Back to Journals » Journal of Asthma and Allergy » Volume 15
Potential Drawbacks of ICS/LABA/LAMA Triple Fixed-Dose Combination Therapy in the Treatment of Asthma: A Quantitative Synthesis of Safety Profile
Authors Rogliani P , Cavalli F, Chetta A, Cazzola M , Calzetta L
Received 12 January 2022
Accepted for publication 20 April 2022
Published 6 May 2022 Volume 2022:15 Pages 565—577
DOI https://doi.org/10.2147/JAA.S283489
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Amrita Dosanjh
Paola Rogliani,1 Francesco Cavalli,1 Alfredo Chetta,2 Mario Cazzola,1 Luigino Calzetta2
1Unit of Respiratory Medicine, Department of Experimental Medicine, University of Rome “Tor Vergata”, Rome, Italy; 2Department of Medicine and Surgery, Respiratory Disease and Lung Function Unit, University of Parma, Parma, Italy
Correspondence: Paola Rogliani, Unit of Respiratory Medicine, Department of Experimental Medicine, University of Rome “Tor Vergata”, Rome, Italy, Email [email protected]
Introduction: Inhaled corticosteroid/long-acting β2-adrenoceptor agonist/long-acting muscarinic antagonist (ICS/LABA/LAMA) fixed-dose combination (FDC) is currently recommended as controller option at asthma Step 4 and as preferred treatment at asthma Step 5, but no research investigated the potential drawbacks of this therapeutic option in a large asthmatic population. Thus, the aim of this study was to quantify the potential drawbacks of triple FDC therapy in asthma.
Methods: A pairwise meta-analysis was performed according to PRISMA-P guidelines to assess the risk of overall serious adverse events (SAEs), cardiovascular SAEs, and pneumonia reported as SAE in asthmatic patients treated with ICS/LABA/LAMA FDC vs ICS/LABA FDC. A pooled analysis was performed to calculate the frequency of SAEs.
Results: Data from 7204 asthmatic patients were extracted from the CAPTAIN, IRIDIUM, TRIMARAN, and TRIGGER studies. Triple FDC vs ICS/LABA FDC did not increase the risk of total SAEs (RR 0.99 95% CI 0.83– 1.18) and cardiac SAEs (RR 0.74 95% CI 0.39– 1.40), whereas the sensitivity analysis performed to resolve heterogeneity resulted in increased risk of vascular SAEs (RR 3.23 95% CI 1.05– 9.90, P< 0.05). The level of ICS dose did not modulate the risk of pneumonia, in any case pneumonia was the most frequent SAE (0.57%). These results were not affected by significant risk of bias.
Conclusion: Triple FDC is a safe pharmacological therapy in severe asthmatic patients; it is characterized by a favourable safety profile and few potential drawbacks, namely, the increased risk of vascular SAEs, that certainly are worthy of future investigations.
Keywords: asthma, cardiovascular, meta-analysis, pneumonia, safety, triple combination
Introduction
The current Global Initiative for Asthma (GINA, 2021)1 recommendations suggest to administer as-needed low dose inhaled corticosteroid (ICS)/formoterol fixed-dose combination (FDC) as preferred controller treatment at asthma Step 1 and 2, and low dose maintenance ICS/formoterol FDC as preferred controller treatment at asthma Step 3 in adults and adolescents.2 The GINA document also recommends to administer ICS/long-acting β2-adrenoceptor agonist (LABA)/long-acting muscarinic antagonist (LAMA) FDC as controller option at asthma Step 4 and as preferred treatment at add on asthma Step 5 in adults and adolescents.2 Effectively, the co-primary endpoints of a large quantitative synthesis of data from randomized controlled trials (RCTs) indicated that triple FDC was significantly effective in preventing the risk of moderate to severe exacerbation and improving lung function.3 These data were confirmed also by the clinical interpretation of efficacy outcomes in pharmacological studies on triple FDC for uncontrolled asthma, reporting that ICS/LABA/LAMA FDC reached the minimal clinically important difference for the risk of exacerbation, lung function, asthma control, and quality of life not only when compared to ICS/LABA FDC, but often also vs the free triple combination of fluticasone/salmeterol plus tiotropium where the ICS was administered at high dose.4
Despite the proved therapeutic efficacy of triple FDC in severe asthma, to date only few evidence originated from research specifically performed to assess the safety profile of ICS/LABA/LAMA FDC in a large asthmatic population.5 Therefore, the aim of this study was to investigate the potential drawbacks of triple FDC therapy in asthma via quantitative synthesis assessing the risk of overall serious adverse events (SAEs), and more specifically the risk of cardiovascular SAEs and pneumonia reported as SAE.
