Back to Journals » International Journal of Nanomedicine » Volume 11
Potential antitumor activity of novel DODAC/PHO-S liposomes
Authors Cássio de Lima Luna A, Kelle Viegas Saraiva G, Mendonça R. Filho O, CHIERICEE GO, Claro Neto S, CUCCOVIA IM, Maria D
Received 20 June 2015
Accepted for publication 19 September 2015
Published 18 April 2016 Volume 2016:11 Pages 1577—1591
DOI https://doi.org/10.2147/IJN.S90850
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Thomas Webster
Arthur Cássio de Lima Luna,1,2 Greice Kelle Viegas Saraiva,3 Otaviano Mendonça Ribeiro Filho,4 Gilberto Orivaldo Chierice,5 Salvador Claro Neto,5 Iolanda Midea Cuccovia,3 Durvanei Augusto Maria1,2
1Biochemistry and Biophysical Laboratory, Butantan Institute, 2Department of Medical Sciences, Medical School, 3Department of Biochemistry, Institute of Chemistry, University of Sao Paulo, Sao Paulo, 4Environmental Health Surveillance, Municipality of Uberaba, Minas Gerais, 5Department of Chemistry and Molecular Physics, University of Sao Paulo, Sao Carlos, Brazil
Abstract: In recent studies, we showed that synthetic phosphoethanolamine (PHO-S) has a great potential for inducing cell death in several tumor cell lines without damage to normal cells. However, its cytotoxic effect and selectivity against tumor cells could increase with encapsulation in cationic liposomes, such as dioctadecyldimethylammonium chloride (DODAC), due to electrostatic interactions between these liposomes and tumor cell membranes. Our aim was to use cationic liposomes to deliver PHO-S and to furthermore maximize the therapeutic effect of this compound. DODAC liposomes containing PHO-S (DODAC/PHO-S), at concentrations of 0.3–2.0 mM, prepared by ultrasonication, were analyzed by scanning electron microscopy (SEM) and dynamic light scattering. The cytotoxic effect of DODAC/PHO-S on B16F10 cells, Hepa1c1c7 cells, and human umbilical vein endothelial cells (HUVECs) was assessed by MTT assay. Cell cycle phases of B16F10 cells were analyzed by flow cytometry and the morphological changes by SEM, after treatment. The liposomes were spherical and polydisperse in solution. The liposomes were stable, presenting an average of ~50% of PHO-S encapsulation, with a small reduction after 40 days. DODAC demonstrated efficient PHO-S delivery, with the lowest values of IC50% (concentration that inhibits 50% of the growth of cells) for tumor cells, compared with PHO-S alone, with an IC50% value of 0.8 mM for B16F10 cells and 0.2 mM for Hepa1c1c7 cells, and without significant effects on endothelial cells. The Hepa1c1c7 cells showed greater sensitivity to the DODAC/PHO-S formulation when compared to B16F10 cells and HUVECs. The use of DODAC/PHO-S on B16F10 cells induced G2/M-phase cell cycle arrest, with the proportion significantly greater than that treated with PHO-S alone. The morphological analysis of B16F10 cells by SEM showed changes such as “bleb” formation, cell detachment, cytoplasmic retraction, and apoptotic bodies after DODAC/PHO-S treatment. Cationic liposomal formulation for PHO-S delivery promoted cytotoxicity more selectively and effectively against B16F10 and Hepa1c1c7 cells. Thus, the DODAC/PHO-S liposomal formulation presents great potential for preclinical studies.
Keywords: hepatocellular carcinoma, lipossomal formulation, synthetic phosphoethanolamine, nanocarriers
Introduction
Synthetic alkylphospholipids are a promising new class of anticancer agents that act on cell membranes rather than on DNA. They selectively modify the turnover of cell membrane, inducing programmed cell death, with high selectivity for tumor cells.1 The alkylphospholipids contain long hydrocarbon chains that are essentially nonpolar.2,3 In a recent report, we showed that synthetic phosphoethanolamine (PHO-S), an amino-ethyl phosphoric ester, has a great potential to induce cell death in B16F10 (melanoma murine) cells and without apparent damage to normal cells, such as fibroblasts and lymphocytes.4,5 We demonstrated that PHO-S is effective in the reduction of DNA synthesis rate and cell proliferation and also induces G2/M cell cycle arrest.5 It caused a decrease in cyclin D1 mRNA, VEGFR1 gene transcription, and VEGFR1 receptor expression. In vitro antiangiogenic activity assays showed that PHO-S inhibits endothelial cell proliferation, migration, and tube formation.6,7 These changes, associated with apoptotic effect, reflected an increased expression of active forms of caspase 3.8 However, the molecular mechanism responsible for the antitumor properties of PHO-S is still under investigation.
