Back to Journals » Journal of Pain Research » Volume 13
Postoperative Analgesia in Neonates and Infants Using Epidural Chloroprocaine and Clonidine
Authors Gibbs A , Kim SS , Heydinger G , Veneziano G , Tobias J
Received 11 September 2020
Accepted for publication 7 October 2020
Published 30 October 2020 Volume 2020:13 Pages 2749—2755
DOI https://doi.org/10.2147/JPR.S281484
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Michael Schatman
Anna Gibbs,1 Stephani S Kim,2 Grant Heydinger,2,3 Giorgio Veneziano,2,3 Joseph Tobias2,3
1The Ohio State University College of Medicine, Columbus, OH, USA; 2Department of Anesthesiology & Pain Medicine, Nationwide Children’s Hospital, Columbus, OH, USA; 3Department of Anesthesiology & Pain Medicine, The Ohio State University College of Medicine, Columbus, OH, USA
Correspondence: Giorgio Veneziano
Nationwide Children’s Hospital, Columbus, OH 43205, USA
Tel +1 (614) 722-4209
Fax +1 (614) 722-4203
Email [email protected]
Introduction: In neonates and infants, epidural analgesia has gained popularity as a means of providing postoperative analgesia, limiting opioid-related adverse effects and improving the postoperative course. In addition to a local anesthetic agent, adjunctive agents may be added to further augment analgesia. Clonidine is an α2-adrenergic agonist that is frequently added to single-shot caudal analgesia, but there are limited data regarding its use in a continuous epidural infusion, especially in patients ≤ 12 months of age.
Methods: We retrospectively reviewed the hospital records of neonates and infants who received postoperative epidural infusions with 2-chloroprocaine, and clonidine was identified over a 4-year period.
Results: The study cohort included 52 neonates and infants ranging in age from 0 to 12 months and in weight from 2.1 to 10.1 kilograms. The catheters were dosed with either 1.5% 2-chloroprocaine (n=47) or 3% 2-chloroprocaine (n=5) with clonidine (median concentration 0.2 μg/mL) infused at a median rate of 0.72 mL/kg/hour. Pain scores were uniformly ≤ 3 at all evaluation points during the first 72 postoperative hours with a limited need for supplemental systemic opioids. No serious adverse effects were noted.
Conclusion: With the recognized limitations of a retrospective study, these preliminary data demonstrate the safety of adding clonidine to an epidural infusion of 2-chloroprocaine in neonates and infants less than 12 months of age. Future studies are needed to determine its analgesic efficacy compared to 2-chloroprocaine alone and the optimal clonidine concentration for postoperative epidural infusions.
Keywords: chloroprocaine, epidural analgesia, clonidine, neonatal anesthesia
Introduction
Regional anesthesia has been shown to be a safe and effective option for intraoperative and postoperative analgesia in neonates, infants, and children.1 In neonates and infants, epidural analgesia has gained popularity as a means of limiting postoperative opioid-related adverse effects, facilitating earlier tracheal extubation, and encouraging the resumption of normal gastrointestinal function.1–3 Although bupivacaine and ropivacaine remain the most commonly used local anesthetic agents for postoperative epidural infusions in infants, clinical concerns exist regarding the potential for local anesthetic systemic toxicity (LAST) with these agents.4–8 Given these concerns, there has been expanding use of 2-chloroprocaine especially for continuous postoperative epidural infusions in neonates and infants.4,9–11 Chloroprocaine is an ester that undergoes rapid metabolism by plasma cholinesterases with a serum half-life of approximately 60 seconds.12
In addition to local anesthetic agents, adjunctive agents such as opioids, ketamine, or clonidine may be added to epidural infusions to potentiate analgesia and limit the infusion requirements for local anesthetic agents.13–15 Clonidine is commonly added to local anesthetic agents for single-shot caudal blockade in infants and children; however, there remains limited data on its addition to solutions for continuous epidural infusions in neonates and infants.15 We retrospectively reviewed our experience with the addition of clonidine to 2-chloroprocaine for continuous postoperative infusions in neonates and infants.
Methods
This study was approved by the Institutional Review Board of Nationwide Children’s Hospital (STUDY00000544) and conducted in accordance with the regulations of the Declaration of Helsinki for research involving human subjects. As a large retrospective cohort study, it was deemed to have no more than minimal risk. With the use of de-identified data for publication, the need for informed consent was waived. Neonates and infants who received postoperative epidural infusions with 2-chloroprocaine and clonidine were identified over the time period from January 2015 to August 2019. The institutional electronic medical record was reviewed for these patients and data regarding each patient’s surgery, anesthesia, and postoperative course was obtained.