Materials and Methods
Search Strategy and Study Eligibility
This quantitative synthesis has been registered to the international prospective register of systematic reviews (PROSPERO registration ID: CRD42022298742, available at https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=298742), and performed in agreement with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Protocols (PRISMA-P).6 The relative flow diagram is shown in Figure 1. This study satisfied all the recommended items reported by the PRISMA-P checklist (Table S1).6
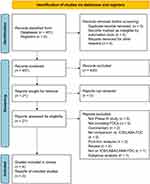 |
Figure 1 PRISMA 2020 flow diagram for the identification of Phase III RCTs included in the meta-analysis concerning the safety profile of triple FDC in asthma. Abbreviations: FDC, fixed-dose combination; ICS, inhaled corticosteroid; LABA, long-acting β2-adrenoceptor agonist; LAMA, long-acting muscarinic antagonist; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; RCT, randomized controlled trial. Note: Adapted from Moher D, Shamseer L, Clarke M, et al.Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4(1):1. Creative Commons license and disclaimer available from: http://creativecommons.org/licenses/by/4.0/legalcod.6 |
A comprehensive literature search was performed for Phase III RCTs written in English and evaluating the impact of FDC therapy for the treatment of asthma. As an example, Table S2 reports the literature search terms used for OVID MEDLINE and Appendix 1 shows the summary text of the identified records.
The PICO (Patient problem, Intervention, Comparison, and Outcome) framework was applied to develop the literature search strategy, as previously reported.7 Namely, the “Patient problem” included patients suffering from asthma; the “Intervention” regarded the administration of triple FDC; the “Comparison” was performed with respect to the same ICS/LABA FDC as in the triple FDC and administered via the same inhaler device; the assessed “Outcome” was the risk of SAEs, and more specifically the risk of cardiovascular SAEs and pneumonia reported as SAE.
The search was performed in ClinicalTrials.gov, Cochrane Central Register of Controlled Trials (CENTRAL), Embase, EU Clinical Trials Register, MEDLINE, Scopus, and Web of Science, in order to provide for relevant studies lasting ≥24 weeks and published up to December 14th, 2021.
The research string was as follows: (((Beclomethasone formoterol glycopyrronium) OR (CHF 5993) OR (CHF5993)) OR (fluticasone furoate vilanterol umeclidinium) OR (mometasone indacaterol glycopyrronium OR (QVM149) OR (QVM 149)) OR ((fluticasone propionate salmeterol tiotropium) OR (ICS LABA tiotropium)) OR triple) AND asthma.
Literature search results were uploaded to Eppi-Reviewer 4 (EPPI-Centre Software. London, UK), a web-based software program for managing and analysing data in literature reviews that facilitates collaboration among reviewers during the study selection process.
Study Selection Funnel
Phase III RCTs that enrolled asthmatic patients, lasting ≥24 weeks, and that included at least one arm assessing the safety of any triple FDC vs the same ICS/LABA FDC included in the triple therapy were included in the meta-analysis. Two reviewers independently examined the studies, and any difference in opinion concerning the selection of relevant Phase III RCTs from literature searches and databases was resolved by consensus.
Data Extraction
Data from the Phase III RCTs included in this quantitative synthesis were extracted from published papers, and/or supplementary files, and/or the public database ClinicalTrials.gov.