Recent studies, utilizing several tumor cell lines, such as EAT cells (ehrlich ascites tumor), MCF-7 cells (human breast cancer), H292 cells (lung cancer), B16F10 cells, and SK-MEL-28, and MeWo cells (human melanoma), support the hypothesis that antitumor activities exerted by PHO-S independent of molecular profiles are resistant and aggressive.4–7
The antineoplastic phospholipids are a new class of antitumor agents, which can cause collateral effects, such as gastrointestinal toxicity, hemolysis, and liver and renal dysfunction. These effects are attributed to agents such as edelfosine, ilmofosine, and miltefosine.2,3,9,10 However, the use of nanocarriers as drug delivery systems for chemotherapeutic agents may be an alternative to decrease the chance of unanticipated adverse effects and maximize the therapeutic effects of encapsulated drugs.11
Recently, the use of liposome-encapsulated paclitaxel and miltefosine provided synergistic effect on human glioblastoma cells resistant to chemotherapeutic agents, with a sustained release of these drugs and reversal of resistance.11 Cationic lipids are amphiphilic molecules that have been used as a delivery system for nucleic acid and have several advantages, such as the fact that these liposomes are endogenous and biodegradable after administration.12–15 This is beneficial because the presence of endogenous enzymes can break down the lipid components of the liposomes.15
The dioctadecyldimethylammonium chloride (DODAC) is a cationic amphiphilic molecule that has been used for gene delivery. Studies have shown that these liposomes exhibit a propensity to selectively target tumor-associated blood vessels.14,16 Thus, due to several advantages exhibited by these liposomes in anticancer treatment, they could be used for PHO-S delivery. In such a case, the cytotoxic effect and selectivity against tumor cells of PHO-S could increase with encapsulation in DODAC due to the electrostatic interaction between these liposomes and the tumor cell membrane. Therefore, our aim was to use cationic liposomes DODAC for the delivery of PHO-S and to furthermore maximize the therapeutic effect of this compound, considering the specificity of the formulation for tumor microenvironments.
Materials and methods
DODAC/PHO-S liposomal formulation
PHO-S was synthesized and supplied by the Laboratory of Chemistry and Polymers Technology, University of Sao Paulo, Sao Carlos, Brazil. A 1 M stock solution of PHO-S was prepared in water and stored at room temperature for in vitro assays.4–8 DODAC was obtained from DODAB (Sigma-Aldrich Co., St Louis, MO, USA) by ion exchange. Primarily, DODAC powder was weighed and suspended in 10 mL of water in order to obtain final concentrations of 0.3, 0.6, 1.0, 1.3, 1.6, and 2.0 mM. Further, PHO-S was included in the solution, at the same concentration of DODAC (DODAC:PHO-S =1/1 molar ratio). In another experiment, we used liposomes with 10 mM DODAC and 0.3–2.0 mM PHO-S. The dispersions were maintained at 57°C, for 20 minutes, until complete homogenization. Samples were vortexed and sonicated using a Braun Sonic 1510 Ultrasonic Apparatus (Branson, MO, USA) equipped with a titanium tip (70 W, 3–4 minutes, at 60°C). After sonication, the samples were centrifuged at 1,500 rpm for 5 minutes to remove residual titanium released from the probe. The liposomes were sterilized by filtration through a 0.22 μm Millipore filter.
Chemical and physical stability of the liposomal suspension
Liposomal suspensions were characterized by hydrodynamic radius (Dh) and zeta potential (ζ). The average diameter of the liposomes of DODAC/PHO-S was calculated using the method of quasielastic light scattering. Aliquots of 1 mL of the preparation were placed into a 1.0 cm quartz cuvette and analyzed by dynamic light scattering (DLS) technique using a Malvern ZetaSizer Nano ZS90 (BioTek, Winooski, VT, USA). For the determination of zeta potential of DODAC/PHO-S and empty DODAC liposomes, 1 mL of the preparation was placed into a 1.0 cm quartz cuvette using the dip cell and analyzed by DLS technique.
Inorganic phosphate determination
Liposomes of DODAC/PHO-S, 10/2 and 2/2 mM, were passed through a column of Sephadex G-25 (34×1.0 cm) to separate liposomes from free PHO-S in the solution, using sucrose 2.0 mM as eluent. Fractions of 1 mL were collected. It was not possible to separate the free and liposome-associated drug by ultracentrifugation, because the surface charge on the membrane does not allow liposome sedimentation. The concentration of PHO-S was quantified by determining the phosphate molecules, as described in the literature.17 Encapsulation efficiency (EE%) was calculated using the following formula: EE% = (Wt/Wi) ×100%, where Wt is the total amount of drug in the nanovesicle suspension and Wi is the total quantity of drug added initially during preparation.
Cell culture
The B16F10 murine melanoma cells (ATCC® CRL 6475) and human umbilical vein endothelial cells (HUVECs; ATCC® CRL 1730) were grown in Roswell Park Memorial Institute (RPMI) 1640 medium (Sigma-Aldrich Co.); for Hepa1c1c7 murine hepatocellular carcinoma, ATCC® CRL 2026 was used as the αMEM medium (LGC Biotecnologia, Cotia, SP, Brazil). The mediums were supplemented with 2 mM L-glutamine (Cultilab, Campinas, SP, Brazil), 10 mM HEPES (Cultilab), 24 mM sodium bicarbonate, 0.01% antibiotics, and 10% fetal bovine serum (Cultilab). Cells were cultivated in 5% CO2 atmosphere at 37°C as monolayer cultures. Cells were checked for viability using trypan blue exclusion test.
The B16F10 cells and HUVECs were used in this study in order to compare the effects of PHO-S and DODAC/PHO-S liposomes with those from other PHO-S studies, which were recently published and used these same cell lines.4–8 Hepa1c1c7 was added in this study with the aim of assessing the potential anticancer capability of DODAC/PHO-S liposomes and PHO-S in hepatocarcinoma cells that enables easy reproducibility for in vivo studies. Ethical approval for the use of human cells was not sought.
