Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 17
Postoperative Pyoderma Gangrenosum in a Patient Presenting with Acute Peripheral Artery Disease Secondary to Antiphospholipid Syndrome: A Case Report
Authors Wei H, Wang K, Huang W, Liu Y
Received 25 November 2023
Accepted for publication 6 February 2024
Published 19 February 2024 Volume 2024:17 Pages 451—455
DOI https://doi.org/10.2147/CCID.S451771
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Jeffrey Weinberg
Haijun Wei,* Ke Wang,* Wei Huang, Yang Liu
Department of Vascular Surgery, Hospital of Chengdu University of Traditional Chinese Medicine, Chengdu, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yang Liu, Department of Vascular Surgery, Hospital of Chengdu University of Traditional Chinese Medicine, Chengdu, People’s Republic of China, Email [email protected]
Abstract: Pyoderma gangrenosum (PG) is a rare autoinflammatory neutrophilic dermatosis clinically characterized by painful nodules, red papules or plaques that rapidly erode into ulcers. We report a 53-year-old febrile male patient with acute peripheral arterial disease who underwent transtibial amputation after failed thrombolysis. Five days after amputation, an ulcer developed around the cannulation site of the right internal jugular vein that was indicative of pathergy. The patient’s fever did not improve after surgery, and purpuric discoloration and punctate ulcers of the skin near the amputation site became apparent, leading to re-debridement. Finally, consultation with a dermatologist raised the possibility of postoperative PG, and additional laboratory tests revealed positive anticardiolipin autoantibodies consistent with antiphospholipid syndrome. The patient was treated with intravenous glucocorticosteroids and antibiotics, and the amputation wound and cannulation site ulcer were found to have healed at the 2-month follow-up. The current report raises the need for vascular surgeons to be aware of this uncommon etiology of arterial thrombosis, and the postoperative appearance of dermatosis and pathergy should alert for PG.
Keywords: antiphospholipid syndrome, case report, peripheral artery disease, pyoderma gangrenosum
Background
Pyoderma gangrenosum (PG) is a relatively rare autoinflammatory neutrophilic dermatosis clinically characterized by painful nodules, red papules or plaques that rapidly erode into ulcers with violaceous undermined border and a purulent base, most commonly on the lower extremities.1 Epidemiological studies that PG typically occurs in mid-40s with an incidence of appropriately 6 cases per million person-years and a mortality rate of 3.2% for those hospitalized.2 Although the exact pathogenesis of PG remains poorly defined, clinical observations noted that more than half of patients with PG have an associated systemic co-morbidities, most commonly inflammatory bowel disease, inflammatory arthritis, and hematologic malignancies.3 Early recognition and prompt multidisciplinary management involving dermatologists, plastic surgeons and immunologists is often required to avoid adverse outcomes. Early interventions with appropriate wound care and fast-acting immunosuppressive drugs (glucocorticosteroids and/or cyclosporine) or biological agents have been shown to decrease patient morbidity and mortality.4 Pathergy, an exaggerated skin injury after minor trauma, has been shown to be present in 32% of PG cases.5 Unfortunately, the diagnosis of pyoderma gangrenosum is quite challenging as no definitive diagnostic criteria have been established and the diagnosis is often based on skin biopsy to exclude other common pathologies. Herein, we report a case of post-operative pyoderma gangrenosum following amputations in a patient with antiphospholipid syndrome presenting with peripheral artery disease. Our case highlighted that lack of awareness of this rare condition may lead to misdiagnosis and repeated surgeries that may incur additional harms for the patient by vascular surgeons.
Case Report
A 53-year-old male was admitted to our hospital for skin ulceration on the left foot with febrile (39°C) for over a week (Figure 1A). Physical examination revealed a blood pressure of 70/30mmHg, elevated skin temperature and blackish nonviable tissue consistent with gangrene in his left foot. Laboratory tests indicated an elevated white blood cell count of 50.47×109/L with a neutrophil ratio of 97.2% and a C-reactive protein level of 267.7 mg/L. Digital subtraction angiography demonstrated patent superficial femoral, posterior tibial, and distal peroneal arteries, with occlusion of the tibiofibular trunk, anterior tibial artery below the knee, and popliteal artery (Figure 1 B-D). The patient was diagnosed with septic shock related to lower extremity gangrene caused by arterial thrombosis. Following failed thrombolysis, the patient underwent an emergency amputation of the lower limb, followed by administration of low-molecular-weight heparin and intravenous antibiotics.