Data were checked for study characteristics and duration, number of analysed patients, treatments with doses of medications, regimen of administration and inhaler device, asthma severity and main inclusion criteria, age, gender, asthma duration, forced expiratory volume in the 1st second (FEV1), level of FEV1 reversibility, exacerbations, blood eosinophil count at baseline, smoking habit, Asthma Control Questionnaire (ACQ), Jadad Score,8 and the Cochrane risk of bias.9
The level of ICS doses (medium-dose and high-dose) included in the FDC was ranked in agreement with the current GINA recommendations10 and the National Institute for Health and Care Excellence (NICE) guidelines.11
Data were extracted in agreement with Data Extraction for Complex Meta-anALysis (DECiMAL) recommendations.12 The inter- and intra-rater reliability for data abstraction was assessed via the Cohen’s Kappa score, as previously described.13 Briefly, Cohen’s Kappa ≥0.80 indicated excellent agreement, coefficients between 0.61 and 0.80 represented substantial agreement, coefficients between 0.41 and 0.61 moderate agreement and <0.41 fair to poor agreement.
Endpoint
The primary endpoint of this meta-analysis was the safety profile of triple FDC vs ICS/LABA FDC expressed as the risk of SAEs, with specific focus on cardiovascular SAEs and pneumonia reported as SAE.
Data Synthesis and Analysis
A pairwise meta-analysis was performed to quantify the risk of SAEs of triple FDC vs ICS/LABA FDC in asthmatic patients. Results were expressed as relative risk (RR) and 95% confidence interval (95% CI).
Since data were selected from a series of studies performed by researchers operating independently and a common effect size cannot be assumed, binary random-effects method (derSimonian and Laird model) was used to balance the study weights and adequately estimate the 95% CI of the mean distribution of drugs effect on the investigated variables.
Subgroup analyses were performed in agreement with the level of ICSs doses included in the FDC.
A pooled analysis was performed to calculate the frequency of SAEs, ranked in agreement with the European Medicine Agency (EMA) document undesirable effect, as follows: very common ≥1/10, common ≥1/100 to <1/10, uncommon ≥1/1000 to <1/100, rare ≥1/10,000 to <1/1000, frequency not known if not calculable from the available data.14
Quality of the Studies, Risk of Bias, and Evidence Profile
The summary of the risk of bias for each included Phase III RCT was analyzed via the Cochrane Risk of Bias 2 (RoB 2)15 and Jadad score.8 The Jadad score ranges from 1 to 5 (score of 5 being the best score), and the quality of studies was ranked as follows: score ≤2, low quality; score =3, medium quality; score ≥4 high quality. The weighted assessment of the risk of bias was analyzed via the Cochrane RoB 2.15
The test for heterogeneity (I2) was performed to quantify the between-study dissimilarity, as previously reported.16 Sensitivity analysis was carried out if studies introduced significant and/or substantial (I2>50%) level of heterogeneity in the quantitative synthesis.17 In sensitivity analysis, after significant and/or substantial heterogeneity was resolved, fixed-effect (Mantel Haenszel) method was used if sparse data were reported.18
Funnel plot and Egger’s test were carried out to assess the origin and risk of publication bias if more than 10 studies were included in the meta-analysis.19–22
The quality of the evidence was assessed in agreement with the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) system, indicating ++++ for high quality of evidence, +++ for moderate quality of evidence, ++ for low quality of evidence, and + for very low quality of evidence.9
Two reviewers independently assessed the quality of studies, risk bias, and evidence profile, and any difference in opinion was resolved by consensus.
Software and Statistical Significance
Open-MetaAnalyst was used to perform the pairwise meta-analysis,16 GRADEpro GDT to assess the quality of evidence,9 and the robvis visualization software to perform the RoB 2 tool.23,24 The statistical significance was assessed for P<0.05.
Results
Study Characteristics
Data obtained from 7204 asthmatic patients (medium-dose ICS/LABA/LAMA FDC: 27.86%; high-dose ICS/LABA/LAMA FDC: 27.79%; high-dose ICS/LABA FDC: 22.18%; medium-dose ICS/LABA FDC: 22.17%) were extracted from 4 Phase III RCTs,25–27 published between 2019 and 2020 as full-text papers, and with a period of treatment of 52 weeks (Table S3). All the four studies were performed in patients suffering from inadequately controlled asthma.25–27
In agreement with the search strategy and study selection criteria, the investigated ICS/LABA/LAMA FDCs included beclomethasone dipropionate (BDP)/formoterol fumarate (FOR)/glycopyrronium bromide (GLY), mometasone furoate (MOM)/indacaterol (IND)/GLY, and fluticasone furoate (FF)/vilanterol (VI)/umeclidinium (UMEC).25–27 The active comparators were the ICS/LABA FDCs BDP/FOR, FF/VI, and MOM/IND.