MTT assay
Cell viability was assessed by MTT assay. Cells were plated in 96-well plates, at a density of 2×104 cells/well, and incubated for 1 day at 37°C, in a humidified incubator at 5% CO2. The cell density reached 80%–90% confluence. The next day, cells were treated with PHO-S (0.3–2.0 mM), DODAC (10 mM)/PHO-S (0.3–2.0 mM), DODAC/PHO-S (1:1) (0.3–2.0 mM), and empty DODAC (0.3–2.0 mM). After 24 hours, cells were exposed to 5 mg/mL MTT for 3 hours and the formazan crystals were solubilized by addition of dimethyl sulfoxide (Sigma-Aldrich Co.). The absorbance was measured at 570 nm on a microplate reader (Thermo Plate; Rayto Life and Analytical Sciences Co., Ltd., Shenzhen, People’s Republic of China). Cytotoxicity was expressed as an IC50% (concentration that inhibits 50% of the growth of cells), which was determined from the concentration–response curve.
Cell cycle phase distribution
The B16f10 cells (1×105 cells/well, when the cell density reached 80%–90% confluence), treated with PHO-S (0.3–2.0 mM), DODAC/PHO-S (1:1) (0.3–2.0 mM), and empty DODAC (0.3–2.0 mM) for 24 hours, were washed with phosphate-buffered saline, resuspended in 1 mL cold GM (glucose 6.1 mM; NaCl 137 mM; KCl 4.4 mM; 14 Na2HPO4 1.5 mM; KH2PO4 0.9 mM; ethylenediaminetetraacetic acid 0.5 mM), and fixed by the addition of 3 mL of 70% ice-cold ethanol. Prior to analysis, cells were incubated with 1.8 μg/mL propidium iodide solution (Sigma-Aldrich Co.) in the dark for 30 minutes. Flow cytometric analysis was performed using BD Biosciences FACSCalibur flow cytometer (Becton Dickinson, San Jose, CA, USA); 10,000 events were collected. DNA content in the cell cycle phases (sub-haploid, G0/G1, S, and G2/M) was analyzed by ModFit LT 3.2 software (Becton Dickinson).
Scanning electron microscopy
Morphology of the liposomes of DODAC/PHO-S (1:1) (0.3–2.0 mM) and empty DODAC (0.3–2.0 mM) was analyzed by scanning electron microscopy (SEM). Aliquots of 5 μL of the liposomal formulation in a glass slides were incubated for 1 hour at 50°C. The samples were gold coated using a Balzers SCD 030 Sputter Coater (Balzers Union Ltd., Balzers, Liechtenstein) and visualized in an FEI Quanta 250 (FEI Company, Hillsboro, OR, USA) SEM.
Aliquots of melanoma B16F10 cells (5×104 cells/well, when the cell density reached 80%–90% confluence) were plated on sterile glass slides. After adhesion, cells were treated with PHO-S, empty DODAC, and DODAC/PHO-S (1:1) (0.3 and 2.0 mM). Then, the cells were fixed by adding 3% glutaraldehyde, incubated for 1 hour at 4°C, and post-fixed in 1% OsO4 for 1 hour at room temperature. After fixation, the cells were washed several times with 0.005 M sodium cacodylate, pH 7.2, and gradually passed through acetone solutions of increasing concentrations ranging from 30% to 100% for 10 minutes. Samples were completely dried in a critical-point drying apparatus using liquid CO2 as the exchange medium. Dehydrated specimens were mounted onto aluminum stubs, coated with carbon/gold and gold-coated using a Balzers SCD 030 Sputter Coater (Balzers Union Ltd.), and visualized in an FEI Quanta 250 (FEI Company) SEM.
Statistical analysis
All values were expressed as mean ± standard deviation (SD). Each value is the mean of at least three independent experiments in each group. One-way analysis of variance (ANOVA) and Tukey–Kramer multiple comparisons test was performed to identify differences among measurements of the groups studied. Graphics were obtained by Prism version 5.0 (CEO and Founder, La Jolla, CA, USA) and ModFit version 3.2 (Verity Software House, Topsham, ME, Estados Unidos) software. P-values <0.05, <0.01, and <0.001 are statistically significant.
Results
Liposome characterization
The hydrodynamic radii (Dh) of DODAC/PHO-S liposomes were determined immediately after the preparation (day 0) and at 1, 8, and 15 days to evaluate the physicochemical stability over time. Initially, the concentrations used were 0.3, 0.6, 1.0, 1.3, 1.6, and 2.0 mM of PHO-S and 10 mM of DODAC. In different liposome preparations, the concentrations of PHO-S and DODAC (0.3–2.0 mM; 1/1 molar ratio) were used. The DODAC (10 mM)/PHO-S (0.3 and 2.0 mM) had a mean Dh of 48.3±3.8 nm, at 0 day (day of preparation). After 15 days, the mean Dh for this liposomal formulation was 62.7±2.8 nm (Figure 1A). The 10 mM DODAC liposomes, without PHO-S, had an average Dh of 66.8±2.3 nm at 0 day, and after 15 days, the average was 80.8±4.5 nm (Figure 1A). The liposomes of DODAC/PHO-S (1:1) had an average Dh of 76.1±26.2 nm at 0 day. The value for the Dh of these liposomes after 15 days was 101.8±31.8 nm (Figure 1B). The Dh of DODAC (0.3–2.0 mM) liposomes, without PHO-S, were 88.8±7.2 and 80.2±7.6 nm, at 0 and 15 days, respectively (Figure 1C).