Patient febrility did not improve after the operation, and purplish discoloration and punctate ulcers of the skin near the amputation site became apparent (Figure 2A). Five days after the amputation, an ulcer developed around the cannulation site of the right internal jugular vein (Figure 2B). In addition, the ulcer continued to expand despite removal of the central venous line. We at that time believed that the patient’s persistent fever was caused by the infection of necrotic tissue remnants that were not completely debrided during the amputation procedure. In response, we performed a thorough wound debridement following the amputation. However, the patient’s hyperthermia still did not resolve postoperatively, even though we conducted multiple blood cultures, all of which turned out to be negative. Postoperative pathologic examinations revealed numerous neutrophil infiltrates with skeletal muscle degeneration (Figure 2C).
A multidisciplinary consultation was requested, and the dermatology specialist suggested that the ulcer around the cannulation site might be a pathergy indicative of pyoderma gangrenosum. After receiving intravenous glucocorticosteroids, the patient’s body temperature returned to normal, and the ulcers and papules at the amputation and cannulation sites began to heal. Subsequent laboratory tests revealed an IgA anticardiolipin antibody titer of 32.80 APLU/mL and an IgA anti-β2 glycoprotein I antibody titer of 84.10 AU/mL. The patient was ultimately diagnosed with post-operative pyoderma gangrenosum following amputations for lower extremity gangrene secondary to antiphospholipid syndrome. At the 2-month post-discharge follow-up, the patient’s amputation wound and cannulation site ulcer were found to have healed (Figure 3).
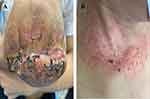 |
Figure 3 Follow-up findings. (A and B) The amputation site and the cannulation site at the right internal jugular vein have healed at 2-months follow-up. |
Discussion
This case highlights that vascular surgeons should be aware of uncommon etiologies for lower extremity thrombosis, and the postoperative appearance of dermatosis and pathergy should raise the possibility of pyoderma gangrenosum.
The clinical manifestations, laboratory tests and even the histopathologic findings of pyoderma gangrenosum are all non-specific. The Su criteria proposed in 2004 required the exclusion of all other possible diagnoses, which is apparently impractical in the clinical setting.6 Subsequently, the Delphi consensus proposed that in addition to a biopsy of the ulcer margin showing neutrophil infiltrates, at least 3 of the following 8 minor criteria must be present for the diagnosis of PG: 1) pathergy; 2) exclusion of infection; 3) peripheral erythema; 4) multiple ulcerations; 5) presence of papules, pustules, or vesicles that rapidly ulcerate; 6) presence of co-morbidities, such as inflammatory bowel disease or arthritis; 7) cribriform scar at sites of healed ulcers; 8) treatment response to immunosuppressive agents or glucocorticosteroids.7 Of note, the PARACELSUS score was developed specifically to discriminate leg venous ulcers from PG.8
In the current case, the patient was afflicted with antiphospholipid syndrome, an autoimmune disorder characterized by the presence of circulating antiphospholipid antibodies that cause venous or arterial thrombotic events. Interestingly, there have been few studies in the literature reporting PG in patients with antiphospholipid syndrome. For example, Schmid et al reported a fulminant case of PG of the right shank presenting with 2 non-healing ulcers in a 64-year-old woman with antiphospholipid syndrome secondary to systemic lupus erythematosus.9 A similar case of PG presenting as leg ulcers was also reported by Choi et al.10
Vascular surgeons may encounter patients with pyoderma gangrenosum, as its association with antiphospholipid syndrome is often accompanied by signs and symptoms of arterial ischemia and venous occlusion. In addition, a special form of PG- postoperative PG- may occur following vascular interventions, as demonstrated in the present case. Most of the prior cases of PG have been reported in the dermatology and burns journals, so vascular surgeons are unfamiliar with this rare condition. The postoperative fever and punctate ulcers led the authors to perform additional operations, highlighting the importance of correct and timely diagnosis. In the largest series of postoperative PG from the Mayo Clinic, Tolkachjov et al found that 83% of patients were female, the most common sites were the breasts and abdomen, and the average time to symptoms was 11 days.11
Glucocorticosteroids and immunosuppressive agents are the mainstay of treatments for PG. For limited cases, local interventions with topical glucocorticosteroids or calcineurin inhibitors may be sufficient.12 However, for extensive or rapidly progressive PG, systemic treatments with glucocorticosteroids, cyclosporine or even biologic agents may be required. Consistent with prior studies reporting favorable outcomes in all 8 patients with PG secondary to antiphospholipid syndrome treated with systemic glucocorticosteroids, warfarin and intravenous cyclosporine,13 the present patient also responded well to systemic glucocorticosteroids. It should be noted that appropriate wound care, such as cleansing and dressing, negative pressure wound therapy and even surgical debridement, are also important aspects of PG management. For instance, the systematic review by Almeida et al showed improvement of wound healing with the use of negative pressure wound therapy in 85.1% of the patients studied.14
Conclusion
In conclusion, we report a case of postoperative PG in a patient with antiphospholipid syndrome presenting with acute peripheral artery disease. The current report raises the need for vascular surgeons to be aware of this uncommon etiology of arterial thrombosis, and the postoperative appearance of dermatosis and pathergy should alert for PG.
