B, Linares A, Hyson D, Kappagoda T. Strawberry modulates LDL oxidation and postprandial lipemia in response to high-fat meal in overweight hyperlipidemic men and women. J Am Coll Nutr. 2010;29(1):46–54. doi:10.1080/07315724.2010.10719816
87. Liu X, Xue Y, Liu C, et al. Eicosapentaenoic acid-enriched phospholipid ameliorates insulin resistance and lipid metabolism in diet-induced-obese mice. Lipids Health Dis. 2013;12(1):1–10. doi:10.1186/1476-511X-12-109
88. Neschen S, Morino K, Dong J, et al. n-3 fatty acids preserve insulin sensitivity in vivoin a Peroxisome Proliferator – activated Receptor-α– dependent Manner. Diabetes. 2007;56(4):1034–1041. doi:10.2337/db06-1206.2-
89. Flachs P, Horakova O, Brauner P, et al. Polyunsaturated fatty acids of marine origin upregulate mitochondrial biogenesis and induce β-oxidation in white fat. Diabetologia. 2005;48(11):2365–2375. doi:10.1007/s00125-005-1944-7
90. Levy BD, Kohli P, Gotlinger K, et al. Protectin D1 Is generated in asthma and dampens airway inflammation and hyperresponsiveness. J Immunol. 2010;178(1):496–502.
91. Mickleborough TD, Tecklenburg SL, Montgomery GS, Lindley MR. Eicosapentaenoic acid is more effective than docosahexaenoic acid in inhibiting proinflammatory mediator production and transcription from LPS-induced human asthmatic alveolar macrophage cells. Clin Nutr. 2009;28(1):71–77. doi:10.1016/j.clnu.2008.10.012
92. Farrell N, Norris G, Lee SG, Chun OK, Blesso CN. Anthocyanin-rich black elderberry extract improves markers of HDL function and reduces aortic cholesterol in hyperlipidemic mice. Food Funct. 2015;6(4):1278–1287. doi:10.1039/c4fo01036a
93. Li D, Zhang Y, Liu Y, Sun R, Xia M. Purified anthocyanin supplementation reduces dyslipidemia, enhances antioxidant capacity, and prevents insulin resistance in diabetic patients. J Nutr. 2015;145(4):742–748. doi:10.3945/jn.114.205674
94. Wolfram S, Raederstorff D, Preller M, et al. Epigallocatechin gallate supplementation alleviates diabetes in rodents. J Nutr. 2006;136(10):2512–2518. doi:10.1093/jn/136.10.2512
95. Cremonini E, Bettaieb A, Haj FG, Fraga CG, Oteiza PI. -)-Epicatechin improves insulin sensitivity in high fat diet-fed mice. Arch Biochem Biophys. 2016;599:13–21. doi:10.1016/j.abb.2016.03.006
96. Moon J, Do HJ, Kim OY, Shin MJ. Antiobesity effects of quercetin-rich onion peel extract on the differentiation of 3T3-L1 preadipocytes and the adipogenesis in high fat-fed rats. Food Chem Toxicol. 2013;58:347–354. doi:10.1016/j.fct.2013.05.006
97. Lee SG, Parks JS, Kang HW. Quercetin, a functional compound of onion peel, remodels white adipocytes to brown-like adipocytes. J Nutr Biochem. 2017;42(2017):62–71. doi:10.1016/j.jnutbio.2016.12.018
98. Gong M, Garige M, Varatharajalu R, et al. Quercetin up-regulates paraoxonase 1 gene expression with concomitant protection against LDL oxidation. Biochem Biophys Res Commun. 2009;379(4):1001–1004. doi:10.1016/j.bbrc.2009.01.015
99. Rivera L, Morón R, Sánchez M, Zarzuelo A, Galisteo M. Quercetin ameliorates metabolic syndrome and improves the inflammatory status in obese Zucker rats. Obesity. 2008;16(9):2081–2087. doi:10.1038/oby.2008.315
100. Luo C, Yang H, Tang C, et al. Kaempferol alleviates insulin resistance via hepatic IKK/NF-κB signal in type 2 diabetic rats. Int Immunopharmacol. 2015;28(1):744–750. doi:10.1016/j.intimp.2015.07.018
101. Zhang Y, Liu D. Flavonol kaempferol improves chronic hyperglycemia-impaired pancreatic beta-cell viability and insulin secretory function. Eur J Pharmacol. 2011;670(1):325–332. doi:10.1016/j.ejphar.2011.08.011
102. Goto T, Teraminami A, Lee J-Y, et al. Tiliroside, a glycosidic flavonoid, ameliorates obesity-induced metabolic disorders via activation of adiponectin signaling followed by enhancement of fatty acid oxidation in liver and skeletal muscle in obese–diabetic mice. J Nutr Biochem. 2012;23(7):768–776. doi:10.1016/j.jnutbio.2011.04.001
103. Priscilla DH, Jayakumar M, Thirumurugan K. Flavanone naringenin: an effective antihyperglycemic and antihyperlipidemic nutraceutical agent on high fat diet fed streptozotocin induced type 2 diabetic rats. J Funct Foods. 2015;14:363–373. doi:10.1016/j.jff.2015.02.005
104. Ahmed OM, Mahmoud AM, Abdel-Moneim A, Ashour MB. Antidiabetic effects of hesperidin and naringin in type 2 diabetic rats. Diabetol Croat. 2012;41(2):53–67.