The zeta potential of DODAC/PHO-S liposomal formulation was determined at 0, 1, 8, and 15 days. The DODAC (10 mM)/PHO-S (0.3–2.0 mM) liposomes had a mean zeta potential of 59.5±5.4 and 40.0±9.4 mV at 0 day and after 15 days, respectively (Figure 1D). The 10 mM DODAC liposomes, without PHO-S, had a mean zeta potential of 65.0±2.3 mV at 0 day and 50.1±3.4 mV after 15 days (Figure 1D).
The DODAC/PHO-S (1:1) had a mean zeta potential of 55.9±7.5 mV at day 0 and 37.8±3.2 mV after 15 days (Figure 1E). The DODAC (0.3–2.0 mM) liposomes, without PHO-S had a mean zeta potential of 55.9±7.5 mV at 0 day and 42.7±3.3 mV after 15 days (Figure 1F).
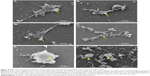
Morphology of DODAC/PHO-S liposomes by SEM
The image obtained by SEM provided an important parameter for evaluation of the morphology and arrangement of liposomes, corroborating the physical and chemical aspects obtained by DLS. In light of this, the SEM was used for morphological observation and distribution of these liposomes.
DODAC/PHO-S (1:1) and empty DODAC (0.3 and 2.0 mM) liposome preparations showed spherical morphology and repulsion due to surface charge. This liposomal formulation exhibited greater homogeneity when compared to the empty DODAC vesicles at the same molar ratio (Figure 2A and B). It was possible to observe large and small liposomes in the same sample. However, samples with 2.0 mM DODAC/PHO-S presented a pattern of smaller liposomes in relation to the 0.3 mM DODAC/PHO-S preparation (Figure 2C and D).
EE% of PHO-S
The PHO-S concentration was determined by measuring the inorganic phosphate in the preparation after sample digestion in perchloric acid (HClO4).14 The encapsulation of 2.0 mM PHO-S on 10 mM DODAC liposomes was 44.9% at 0 day (Figure 3A) and 44.7% after 40 days (Figure 3B). The liposomes of DODAC/PHO-S (1:1) (2.0 mM) had a mean encapsulation of 51.4% at 0 day (Figure 3C) and 49.6% after 40 days (Figure 3D).
Cytotoxic effects of PHO-S and DODAC/PHO-S on tumor and normal cells
B16F10 cells showed morphological changes after treatment with 0.3 and 2.0 mM of PHO-S, such as cytoplasmic retraction and formation of apoptotic bodies, resulting in cell adhesion loss. Cells treated with DODAC/PHO-S (1:1) liposomes exhibited larger morphological changes when compared to cells treated with PHO-S at the same molar ratio, such as cytoplasmic retraction, formation of precipitate, cell fragmentation, and formation of a large number of apoptotic bodies (Figure 4A–E). Cytotoxic effects of PHO-S, empty DODAC, and DODAC/PHO-S liposomes at different concentrations were evaluated 24 hours after treatment on B16F10 and Hepa1c1c7 cell lines by MTT assay. The results showed that, 24 hours after treatment, the PHO-S resulted in significant cytotoxicity at 1.3–2.0 mM on B16F10. The IC50% value to B16F10 was 4.4 mM (Figure 4F). DODAC (10 mM)/PHO-S (0.3–2.0 mM) liposomal formulation induced cytotoxic effects against B16F10 cells at all concentrations, resulting in IC50% of 1.2 mM (Figure 4G). Empty DODAC (10 mM) also resulted in significant cytotoxicity (Figure 4G). DODAC/PHO-S (1:1) liposomal formulation was cytotoxic at all concentrations, resulting in IC50% of 0.8 mM for this cell line (Figure 4H). The treatment with empty DODAC liposomes (0.3–2.0 mM) did not show significant cytotoxic effect (Figure 4I).
Hep1c1c7 cells treated with PHO-S and DODAC/PHO-S (1:1) (0.3–2.0 mM) showed similar morphological changes to B16F10, as described earlier (Figure 5A–E). The treatment with PHO-S resulted in significant cytotoxicity at 1.6 and 2.0 mM in this cell line, with an IC50% value of 2.9 mM (Figure 5F). The treatment with DODAC (10 mM)/PHO-S (0.3–2.0 mM) resulted in a significant decrease in cell viability and presented IC50% of 1 μM (Figure 5G). Empty DODAC (10 mM) also resulted in significant cytotoxicity (Figure 5G). The cells treated with DODAC/PHO-S (0.3–2.0 mM) had a significant decrease of 64.2%±12.7% in cell viability, already in the lower concentration (Figure 5H). Empty DODAC (0.3–2.0 mM) liposomes induced cytotoxic effects on Hepa1c1c7 cells, with a decrease of 41.6%±3.4% in cell viability, in the higher concentration (Figure 5I).