Ethics Statement
The patient provided written informed consent for publication of this report and accompanying images. The Hospital Ethics Committees of the Hospital of Chengdu University of Traditional Chinese Medicine approved publishing the case details.
Funding
There is no funding to report.
Disclosure
The authors have no conflicts of interest to declare for this work.
References
1. Maronese CA, Pimentel MA, Li MM, Genovese G, Ortega-Loayza AG, Marzano AV. Pyoderma gangrenosum: an updated literature review on established and emerging pharmacological treatments. Am J Clin Dermatol. 2022;23(5):615–634. doi:10.1007/s40257-022-00699-8
2. Maverakis E, Marzano AV, Le ST, et al. Pyoderma gangrenosum. Nat Rev Dis Primers. 2020;6(1):81. doi:10.1038/s41572-020-0213-x
3. Ye MJ, Ye JM. Pyoderma gangrenosum: a review of clinical features and outcomes of 23 cases requiring inpatient management. Dermatol Res Pract. 2014;2014:461467. doi:10.1155/2014/461467
4. Langan SM, Groves RW, Card TR, Gulliford MC. Incidence, mortality, and disease associations of pyoderma gangrenosum in the United Kingdom: a retrospective cohort study. J Invest Dermatol. 2012;132(9):2166–2170. doi:10.1038/jid.2012.130
5. Binus AM, Qureshi AA, Li VW, Winterfield LS. Pyoderma gangrenosum: a retrospective review of patient characteristics, comorbidities and therapy in 103 patients. Br J Dermatol. 2011;165(6):1244–1250. doi:10.1111/j.1365-2133.2011.10565.x
6. Su WP, Davis MD, Weenig RH, Powell FC, Perry HO. Pyoderma gangrenosum: clinicopathologic correlation and proposed diagnostic criteria. Int J Dermatol. 2004;43(11):790–800.
7. Maverakis E, Ma C, Shinkai K, et al. Diagnostic criteria of ulcerative pyoderma gangrenosum: a delphi consensus of international experts. JAMA Dermatol. 2018;154(4):461–466. doi:10.1001/jamadermatol.2017.5980
8. Jockenhöfer F, Wollina U, Salva KA, Benson S, Dissemond J. The PARACELSUS score: a novel diagnostic tool for pyoderma gangrenosum. Br J Dermatol. 2019;180(3):615–620.
9. Schmid MH, Hary C, Marstaller B, Konz B, Wendtner CM. Pyoderma gangrenosum associated with the secondary antiphospholipid syndrome. Eur J Dermatol. 1998;8(1):45–47.
10. Choi YJ, Yoo WH. Pyoderma gangrenosum masquerading as an ulcer related to antiphospholipid antibodies in a patient with systemic lupus erythematosus. Lupus. 2018;27(13):2174–2176. doi:10.1177/0961203318793705
11. Tolkachjov SN, Fahy AS, Wetter DA, et al. Postoperative pyoderma gangrenosum (PG): the mayo clinic experience of 20 years from 1994 through 2014. J Am Acad Dermatol. 2015;73(4):615–622. doi:10.1016/j.jaad.2015.06.054
12. Thomas KS, Ormerod AD, Craig FE, et al. Clinical outcomes and response of patients applying topical therapy for pyoderma gangrenosum: a prospective cohort study. J Am Acad Dermatol. 2016;75(5):940–949. doi:10.1016/j.jaad.2016.06.016
13. Cañas CA, Durán CE, Bravo JC, Castaño DE, Tobón GJ. Leg ulcers in the antiphospholipid syndrome may be considered as a form of pyoderma gangrenosum and they respond favorably to treatment with immunosuppression and anticoagulation. Rheumatol Int. 2010;30(9):1253–1257. doi:10.1007/s00296-010-1418-1
14. Almeida IR, Coltro PS, Gonçalves HOC, et al. The role of negative pressure wound therapy (NPWT) on the treatment of pyoderma gangrenosum: a systematic review and personal experience. Wound Repair Regen. 2021;29(3):486–494. doi:10.1111/wrr.12910
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