105. Liu Z, Liu T, Lei C, et al. Novel role of hesperidin improve obesity in HFD mice by modulating the composition of the gut microbiota. Res Sq. 2020. doi:10.21203/rs.2.21089/v1
106. El-Bassossy HM, Abo-Warda SM, Fahmy A. Chrysin and luteolin attenuate diabetes-induced impairment in endothelial-dependent relaxation: effect on lipid profile, AGEs and NO generation. Phytother Res. 2013;27(11):1678–1684. doi:10.1002/ptr.4917
107. El-Bassossy HM, Abo-Warda SM, Fahmy A. Chrysin and luteolin alleviate vascular complications associated with insulin resistance mainly through PPAR-γ activation. Am J Chin Med. 2014;42(5):1153–1167. doi:10.1142/S0192415X14500724
108. Zhang L, Han Y-J, Zhang X, et al. Luteolin reduces obesity-associated insulin resistance in mice by activating AMPKα1 signalling in adipose tissue macrophages. Diabetologia. 2016;59(10):2219–2228. doi:10.1007/s00125-016-4039-8
109. Fu Z, Liu D. Long-term exposure to genistein improves insulin secretory function of pancreatic β-cells. Eur J Pharmacol. 2009;616(1–3):321–327. doi:10.1016/j.ejphar.2009.06.005
110. Hsu CL, Wu CH, Huang SL, Yen GC. Phenolic compounds rutin and o-coumaric acid ameliorate obesity induced by high-fat Diet in rats. J Agric Food Chem. 2009;57(2):425–431. doi:10.1021/jf802715t
111. Abdel-Moneim A, SMA E-T, Yousef AI, Reheim ESA, Ashour MB. Modulation of hyperglycemia and dyslipidemia in experimental type 2 diabetes by gallic acid and p-coumaric acid: the role of adipocytokines and PPARγ. Biomed Pharmacother. 2018;105:1091–1097. doi:10.1016/j.biopha.2018.06.096
112. Ibitoye OB, Ajiboye TO. Dietary phenolic acids reverse insulin resistance, hyperglycaemia, dyslipidaemia, inflammation and oxidative stress in high-fructose diet-induced metabolic syndrome rats. Arch Physiol Biochem. 2018;124(5):410–417. doi:10.1080/13813455.2017.1415938
113. Antunes AEC, Vinderola G, Xavier-Santos D, Sivieri K. Potential contribution of beneficial microbes to face the COVID-19 pandemic. Food Res Int. 2020;136(June):109577. doi:10.1016/j.foodres.2020.109577
114. Dhar D, Mohanty A. Gut microbiota and Covid-19- possible link and implications. Virus Res. 2020;285:198018. doi:10.1016/j.virusres.2020.198018
115. Berggren A, Lazou Ahrén I, Larsson N, Önning G. Randomised, double-blind and placebo-controlled study using new probiotic lactobacilli for strengthening the body immune defence against viral infections. Eur J Nutr. 2011;50(3):203–210. doi:10.1007/s00394-010-0127-6
116. de Vrese M, Winkler P, Rautenberg P, et al. Probiotic bacteria reduced duration and severity but not the incidence of common cold episodes in a double blind, randomized, controlled trial. Vaccine. 2006;24(44–46):6670–6674. doi:10.1016/j.vaccine.2006.05.048
117. Boge T, Rémigy M, Vaudaine S, Tanguy J, Bourdet-Sicard R. van der Werf S. A probiotic fermented dairy drink improves antibody response to influenza vaccination in the elderly in two randomised controlled trials. Vaccine. 2009;27(41):5677–5684. doi:10.1016/j.vaccine.2009.06.094
118. National US. Library of Medicine. Evaluation of the Probiotic Lactobacillus Coryniformis K8 on COVID-19 Prevention in Healthcare Workers. 2020. Available from: https://clinicaltrials.gov/ct2/show/NCT04366180.