The level of ICS doses in the FDC is shown in Table 1. The inter- and intra-rater reliability for data abstraction was generally excellent (Cohen’s Kappa >0.90).
 |
Table 1 Level of ICS Doses in Agreement with the Daily Doses of Medications in Adults in the Phase III RCTs Included in the Meta-Analysis as Reported by Current GINA Recommendations10 and NICE Guidelines.11 |
Safety Profile
Risk of Total SAEs
Triple FDC did not significantly increase the risk of total SAEs compared to ICS/LABA FDC (RR 0.99 95% CI 0.83–1.18, P>0.05; GRADE +++); the subgroup analysis did not show significant (P>0.05) difference between medium-dose ICS and high-dose ICS (Figure 2).
Neither significant (P>0.05) nor substantial heterogeneity was reported for triple FDC vs ICS/LABA FDC in the subgroup high-dose ICS (GRADE +++). Conversely, in the subgroup medium-dose ICS heterogeneity was substantial (I2=72%), although not significant (P>0.05; GRADE ++).
The sensitivity analysis indicated that FF/VI/UMEC 100/25/31.25 vs FF/VI 100/25 was the comparison in the CAPTAIN25 study that introduced substantial heterogeneity when comparing triple FDC vs ICS/LABA FDC in the subgroup medium-dose ICS (I2=0% after sensitivity analysis). When substantial heterogeneity was resolved by sensitivity analysis, no significant differences were detected for the risk of total SAEs between triple FDC and ICS/LABA FDC (RR 1.02 95% CI 0.85–1.23, P>0.05; GRADE ++++), regardless of the ICS doses.
Risk of Cardiovascular SAEs
The overall analysis of cardiovascular SAEs reported no significant increased risk when comparing triple FDC with ICS/LABA FDC (RR 1.03 95% CI 0.61–1.73, P>0.05; GRADE +++), regardless of the ICS doses.
The specific analysis of cardiac SAEs showed no significant difference between triple FDC and ICS/LABA FDC (RR 0.74 95% CI 0.39–1.40, P>0.05; GRADE +++), regardless of the ICS doses, with neither significant (P>0.05) nor substantial heterogeneity (Figure 3A).
Triple FDC did not apparently modulate the risk of vascular SAEs compared to ICS/LABA FDC (RR 1.91 95% CI 0.71–5.17, P>0.05; GRADE +++) (Figure 3B). While neither significant (P>0.05) nor substantial heterogeneity resulted in the subgroup medium-dose ICS (GRADE +++), when triple FDC was compared to ICS/LABA FDC in the subgroup high-dose ICS substantial, although not significant (P>0.05), heterogeneity was detected (I2=52%; GRADE ++). The sensitivity analysis revealed that the comparison between FF/VI/UMEC 200/25/31.25 and FF/VI 200/25 in the CAPTAIN25 study caused substantial heterogeneity in the subgroup high-dose ICS (I2=0% after sensitivity analysis). When substantial heterogeneity was resolved by sensitivity analysis, triple FDC vs ICS/LABA FDC resulted in significant (P<0.05) greater risk of vascular SAEs (RR 3.23 95% CI 1.05–9.90; GRADE ++++) (Figure 3B’ and S1).
Risk of Pneumonia
Triple FDC did not significantly enhance the risk of pneumonia compared to ICS/LABA FDC (RR 0.79 95% CI 0.40–1.55, P>0.05; GRADE +++), regardless of the ICS doses, with neither significant (P>0.05) nor substantial heterogeneity (Figure 4).
High-dose triple FDC vs medium-dose ICS/LABA FDC and medium-dose triple FDC vs high-dose ICS/LABA FDC did not significantly (P>0.05) modulate the risk of pneumonia (RR 1.14 95% CI 0.40–3.24 and RR 0.86 95% CI 0.26–2.23, respectively; GRADE +++).