Data collected included the following patient demographic characteristics: age at time of procedure, gestational age, height, weight, gender, race, and ethnicity. Surgical data collected included the primary diagnosis, surgical procedure, American Society of Anesthesiologists (ASA) physical status, and length of surgery. Epidural data collection consisted of location of epidural catheter placement, whether the epidural infusion was started intraoperatively or postoperatively, length of endotracheal intubation and timing of tracheal extubation, duration of epidural therapy, adverse effects related to the epidural infusion (hypotension, bradycardia, or excessive sedation) and the reason for removal of the epidural catheter. Data were obtained on the administration of bolus epidural doses and the administration of analgesic or sedative adjuvants including acetaminophen, lorazepam, diazepam, and ketorolac. Consumption of fentanyl, morphine, hydromorphone, and oxycodone were determined in 24-hour intervals up to 72 hours after surgery (postoperative days 1, 2, and 3). Morphine, hydromorphone, and oxycodone were combined and converted to oral morphine equivalents (OMEs) in mg/kg/day.16 Intravenous fentanyl administration was calculated separately as µg/kg/day. The consumption of fentanyl was tabulated separately due to its comparatively divergent half-life and potency, particularly in neonates and infants. Two validated methods were used to assess pain levels in our study population. These assessment tools were the Faces, Legs, Agitation, Cry, and Consolability (FLACC) Scale and the Neonatal Pain, Agitation and Sedation Scale (NPASS).17,18 Both tools are based on a 10-point scale with zero being most comfortable or no pain.
Information gathered on each patient was stored on the secure, on-site server REDCap. Patient and procedure characteristics are described as median and interquartile range (IQR) for continue variables or count and percentage for categorical variables. Medications and pain scores are described as median (IQR). All analyses were performed using SAS 9.4 (Cary, NC).
Results
The study cohort included 52 neonates and infants ranging in age from 0 to 12 months and in weight from 2.1 to 10.1 kilograms. Four patients were ≤1 month of age and 19 were ≤3 months of age. Gestational age ranged from 23 to 41 weeks. Forty-three patients (83%) identified as white (96%), four identified as Black or African American (8%), and five as other (9%). During their preoperative evaluation, most patients were assigned an ASA physical status of II (35%) or III (48%). The demographic data are outlined in Table 1. The epidural catheter was placed in the operating room after the induction of anesthesia and endotracheal intubation in all patients. There were ten surgical procedures on the airway, lung or diaphragm; thirty-eight on the gastrointestinal tract; and four on the genito-urologic tract. Twenty catheters were placed from a caudal approach and threaded cephalad so that the tip of the catheter was positioned at T5-T11, 30 catheters were placed at the thoracic level (T6-T10), and two catheters were placed at the lumbar level (L2-3). The decision regarding the eventual location of the tip of the catheter was based on the site of the surgical incision and the dermatomes involved. Documentation of the appropriate placement of the epidural catheter was verified by real-time ultrasound in the operating room or by a postoperative radiograph. Forty-four patients (85%) had their tracheas extubated in the operating room while 8 (15%) required postoperative mechanical ventilation. The tracheas of four of these patients were extubated within 72 hours while the four others required a more prolonged course of mechanical ventilation (6, 9, 15, and 24 days) due to the surgical procedure, secondary medical issues or associated comorbid conditions.