The cytological changes of the HUVECs treated with 0.3 and 2.0 mM PHO-S were discrete morphological changes and produced a lower number of apoptotic bodies. The treatment with DODAC/PHO-S (1:1) (0.3 and 2.0 mM) exhibited discrete precipitate formation and a lower number of apoptotic bodies (Figure 6A–E). The viability of HUVECs treated with PHO-S and DODAC/PHO-S liposomal formulation for 24 hours was also evaluated by MTT assay. Cells treated with 2.0 mM PHO-S did not induce significant cytotoxicity and reductions of 19.43%±5.9% in the number of viable cells (Figure 6F). The DODAC (10 mM)/PHO-S (2.0 mM) liposomal formulation reduced the number of viable cells by 57.5%±6.2% (Figure 6G). The cells treated with empty DODAC (10 mM) liposomes had a significant decrease of 30.2%±6.8%. On the other hand, the cytotoxic effects of DODAC/PHO-S (1:1) liposomal formulation were lower, reducing the number of viable cells by 27.9%±7.9% at the highest concentration (Figure 6H). The treatment with empty DODAC (0.3–2.0 mM) did not show significant cytotoxic effect (Figure 6I).
DODAC/PHO-S liposomal formulation induces cell cycle arrest in G2/M on B16F10 cells
In order to assess whether PHO-S and DODAC/PHO-S induce changes in the cell cycle of B16F10 cells, cell cycle phases were analyzed using flow cytometry. Cells were treated with PHO-S, as well as with empty DODAC and DODAC/PHO-S (1:1) (0.3 and 2.0 mM). The cell cycle analysis results are shown in Figure 7.
Treatment with empty DODAC (0.3 and 2.0 mM) and DODAC/PHO-S (1:1) (0.3 mM) also resulted in increased percentages of cells in the sub-G1 phase, with values of 28.3%±2.8%, 43.0%±5.6%, and 25.6%±2.6%, respectively. A significant decrease in the percentage of cells in G0/G1 phase was observed for all treatments compared to the control group.
The cells treated with 0.3 and 2.0 mM of PHO-S promoted a significant increase in the population of cells in S-phase, with a percentage of 9.8%±1.3% and 13.1%±1.4%, respectively. The PHO-S (0.3 and 2.0 mM) and DODAC/PHO-S (1:1) at 2.0 mM promoted a significant increase in the population of cells in the G2/M phase with an average percentage of 9.6%±1.4%, 14.4%±1.3%, and 20.5%±1.2%, respectively. The DODAC (10 mM)/PHO-S (0.3–2.0 mM) liposomes treatment promoted a decrease in cell populations in this phase. The treatment with empty DODAC liposomes (0.3–2.0 mM) did not promote an increase in cell populations in this phase.
Morphology of cells treated by SEM
B16F10 tumor cells treated with DODAC/PHO-S (1:1) (0.3 and 2.0 mM) liposomal formulation for 24 hours were evaluated to assess morphological changes and interaction with liposome by SEM. The lipid aggregate remained affixed to the cell surface and maintained spherical morphology characteristic of the liposome. Lipid aggregates spread in the culture medium were also observed. B16F10 cells showed morphological changes after DODAC/PHO-S treatment, such as “blebs” formation, cell detachment, cytoplasmic retraction, and apoptotic bodies (Figure 8A–F).
Discussion
Recent important advances in employing nanotechnology for drug delivery have provided important strategies to overcome limitations, such as solubility in biological fluids, physical and chemical stability, bioavailability for the targeted tissues, and toxicity to normal cells.18,19 Among these systems for drug delivery, the DODAC has several advantages as a carrier of PHO-S. First, the results showed that DODAC molecules interact with the PHO-S in aqueous solution after being subjected to phase transition temperature and subsequent sonication. This afforded a liposomal DODAC/PHO-S formulation, which was stable for up to 15 days after the preparation at room temperature.
The results found evidence that size and zeta potential of DODAC/PHO-S liposomes varied in the assessed days (1–15 days), as well as in the different concentrations of the compounds used in the formulation. However, these variations did not show any significant correlation with valuation day and concentrations used, both from DODAC and PHO-S. The system is not fully stable and interactions between the different molecules occur constantly. For example, the number of water molecules that hydrate the lipid heads and the power of the water–lipid interactions strongly influence the formation and properties of liposomes.
Although there was no significant correlation between the concentration of PHO-S with the diameter and zeta potential of the vesicles, DODAC liposomes were effective in the PHO-S encapsulation with an encapsulation average rate >50% and with small reductions after 40 days.