Pooled Analysis of SAEs Frequency
The pooled analysis reported consistency in the frequency of SAEs across medium- and high-dose ICS/LABA/LAMA FDC and medium- and high-dose ICS/LABA FDC. The total SAEs were common, whereas the cardiovascular SAEs and pneumonia were uncommon. Overall, the most frequent SAEs were pneumonia (0.57%), followed by angina unstable and myocardial infarction (0.13%), and by acute coronary syndrome, atrial fibrillation, deep vein thrombosis, hypertension, hypertensive crisis, and varicose vein (0.10%); other SAEs were ranked as rare (<0.10%). Detailed information on the frequency of SAEs are shown in Table 2.
 |
Table 2 Pooled Analysis of Total SAEs, Cardiovascular SAEs, and Pneumonia with Number of Events, Frequency, and Rank According to EMA Guidelines.14 |
Risk of Bias and Quality of Evidence
The traffic light plot for the assessment of each included RCT is reported in Figure 5A and the weighted plot for the assessment of the overall risk of bias by domains is shown in Figure 5B. All the Phase III RCTs had a low risk of bias for the randomization process, deviations from intended intervention, missing outcome data, measurement of the outcomes, and selection of the reported results (4 [100.0%]). All four studies (100.0%) included in this meta-analysis were ranked as being of high quality in agreement with the Jadad score (Table S3). Funnel plot and Egger’s test were not performed since less than 10 studies were included in the meta-analysis.
Discussion
This meta-analysis provides the moderate to high quality evidence that ICS/LABA/LAMA triple FDC is characterized by an favourable safety profile when compared to ICS/LABA FDC with respect to the risk of total SAEs, regardless of ICS dose. The safety profile was also confirmed for the risk of cardiac SAEs and pneumonia reported as SAEs. However, the sensitivity analysis reported significant increased risk of vascular SAEs related to triple FDC vs ICS/LABA FDC.
The treatment comparison that introduced a bias leading to reduced risk of vascular SAEs included FF/VI/UMEC 200/25/31.25, a triple FDC in which the LAMA was administered at lower dose. Evidently, the dose of LAMA in the FDC may modulate the risk of vascular SAEs. This finding originates from results obtained by specific meta-analysis technique pertinent to conditions in which very little heterogeneity exists and, above all, data are sparse.18 In this regard, the frequency of cardiovascular SAEs and pneumonia reported as SAEs were rare to uncommon across all the meta-analysis, and often no events were reported for some treatments.
Indeed, the sparsity of data poses the question whether this meta-analysis based on RCTs lasting only one year may be strong enough to quantify the real potential drawbacks of triple FDC in the treatment of asthma. Unfortunately, neither longer RCTs nor larger observational studies are currently available on this topic. In any case, the number of patients per treatment (N>1000) included in the pooled analysis of CAPTAIN, IRIDIUM, TRIMARAN, and TRIGGER25–27 studies allowed intercepting rare SAEs, a condition characterized by a level of frequency of less than 0.1% according to EMA guidelines.14 This evidence confirms that, in some specific areas of healthcare such as the study of sparse data, meta-analysis may be a suitable way to quantify the impact of interventions on rare events, by giving more precise answer to a research question than a single RCT and detecting an effect with greater statistical power.28
This study generally supports the safety profile resulting from a previous network meta-analysis on triple therapy in uncontrolled asthma3 that, however, considered the cardiovascular risk as pooled data and not differentiated as cardiac SAEs and vascular SAEs. This is the reason why in the present meta-analysis, specifically focused on the safety profile, we were able to detect a significant increased risk associated with triple FDC specifically for vascular SAEs.
However, since meta-analytical methods are based on large sample approximations, it is possible that they may provide misleading results on rare but important outcomes such as the risk of SAEs. This may happen according to the underlying event rate, likely size of the treatment effect, and balance in the numbers of treated and control participants in the RCTs.28,29 The discrepancy between the selected patients fitting the criteria of RCTs and real-life asthmatic patients3,30 may limit the possible application of results of this meta-analysis in daily clinical practice. Therefore, further well-permed real-life studies are needed to assess if the treatment of asthmatic patients with triple FDC is really associated to increased vascular risk.