119. U.S. National Library of Medicine. Bacteriotherapy in the Treatment of COVID-19 (BACT-Ovid).. Https://Clinicaltrials.gov/Ct2/Show/Nct04368351. 2020. Available from:.
120. Corrêa-Oliveira R, Fachi JL, Vieira A, Sato FT, Vinolo MAR. Regulation of immune cell function by short-chain fatty acids. Clin Transl Immunol. 2016;5(4):1–8. doi:10.1038/cti.2016.17
121. Li M, BCAM VE, Wagenaar GTM, Garssen J, Folkerts G, Henricks PAJ. Pro- and anti-inflammatory effects of short chain fatty acids on immune and endothelial cells. Eur J Pharmacol. 2018;831:52–59. doi:10.1016/j.ejphar.2018.05.003
122. Venkatakrishnan K, Chiu HF, Wang CK. An extensive review on popular functional foods and nutraceuticals against obesity and its related complications with a special focus on randomized clinical trials. Food Funct. 2019. doi:10.1039/C9FO00293F.Food
123. Chakrabarti S, Wu J. Milk-derived tripeptides IPP (Ile-Pro-Pro) and VPP (Val-Pro- Pro) promote adipocyte differentiation and inhibit inflammation in 3T3-F442A cells. PLoS One. 2015;10(2):1–15. doi:10.1371/journal.pone.0117492
124. Ortega-González M, Capitán-Cañadas F, Requena P, et al. Validation of bovine glycomacropeptide as an intestinal anti-inflammatory nutraceutical in the lymphocyte-transfer model of colitis. Br J Nutr. 2014;111(7):1202–1212. doi:10.1017/S0007114513003590
125. Nakamura T, Hirota T, Mizushima K, et al. Milk-derived peptides, Val-Pro-Pro and Ile-Pro-Pro, attenuate atherosclerosis development in apolipoprotein E-deficient mice: A preliminary study. J Med Food. 2013;16(5):396–403. doi:10.1089/jmf.2012.2541
126. Iskandar MM, Dauletbaev N, Kubow S, Mawji N, Lands LC. Whey protein hydrolysates decrease IL-8 secretion in lipopolysaccharide (LPS)-stimulated respiratory epithelial cells by affecting LPS binding to Toll-like receptor 4. Br J Nutr. 2013;110(1):58–68. doi:10.1017/S0007114512004655
127. Majumder K, Chakrabarti S, Davidge ST, Wu J. Structure and activity study of egg protein ovotransferrin derived peptides (IRW and IQW) on endothelial inflammatory response and oxidative stress. J Agric Food Chem. 2013;61(9):2120–2129. doi:10.1021/jf3046076
128. de Mejia EG, Dia VP. Lunasin and lunasin-like peptides inhibit inflammation through suppression of NF-κB pathway in the macrophage. Peptides. 2009;30(12):2388–2398. doi:10.1016/j.peptides.2009.08.005
129. Kovacs-Nolan J, Zhang H, Ibuki M, et al. The PepT1-transportable soy tripeptide VPY reduces intestinal inflammation. Biochim Biophys Acta - Gen Subj. 2012;1820(11):1753–1763. doi:10.1016/j.bbagen.2012.07.007
130. Bjørndal B, Berge C, Ramsvik MS, et al. A fish protein hydrolysate alters fatty acid composition in liver and adipose tissue and increases plasma carnitine levels in a mouse model of chronic inflammation. Lipids Health Dis. 2013;12(1):1–11. doi:10.1186/1476-511X-12-143
131. Zhang Y, Kouguchi T, Shimizu K, Sato M, Takahata Y, Morimatsu F. Chicken collagen hydrolysate reduces proinflammatory cytokine production in C57BL/6.KOR-ApoEsh1 mice. J Nutr Sci Vitaminol (Tokyo). 2010;56(3):208–210. doi:10.3177/jnsv.56.208
132. Suzuki Y, Asano M, Sato K, et al. Wheat gluten hydrolysate alters the progress of hepatic pathology induced by prolonged carbon tetrachloride administration in rat. Biomed Res. 2011;22(4):481–488.