 |
Table 1 Demographic Data of the Study Cohort (n=52) |
The catheters were dosed with either 1.5% 2-chloroprocaine (47 patients or 90%) or 3% 2-chloroprocaine (5 patients or 10%). The clonidine concentration in the epidural solution varied from 0.1 to 0.4 µg/mL (median concentration of 0.2 µg/mL). No other adjunctive agents were added to the epidural infusions. The epidural infusion was dosed and administered intraoperatively in two patients, intraoperatively and postoperatively in 31 patients or only postoperatively in 19 patients. Nineteen patients (37%) received a bolus dose of 2-chloroprocaine followed by an infusion while the remaining 33 patients (63%) had the infusion started without a bolus dose. During the entire course and subsequent use of the catheter, seven patients received one bolus dose, five patients received two bolus doses, and seven patients received three bolus doses. Per our usual clinical practice, the initial bolus dose in the operating room after the epidural catheter was placed included only 2-chloroprocaine (1.5% or 3%). The bolus doses administered during the postoperative course used the 2-chloroprocaine with clonidine solution. The median bolus dose was 2 mL (range 1–3 mL, IQR 2–2.5 mL). The continuous infusion was started at a median dose of 0.73 mL/kg/hour (range 0.32–1.67 mL/kg/hour, IQR 0.59–0.93 mL/kg/hour). Table 2 shows the duration of catheter use in the 52 patients. The catheter was discontinued because it was no longer needed in 47 patients (90%) and due to concerns regarding sterility or status of the dressing in five patients (10%). No change was required in the infusion rate in 29 patients (56%), one change in 18 patients (34%), two changes in five patients (10%). In all patients that had a change in the infusion rate, the result was an increase in the infusion to improve the level of analgesia. The starting infusion rate and the subsequent rates after the first and second change in the infusion are outlined in Table 3. No adverse effects including excessive sedation, bradycardia or hypotension were noted in the study cohort following bolus dosing or during the continuous infusion. None of the patients required a decrease in the infusion rate, discontinuation of the infusion or change in the infusion medications due to adverse effects.
 |
Table 2 Duration of Catheter Use |
 |
Table 3 Infusion and Subsequent Infusion Rates |
Postoperative analgesia was supplemented as needed by the administration of opioids including intravenous morphine, fentanyl, hydromorphone, and oral oxycodone (Table 4). In the entire cohort of 52 patients, the median (IQR) consumption of OMEs for the first, second, and third 24-hour postoperative periods were 0.3 (0, 0.7), 0.3 (0–0.6), and 0.15 (0–0.5) mg/kg (Figure 1). To exclude sedation and analgesia needs impacted by the presence of an endotracheal tube and ongoing mechanical ventilation, the consumption of opioids was also calculated for 72 postoperative hours in the 44 patients who had their tracheas extubated in the operating room resulting in OMEs use of 0.4 (0–0.7), 0.3 (0–0.6), and 0.2 (0–0.5) mg/kg, respectively, on postoperative days 1, 2, and 3. For fentanyl consumption, the median (IQR) for the first, second, and third 24-hour postoperative periods was 0 (0, 1.1), 0 (0, 0.4), and 0 (0, 0) µg/kg/day, respectively, on postoperative days 1, 2, and 3 (Figure 2).
 |
Table 4 Postoperative Opioid Use |
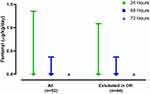
In addition to the opioids noted, the following analgesic or sedative adjunctive agents were administered. Acetaminophen (intravenous or enteral) was administered around the clock every six hours to 51 of 52 patients (98%), ketorolac was administered every 6–8 hours to 19 patients (36%), and a benzodiazepine (diazepam or lorazepam) was administered as needed to 14 patients (27%). Pain scores using either the N-PASS or FLACC scoring are listed in Figure 3. Values were uniformly less than 3 of 10 at all assessment points. Neonates were more commonly assessed using the N-PASS pain scale, while infants and children were more commonly assessed using the FLACC pain scale.