Another drawback of adding a LAMA to ICS/LABA FDC in asthma is related to the risk of prescribing triple therapy on “one size fits all” basis, whereas the trend should be towards a personalized medicine approach even in severe asthma.31,32 In this respect, the response to triple FDCs should be at least assessed according to the sex and body mass index (BMI), even before considering the difference in asthma phenotypes.31,32 In fact, in women with chronic obstructive pulmonary disease the bronchorelaxant effect of inhaled antimuscarinic agents resulted inversely correlated to BMI, whereas in men the impact of BMI was of little extent.33 Moreover, lower expression of M2 muscarinic acetylcholine receptor (mAChR) was found in women than in men, leading to a greater M3/M2 mAChR receptor ratio.33 Indeed, the inhibition of M2 mAChR expressed at the level of cardiovascular system is related to adverse events such as such as tachycardia and prolonged QT,34 thus administering a triple FDC including a LAMA should be well assessed in those asthmatic patients that may have an altered response to muscarinic antagonists.
Although the safety profile of ICS is markedly better than that of oral corticosteroids, ICS are however absorbed from the lungs into the systemic circulation, leading to osteoporosis and increase the risk of cataracts, glaucoma, skin atrophy, and vascular alterations.35 The possibility of modulating the dose of ICS in the triple FDC and the evidence that medium-dose triple FDC may be effective even when compared to high-dose ICS/LABA3 opens new perspectives of asthma treatment. We should consider the opportunity of shifting from high-dose ICS/LABA to medium-dose ICS/LABA/LAMA in patients with airflow obstruction and few exacerbations, as well as the option of de-escalating the ICS dose in the triple FDC according to the patient’s clinical response to therapy.2
Conclusion
Triple FDC is a pharmacological therapy to treat severe asthma characterized by a favourable safety profile and few potential drawbacks that, however, are worthy of future investigations. The possibility of adding a LAMA to ICS/LABA FDC along with the option of modulating the dose of ICS in the triple FDC make ICS/LABA/LAMA therapy a flexible pharmacological tool suitable not only for difficult-to-treat asthma, but also for patients suffering from moderate forms of the disease. Finally, but not less important, FDC including a LAMA at lower dose such as UMEC at 31.25 µg instead of 62.25 µg may improve the safety profile of the combination and further improve the possible therapeutic options in asthma. However, EMA did not approve asthma label expansion for FF/VI/UMEC since the CAPTAIN25 study did not clearly show that the medicine was effective at reducing asthma attacks or controlling symptoms,36 a missing opportunity to optimize the benefit-risk ratio of ICS/LABA/LAMA FDC in the treatment of asthma.
Acknowledgments
We thank dr. Beatrice Ludovica Ritondo for her support in PRISMA-P.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This research was not funded.
Disclosure
PR reported grants and personal fees from Almirall, AstraZeneca, Biofutura, Boehringer Ingelheim, Chiesi Farmaceutici, GlaxoSmithKline, Menarini Group, MSD, Mundipharma, and Novartis, and participated as a lecturer and advisor in scientific meetings sponsored by Almirall, AstraZeneca, Biofutura, Boehringer Ingelheim, Chiesi Farmaceutici, Edmond Pharma, GlaxoSmithKline, Menarini Group, Mundipharma, and Novartis. Her department was funded by Almirall, Boehringer Ingelheim, Chiesi Farmaceutici, Novartis, and Zambon. AC reports grants from Menarini and Astra Zeneca; personal fee from Chiesi.
MC reports grants and personal fees from Boehringer Ingelheim, grants and personal fees from Novartis, personal fees from AstraZeneca, personal fees from Chiesi Farmaceutici, grants and personal fees from Almirall, personal fees from ABC Farmaceutici, personal fees from Edmond Pharma, grants and personal fees from Zambon, personal fees from Verona Pharma, personal fees from Ockham Biotech, personal fees from Biofutura, personal fees from GlaxoSmithKline, personal fees from Menarini, personal fees from Lallemand, personal fees from Mundipharma, personal fees from Pfizer, outside the submitted work.
LC participated as an advisor in scientific meetings sponsored by Boehringer Ingelheim and Novartis, received non-financial support from AstraZeneca, a research grant partially funded by Chiesi Farmaceutici, Boehringer Ingelheim, Novartis, and Almirall, and is or was a consultant to ABC Farmaceutici, MSD, Recipharm, Zambon, Verona Pharma and Ockham Biotech. His department was funded by Almirall, Boehringer Ingelheim, Chiesi Farmaceutici, Novartis and Zambon. The authors report no other conflicts of interest in this work.