133. Hirai S, Horii S, Matsuzaki Y, et al. Anti-inflammatory effect of pyroglutamyl-leucine on lipopolysaccharide-stimulated RAW 264.7 macrophages. Life Sci. 2014;117(1):1–6. doi:10.1016/j.lfs.2014.08.017
134. Udechukwu MC, Tsopmo A, Mawhinney H, He R, Kienesberger PC, Udenigwe CC. Inhibition of ADAM17/TACE activity by zinc-chelating rye secalin-derived tripeptides and analogues. RSC Adv. 2017;7(42):26361–26369. doi:10.1039/c6ra26678a
135. RML CB, Berrill M, Marik PE. The antiviral properties of vitamin C. Expert Rev Anti Infect Ther. 2020;18:2. doi:10.1080/14787210.2020.1706483
136. Chiscano-Camón L, Ruiz-Rodriguez JC, Ruiz-Sanmartin A, Roca O, Vitamin FR. C levels in patients with SARS-CoV-2-associated acute respiratory distress syndrome. Crit Care. 2020;24:1. doi:10.1186/s13054-020-03249-y
137. Feyaerts AF, Luyten W. Vitamin C as prophylaxis and adjunctive medical treatment for COVID-19? Nutrition. 2020;110948:79–80. doi:10.1016/j.nut.2020.110948
138. Hiedra R, Lo KB, Elbashabsheh M, et al. The Use of IV vitamin C for patients with COVID-19: a single center observational study. Expert Rev Anti Infect Ther. 2020;18(12):1259–1261. doi:10.1080/14787210.2020.1794819
139. RML CB, Berrill M, Catravas JD, Marik PE. Quercetin and Vitamin C: an experimental, synergistic therapy for the prevention and treatment of SARS-CoV-2 related disease (COVID-19). Front Immunol. 2020. doi:10.3389/fimmu.2020.01451
140. Davis JM, Murphy EA, McClellan JL, Carmichael MD, Gangemi JD. Quercetin reduces susceptibility to influenza infection following stressful exercise. Am J Physiol - Regul Integr Comp Physiol. 2008;295(2):505–509. doi:10.1152/ajpregu.90319.2008
141. Arslan B, Ucuncu Ergun N, Topuz S, et al. Synergistic Effect of Quercetin and Vitamin C Against COVID-19: is a Possible Guard for Front Liners. SSRN Electron J. 2020. doi:10.2139/ssrn.3682517
142. Grant WB, Lahore H, McDonnell SL, et al. Evidence that vitamin D supplementation could reduce risk of influenza and covid-19 infections and deaths.. Nutrients. 2020;12(4):1–19. doi:10.3390/nu12040988
143. Arboleda JF, Urcuqui-Inchima S. Coronavirus covid-19 has killed more people than SARS and MERS combined, despite lower case fatality rate. Front Immunol. 2020;11(1523):1–4. doi:10.1136/bmj.m641
144. Ebadi M, Montano-Loza AJ. Perspective: improving vitamin D status in the management of COVID-19. Eur J Clin Nutr. 2020;74(6):856–859. doi:10.1038/s41430-020-0661-0
145. Ghavideldarestani M, Honardoost M, Khamseh ME. Role of Vitamin D in Pathogenesis and Severity of Coronavirus Disease 2019 (COVID-19) Infection. Pakistan J Med Heal Sci. 2020;14(2):462–465. doi:10.20944/preprints202004.0355.v1
146. U.S. National Library of Medicine. Utility of Lactoferrin as an Adjunct Therapeutic Agent for COVID-19; 2020. Available from:: https://clinicaltrials.gov/ct2/show/NCT04421534.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.