Sonication is perhaps the most extensively used method for the preparation of small unilamellar vesicles. The main disadvantages of this method are very low internal volume/encapsulation efficacy and some compounds such as DNA are degraded during sonication.18,19 Systems for DNA delivery, known as stabilized plasmid lipid particles, consist of plasmid DNA encapsulated within a lipid bilayer composed of dioleoylphosphatidylethanolamine, a cationic lipid (usually DODAC), and polyethylene glycol ceramide. Stabilized plasmid lipid particles are formed by a procedure in which mixtures of plasmid and lipid are cosolubilized at a specific ionic strength by the detergent octyl-glucopyranoside, which is then removed by dialysis. The particles are purified by sucrose density gradient centrifugation.18,19 When conditions are optimized, high plasmid encapsulation efficiencies are achieved (50%–70%).19 Therefore, the EE% of the PHO-S (50%) by DODAC was considered optimal. In another liposomal formulation, researchers proposed the use of a negatively charged liposomal fluid to encapsulate an oligonucleotide “antisense” (anti-β-galactosidase), as well as the plasmid “pUC18.”20 In the study, the liposomes were formulated with synthetic phospholipids dipalmitoylphosphatidylcholine, and dimyristoyl phosphatidylglycerol in a molar ratio of 10:1, in the presence of a solution of a monovalent salt (NaCl, KCl, NaHCO3, or phosphate buffer), and the rate of encapsulation and activity “antisense” were measured by Escherichia coli CSH36. As a result, levels of encapsulation of 48.9% and 43.5% were found in the purified plasmid and oligonucleotide “antisense,” respectively.20 Other studies showed that, in dioctadecyldimethylammonium bromide bilayers, the preference of the monomeric amphotericin B was for the gel phase to be incorporated by 40%, compared to 23% in the fluid phase.21
As in the DODAC/PHO-S liposomal formulation, the concentrations of PHO-S are not greater than the DODAC concentration. It is not possible to observe a direct correlation between the sizes of these formulations with the molar mass of the PHO-S. This may be due to the number of water molecules that hydrate the lipid heads and the power of the water–lipid interactions, which strongly influence the formation and properties of liposomes. Hydration forces cause an energy barrier that prevents the approach of two liposomes.22,23 However, it was observed by SEM and through the DLS that the liposomes with DODAC/PHO-S (1:1) (2.0 mM) tend to form liposomes with smaller diameters when compared to the lower concentration liposomes. The sizes of the liposomes DODAC/PHO-S, ranging between 60 and 100 nm, are ideal for antitumoral therapy because the vascular endothelium of healthy tissues is formed by fenestrations from 5 to 10 nm, whereas the neovascularization of tumors exhibits much larger fenestrations (100–780 nm).24,25 Fenestrations in liver sinusoids are well in excess of the 5–10 nm, with diameters in size range between 50 and 150 nm.24,25 Therefore, DODAC/PHO-S nanoparticles with an average size of 100–200 nm are able to permeate the wider fenestration of tumor vessels, but they do not penetrate the majority of the narrow fenestrated endothelium of healthy tissue, resulting in greater accumulation of the nanoparticles, especially in tumor tissues.26–28
Confirming the data, PHO-S was cytotoxic to all tumor cell lines, without promoting significant cytotoxic effects on endothelial cells (HUVECs). As already described by our group, our data supported the hypothesis that the sensitivity and the IC50% values obtained from the tumor cells were irrespective of the molecular profile, such as resistance and aggressiveness.4–8 These results have been confirmed in several cell lines and more recently in KG-1 (human myeloid), K562 (human erythromyeloblastoid leukemia), and Jurkat (human T-cell leukemia).6
The formulation with cationic amphiphilic DODAC demonstrated efficiency for PHO-S delivery, with the lowest values of IC50% for all the tumor cells compared with those that were treated with free PHO-S. Assuming that chemotherapy should be selective for tumor cells, the high cytotoxic effect of DODAC (10 mM)/PHO-S (0.3 and 2.0 mM) liposomal formulation on HUVECs precludes its use at this concentration. However, the DODAC/PHO-S (1:1) (0.3 and 2.0 mM) liposomal formulation promoted more effective cytotoxicity in tumor cell lines, without significant effects on normal cells.
The Hepa1c1c7 cells showed greater sensitivity to the DODAC/PHO-S formulation when compared to B16F10 cells and HUVECs. Several studies showed that cationic liposomal formulations can accumulate in the parenchyma of the lung, liver, and spleen.29 This accumulation can be explained by the fact that these systems require high concentrations of the complexes at the therapeutic dose.29 A well-known target of the liposomes is the liver, because many low-density lipoprotein (LDL), high-density lipoprotein (HDL), and asialoglycoprotein receptors are expressed on the surface of hepatocytes.13 The HDLs and LDLs show high affinity for accumulating in tumor cells, and for this reason they are used as carriers for anticancer drug delivery for the treatment of hepatocellular carcinoma.13,29–31 Studies showed that anticancer drugs using HDL and LDL are great target carriers on tumor cells, which show a high activity for lipoprotein receptors in animal models.29–33 We demonstrated earlier that the higher level of LDL receptor expression had been ascribed to the accelerated mitosis rates that are pivotal in cancer pathophysiology.34 Anticancer complexes with HDL and LDL do not affect the characteristic of the drug.32,35 Therefore, the higher sensitivity of the Hepa1c1c7 cells to treatment with the liposomal formulation may be correlated to the interaction of the DODAC with lipid receptors of these cells, enabling greater availability of the DODAC/PHO-S in the cell membrane and resulting in a larger action of the PHO-S in these cells.