References
1. GINA main report - Global Initiative for Asthma; 2021. Available from: https://ginasthma.org/wp-content/uploads/2021/05/GINA-Main-Report-2021-V2-WMS.pdf.
2. Rogliani P, Ritondo BL, Facciolo F, Matera MG, Nikolaev I, Calzetta L. Indacaterol, glycopyrronium, and mometasone: pharmacological interaction and anti-inflammatory profile in hyperresponsive airways. Pharmacol Res. 2021;172:105801. doi:10.1016/J.PHRS.2021.105801
3. Rogliani P, Ritondo BL, Calzetta L. Triple therapy in uncontrolled asthma: a network meta-analysis of phase III studies. Eur Respir J. 2021;58(3):2004233. doi:10.1183/13993003.04233-2020
4. Rogliani P, Calzetta L. Clinical Interpretation of efficacy outcomes in pharmacological studies on triple fixed-dose combination therapy for uncontrolled asthma: assessment of IRIDIUM and ARGON studies. J Exp Pharmacol. 2022;14:1–5. doi:10.2147/JEP.S336304
5. Scosyrev E, van Zyl-smit R, Kerstjens H, et al. Cardiovascular safety of mometasone/indacaterol and mometasone/indacaterol/glycopyrronium once-daily fixed-dose combinations in asthma: pooled analysis of Phase 3 trials. Respir Med. 2021;180:106311. doi:10.1016/j.rmed.2021.106311
6. Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4(1):1. doi:10.1186/2046-4053-4-1
7. Schardt C, Adams MB, Owens T, Keitz S, Fontelo P. Utilization of the PICO framework to improve searching PubMed for clinical questions. BMC Med Inf Decis Mak. 2007;7(1):16. doi:10.1186/1472-6947-7-16
8. Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17(1):1–12. doi:10.1016/0197-2456(95)00134-4
9. Guyatt G, Oxman AD, Akl EA, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64(4):383–394. doi:10.1016/j.jclinepi.2010.04.026
10. Global Initiative for Asthma (GINA). Global strategy for asthma management and prevention. Available from: https://ginasthma.org/wp-content/uploads/2021/05/GINA-Main-Report-2021-V2-WMS.pdf.
11. National Institute for Health and Care Excellence (NICE). Inhaled corticosteroid doses for NICE’s asthma guideline; 2018. Available from: www.nice.org.uk/guidance/ng80/resources/inhaled-corticosteroid-doses-pdf-4731528781.
12. Pedder H, Sarri G, Keeney E, Nunes V, Dias S. Data extraction for complex meta-analysis (DECiMAL) guide. Syst Rev. 2016;5(1):212. doi:10.1186/s13643-016-0368-4
13. Gianinazzi ME, Rueegg CS, Zimmerman K, Kuehni CE, Michel G; Swiss Paediatric Oncology G. Intra-rater and inter-rater reliability of a medical record abstraction study on transition of care after childhood cancer. PLoS One. 2015;10(5):e0124290. doi:10.1371/journal.pone.0124290
14. European Medicines Agency. Section 4.8 Undesirable effects. Available from https://www.ema.europa.eu/en/documents/presentation/presentation-section-48-undesirable-effects_en.pdf.
15. Higgins JPT, Savović J, Page MJ, Elbers RG, Sterne JAC. Chapter 8: assessing risk of bias in a randomized trial. In: Cochrane Handbook for Systematic Reviews of Interventions Version 6.0 (Updated July 2019). Cochrane, 2019; 2019:205–228. Available from www.training.cochrane.org/handbook.
16. Wallace BC, Dahabreh IJ, Trikalinos TA, Lau J, Trow P, Schmid CH. Closing the gap between methodologists and end-users: r as a computational back-end. J Stat Softw. 2012;49(5):1–15. doi:10.18637/jss.v049.i05
17. Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Br Med J. 2003;327(7414):557–560. doi:10.1136/bmj.327.7414.557
18. Sankey SS, Weissfeld LA, Fine MJ, Kapoor W. An assessment of the use of the continuity correction for sparse data in meta-analysis. Commun Stat Part B Simul Comput. 1996;25(4):1031–1056. doi:10.1080/03610919608813357
19. Sterne JAC, Gavaghan D, Egger M. Publication and related bias in meta-analysis: power of statistical tests and prevalence in the literature. J Clin Epidemiol. 2000;53(11):1119–1129. doi:10.1016/S0895-4356(00)00242-0
20. Sterne JAC, Egger M. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol. 2001;54(10):1046–1055. doi:10.1016/S0895-4356(01)00377-8
21. Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. Br Med J. 1997;315(7109):629–634. doi:10.1136/bmj.315.7109.629
22. 10.4.3.1 Recommendations on testing for funnel plot asymmetry. Available from: https://handbook-5-1.cochrane.org/chapter_10/10_4_3_1_recommendations_on_testing_for_funnel_plot_asymmetry.htm.