The data obtained demonstrated that the use of the DODAC as a carrier can maximize the availability of the PHO-S in the cell membrane and consequently maximize cytotoxicity potential in tumor cells. Recent studies conducted by our group have shown that the PHO-S inhibited the proliferation and migration, leading to accumulation of cells in G2/M phase.5 The evaluation of the cell cycle phases of B16F10 cells demonstrated that the cytotoxic effects of the DODAC/PHO-S formulation at the concentration of 2.0 mM are mediated by PHO-S. It was observed that the use of the DODAC (2.0 mM) as a carrier of the PHO-S (2.0 mM) promoted a G2/M-phase cell cycle arrest with proportion significantly greater than the treatment with PHO-S. However, the 0.3 mM DODAC/PHO-S (1:1) liposomal formulation did not provide this effect. As the concentrations of the DODAC and the PHO-S used were proportional in this formulation – ie, the same molar ratio – the influence of the concentration of the number of water molecules that hydrate the lipid heads and the power of the water–lipid interaction may have strongly influenced the formation and the property of the liposomes 0.3 mM DODAC/PHO-S (1:1), as described earlier.22,23 The treatments performed with DODAC only provided an increase in the number of cells in the sub-G1 phase but not in the G2/M phase, demonstrating again the nonspecific effect of the DODAC in causing toxicity. Nonetheless, when comparing the PHO-S associated with the carrier, there was an increase in the S and G2/M phases. The PHO-S can reduce the nonspecific cytotoxicity of the carrier, providing target-specific antitumor effect.5,8
The morphological analysis of cells by SEM showed that melanoma murine B16F10 cells die after the activation of pro-apoptotic mechanisms. The changes in the plasma membrane during apoptosis are mainly of a biochemical nature, with no involvement of morphological changes except for the loss of microvilli and consequent cytoplasmic retraction, which was observed in cells treated with the liposomal formulation. These were the key features of apoptosis. The presence of the lipid aggregates adhered to the membrane of the B16F10 cells, as well as spread in the culture medium after 24 hours of exposure, which can promote the maintenance of cytotoxic activity. The results show that the electrostatic interaction between DODAC and PHO-S, DODAC and PHO-S, and cell membrane can maximize the antitumor effects mediated by PHO-S. Thus, the set of results obtained in vitro demonstrate that DODAC/PHO-S liposomal formulation presents great potential for the treatment of melanoma and hepatocellular carcinoma.
Conclusion
The liposomal formulation with cationic amphiphilic DODAC was effective as PHO-S delivery agents, considering that IC50% values for tumor cells treated with this liposome were significantly lower compared with PHO-S treatment. DODAC liposomes involved in the delivery of PHO-S promoted cytotoxicity more selectively and effectively against murine B16F10 melanoma and Hepa1c1c7 hepatocellular carcinoma. Thus, the DODAC/PHO-S liposomal formulation presents vast potential for preclinical studies.
Acknowledgment
This study was funded by São Paulo Research Foundation, process number: 2015/02950-1.
Disclosure
The authors report no conflicts of interest in this work
References
Ríos-Marco P, Jiménez-López JM, Marco C, Segovia JL, Carrasco MP. Antitumoral alkylphospholipids induce cholesterol efflux from the plasma membrane in HepG2 cells. J Pharmacol Exp Ther. 2011;336(3):866–873. | ||
Van Blitterswijk WJ, Verheij M. Anticancer alkylphospholipids: mechanisms of action, cellular sensitivity and resistance, and clinical prospects. Curr Pharm Des. 2008;14(21):2061–2074. | ||
Van Blitterswijk WJ, Verheij M. Anticancer mechanisms and clinical application of alkylphospholipids. Biochim Biophys Acta. 2013;1831(3):663–674. | ||
Ferreira AK, Meneguelo R, Neto SC, Chierice GO, Maria DA. Synthetic phosphoethanolamine induces apoptosis through caspase-3 pathway by decreasing expression of bax/bad protein and changes cell cycle in melanoma. J Cancer Sci Ther. 2011;3(1):53–59. | ||
Ferreira AK, Meneguelo R, Marques FL, et al. Synthetic phosphoethanolamine a precursor of membrane phospholipids reduce tumor growth in mice bearing melanoma B16-F10 and in vitro induce apoptosis and arrest in G2/M phase. Biomed Pharmacother. 2012;66(7):541–548. | ||
Ferreira AK, Santana-Lemos BA, Rego EM, Filho OM, Chierice GO, Maria DA. Synthetic phosphoethanolamine has in vitro and in vivo anti-leukemia effects. Br J Cancer. 2013;109(11):2819–2828. | ||
Ferreira AK, Freitas VM, Levy D, et al. Anti-angiogenic and anti-metastatic activity of synthetic phosphoethanolamine. PLoS One. 2013;8(3):e57937. | ||
Ferreira AK, Meneguelo R, Pereira A, Mendonça Filho O, Chierice GO, Maria DA. Anticancer effects of synthetic phosphoethanolamine on Ehrlich ascites tumor: an experimental study. Anticancer Res. 2012;32(1):95–104. | ||
Jiménez-López JM, Ríos-Marco P, Marco C, Segovia JL, Carrasco MP. Alterations in the homeostasis of phospholipids and cholesterol by antitumor alkylphospholipids. Lipids Health Dis. 2010;25:9–33. | ||
Dorlo TP, Balasegaram M, Beijnen JH, De Vries PJ. Miltefosine: a review of its pharmacology and therapeutic efficacy in the treatment of leishmaniasis. J Antimicrob Chemother. 2012;67(11):2576–2597. | ||
Thakur A, Joshi N, Shanmugam T, Banerjee R. Proapoptotic miltefosine nanovesicles show synergism with paclitaxel: implications for glioblastoma multiforme therapy. Cancer Lett. 2013;334(2):274–283. | ||
Oliveira AC, Martens TF, Raemdonck K, et al. Dioctadecyldimethylammonium: monoolein nanocarriers for efficient in vitro gene silencing. ACS Appl Mater Interfaces. 2014;6(9):6977–6989. | ||
El-aneed A. An overview of current delivery systems in cancer gene therapy. J Control Release. 2004;94(1):1–14. | ||
Carmona-Ribeiro AM. Bilayer-forming synthetic lipids: drugs or carriers? Curr Med Chem. 2003;10(22):2425–2446. | ||
Shim G, Kim M, Park JY, Oh YK. Application of cationic liposomes for delivery of nucleic acids. Asian J Pharm Clin Res. 2013;8(2):72–80. | ||
Ho EA, Ramsay E, Ginj M, et al. Characterization of cationic liposome formulations designed to exhibit extended plasma residence times and tumor vasculature targeting properties. J Pharm Sci. 2010;99(6):2839–2853. | ||
Manzini MC, Perez KR, Riske KA, et al. Peptide:lipid ratio and membrane surface charge determine the mechanism of action of the antimicrobial peptide BP100. Conformational and functional studies. Biochim Biophys Acta. 2014;1838(7):1985–1999. | ||
Akbarzadeh A, Rezaei-Sadabady R, Davaran S, et al. Liposome: classification, preparation, and applications. Nanoscale Res Lett. 2013;8(1):102. | ||
Fenske DB, Chonn A, Cullis PR. Liposomal nanomedicines: an emerging field. Toxicol Pathol. 2008;36(1):21–29. | ||
Fillion P, Desjardins A, Sayasith K, Lagacé J. Encapsulation of DNA in negatively charged liposomes and inhibition of bacterial gene expression with fluid liposome-encapsulated antisense oligonucleotides. Biochim Biophys Acta. 2001;1515(1):44–54. | ||
Oliveira TR, Benatti CR, Lamy MT. Structural characterization of the interaction of the polyene antibiotic Amphotericin B with DODAB bicelles and vesicles. Biochim Biophys Acta. 2011;1808(11):2629–2637. | ||
Hauser H. Nucleic acids, peptides, and proteins. In: Franks F, editor. Water, a Comprehensive Treatise. New York: Plenum Press; 1975:209–303. | ||
Hauser H. Water/phospholipid interactions. In: Duckworth RB, editor. Water Relations of Foods. London: Academic Press; 1975:37–41. | ||
Fang J, Nakamura H, Maeda H. The EPR effect: unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect. Adv Drug Deliv Rev. 2011;63(3):136–151. | ||
Taurin S, Nehoff H, Greish K. Anticancer nanomedicine and tumor vascular permeability; Where is the missing link? J Control Release. 2012;164(3):265–275. | ||
Hyodo K, Yamamoto E, Suzuki T, Kikuchi H, Asano M, Ishihara H. Development of liposomal anticancer drugs. Biol Pharmaceut Bull. 2013;36(5):703–707. | ||
Oliveira LC, Taveira EJF, Souza LG, Marreto RN, Lima EM, Taveira SF. [Applications of lipid nanoparticles in the treatment of solid tumors: review of literature]. Aplicações das Nanopartículas Lipídicas no Tratamento de Tumores Sólidos: Revisão de Literatura. Rev Bras Cancerol. 2012;58(4):695–701. Portugese. | ||
Trédan O, Galmarini CM, Patel K, Tannock IF. Drug resistance and the solid tumor microenvironment. J Natl Cancer Inst. 2007;99(19):1441–1454. | ||
Morille M, Passirani C, Vonarbourg A, Clavreul A, Benoit JP. Progress in developing cationic vectors for non-viral systemic gene therapy against cancer. Biomaterials. 2008;29(24–25):3477–3496. | ||
Tokui T, Takatori T, Shinozaki N, et al. Delivery and cytotoxicity of RS-1541 in St-4 human gastric cancer cells in vitro by the low-density-lipoprotein pathway. Cancer Chemother Pharmacol. 1995;36(1):1–6. | ||
Versluis AJ, Van Geel PJ, Oppelaar H, Van Berkel TJ, Bijsterbosch MK. Receptor-mediated uptake of low-density lipoprotein by B16 melanoma cells in vitro and in vivo in mice. Br J Cancer. 1996;74(4):525–532. | ||
Masquelier M, Vitols S, Peterson C. Low-density lipoprotein as a carrier of antitumoral drugs: in vivo fate of drug-human low-density lipoprotein complexes in mice. Cancer Res. 1986;46(8):3842–3847. | ||
Moura JA, Valduga CJ, Tavares ER, Kretzer IF, Maria DA, Maranhão RC. Novel formulation of a methotrexate derivative with a lipid nanoemulsion. Int J Nanomedicine. 2011;6(1):2285–2295. | ||
Contente TC, Kretzer IF, Filippin-Monteiro FB, Maria DA, Maranhão RC. Association of daunorubicin to a lipid nanoemulsion that binds to low-density lipoprotein receptors enhances the antitumour action and decreases the toxicity of the drug in melanoma-bearing mice. J Pharm Pharmacol. 2014;66(12):1698–1709. | ||
Kretzer IF, Maria DA, Maranhão RC. Drug-targeting in combined cancer chemotherapy: tumor growth inhibition in mice by association of paclitaxel and etoposide with a cholesterol-rich nanoemulsion. Cell Oncol. 2012;35(6):451–460. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.