23. Sterne JAC, Savovic J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi:10.1136/bmj.l4898
24. McGuinness LA. robvis: an R package and web application for visualising risk-of-bias assessments; 2020.
25. Lee LA, Bailes Z, Barnes N, et al. Efficacy and safety of once-daily single-inhaler triple therapy (FF/UMEC/VI) versus FF/VI in patients with inadequately controlled asthma (CAPTAIN): a double-blind, randomised, phase 3A trial. Lancet Respir Med. 2021;9(1):69–84. doi:10.1016/S2213-2600(20)30389-1
26. Kerstjens HAM, Maspero J, Chapman KR, et al. Once-daily, single-inhaler mometasone–indacaterol–glycopyrronium versus mometasone–indacaterol or twice-daily fluticasone–salmeterol in patients with inadequately controlled asthma (IRIDIUM): a randomised, double-blind, controlled phase 3 study. Lancet Respir Med. 2020;8(10):1000–1012. doi:10.1016/S2213-2600(20)30190-9
27. Virchow JC, Kuna P, Paggiaro P, et al. Single inhaler extrafine triple therapy in uncontrolled asthma (TRIMARAN and TRIGGER): two double-blind, parallel-group, randomised, controlled phase 3 trials. Lancet. 2019;394(10210):1737–1749. doi:10.1016/S0140-6736(19)32215-9
28. Bradburn MJ, Deeks JJ, Berlin JA, Localio AR. Much ado about nothing: a comparison of the performance of meta-analytical methods with rare events. Stat Med. 2007;26(1):53–77. doi:10.1002/SIM.2528
29. 16.9.5 Validity of methods of meta-analysis for rare events. Available from: https://handbook-5-1.cochrane.org/chapter_16/16_9_5_validity_of_methods_of_meta_analysis_for_rare_events.htm.
30. Usmani OS. Small-airway disease in asthma: pharmacological Considerations. Curr Opin Pulm Med. 2015;21(1):55–67. doi:10.1097/MCP.0000000000000115
31. Canonica GW, Ferrando M, Baiardini I, et al. Asthma: personalized and precision medicine. Curr Opin Allergy Clin Immunol. 2018;18(1):51–58. doi:10.1097/ACI.0000000000000416
32. Heffler E, Canonica GW, Diamant Z, Fonseca J, Malinovschi A. Personalized approach to severe asthma. Biomed Res Int. 2018;2018:1–2. doi:10.1155/2018/2465172
33. Calzetta L, Puxeddu E, Rogliani P. Gender-related responsiveness to the pharmacological treatment of COPD: a first step towards the personalized medicine. EBioMedicine. 2017;19:14–15. doi:10.1016/j.ebiom.2017.04.035
34. Ora J, Coppola A, Cazzola M, Calzetta L, Rogliani P. Long-acting muscarinic antagonists under investigational to treat chronic obstructive pulmonary disease. J Exp Pharmacol. 2020;12:559–574. doi:10.2147/JEP.S259330
35. Allen DB, Bielory L, Derendorf H, Dluhy R, Colice GL, Szefler SJ. Inhaled corticosteroids: past lessons and future issues. J Allergy Clin Immunol. 2003;112(3):S1–S40. doi:10.1016/S0091-6749(03)01859-1
36. European Medicine Agency. Refusal of change to the marketing authorisation for Trelegy Ellipta (fluticasone furoate / umeclidinium / vilanterol). Available from: https://www.ema.europa.eu/en/documents/smop/questions-answers-refusal-change-marketing-authorisation-trelegy-ellipta-fluticasone-furoate/umeclidinium/vilanterol_en.pdf.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