Discussion
Despite the benefits of continuing epidural analgesia into the postoperative period, neonates and infants present unique challenges. In the early 1990s, the expanded use of continuous epidural analgesia resulted in several instances of LAST in the pediatric population.5,19,20 Dose restrictions with consideration of the volume and concentration is needed in neonates and infants when using continuous infusions of epidural bupivacaine or ropivacaine.7,21 Even with these considerations, toxicity may occur as more recent information has demonstrated that with epidural infusion rates of bupivacaine at 0.2 mg/kg/hour, increasing serum concentrations were noted at 48 hours leading the authors to caution against infusions beyond that time period.21
Concerns with the variable pharmacokinetics of amide local anesthetic agents during prolonged infusions in neonates and infants have led to a resurgence with interest in the use of 2-chloroprocaine for continuous epidural infusions. These studies have demonstrated effective analgesia with acceptable pain scores and limitation of postoperative opioid needs with the use of a postoperative epidural infusion with 2-chloroprocaine.9–11 The reader is referred to the manuscript of Veneziano and Tobias for a full review of the use of chloroprocaine for epidural infusions in neonates, infants, and children.9
The addition of adjunctive agents is frequently chosen to improve analgesia and limit local anesthetic dosing requirements (volume and concentration) of postoperative epidural infusions. Clonidine is a centrally acting α2-adrenergic agonist, that provides neuraxial analgesia through its effects on α2-adrenergic receptors in the dorsal horn of spinal cord, central nervous system, and peripheral nervous system. It was first used for epidural analgesia in 1984, and since then has been shown to be a valuable adjunct for continuous epidural analgesia in adults in various clinical scenarios.22–25 Similar efficacy was subsequently demonstrated in infants and children with clonidine added as an adjunct to caudal analgesia.26–28 However, there are limited data regarding the use of epidural clonidine in a continuous epidural infusion in infants and children.29–31 In an open-label, prospective trial, clonidine was evaluated for epidural analgesia following abdominal surgery in a cohort of 40 children, less than three years of age.29 The children were randomly allocated to receive a bolus of epidural clonidine (2 µg/kg) followed by a 24-hour epidural infusion of clonidine (1 µg/mL) at 0.2 mL/kg/hour or clonidine added to a 0.1% ropivacaine infused at 0.2 mL/kg/hour. Approximately 77% of the clonidine group and 59.3% of the clonidine and ropivacaine group required ≤1 dose of tramadol over a 24-hour period. Except for those patients who exhibited frequent coughing during the night (four patients in the clonidine group and five patients in the clonidine-ropivacaine group), no patient required supplemental analgesia and all had good sleep quality during the first postoperative night. Sedation and decreased systolic blood pressure were observed after the administration of the initial epidural clonidine bolus. A subsequent prospective study sought to determine the optimal concentration of clonidine when added to epidural 0.1% ropivacaine for postoperative analgesia.31 The authors concluded that the addition of clonidine (0.08–0.12 µg/kg/hour) was the optimal concentration and was found to improve postoperative pain relief in children without clinically significant adverse effects.
However, there remains no information regarding epidural clonidine infusions in neonates and the suggestion that its use in children less than six months of age may be limited in current clinical practice.32 The current retrospective study evaluates a practice change in epidural analgesia at our institution with the addition of clonidine to continuous 2-chloroprocaine infusions. As noted above, 2-chloroprocaine is chosen given its improved safety profile when compared to local anesthetics of the amide class. Given the popularity and efficacy of adding clonidine to local anesthetic solutions for single-shot caudal epidural blockade, we decided to evaluate the addition of this agent to our continuous epidural infusions in patients less than one year of age. As there is no control group, an evaluation of its efficacy is not feasible; however, the current practice which we described demonstrates acceptable pain scores without the need for excessive supplementation with systemic opioids. Pain scores were uniformly ≤3 at all evaluation points during the first 72 postoperative hours. While the need for systemic opioids was limited, supplemental parental agents must be available to treat agitation in patients that are nil per os, for patient agitation related to other factors (presence of an endotracheal tube) or in the event that epidural analgesia is not complete.
Of note in our cohort was the lack of adverse effects. Although generally safe and effective, adverse effects reported with epidural clonidine related to direct neuraxial actions or systemic absorption have included bradycardia, hypotension, respiratory depression or apnea.33–37 These effects are more likely in neonates and infants, with higher doses, and following bolus administration.33,37,38 In our patient population, no hemodynamic effects were noted and none of the infusions were discontinued or the rates decreased due to hypotension or bradycardia. The majority of the cohort had their tracheas extubated in the operating room and breathed spontaneously during the administration of epidural clonidine. This was feasible with epidural clonidine infusion rates at a median dose of 0.15–0.16 µg/kg/hour (0.73–0.79 mL/kg/hour with a clonidine concentration of 0.2 µg/mL).
Given the retrospective nature of this study, there are specific limitations that must be recognized. There was variability in practice according to the physicians on the Acute Pain Service including the starting infusion rate of the epidural infusion, the concentration of 2-chloroprocaine used (1.5% or 3%), and the concentration of clonidine in the epidural solution. However, in the majority of patients, the starting dose for clonidine was 0.1–0.2 µg/kg/hour. Although pain scores (FLACC or N-PASS) were recorded, assessments did not routinely include the use of a separate sedation score. Additionally, given that the identification of adverse effects may be problematic during a retrospective chart review, our ability to definitely comment on the adverse profile of epidural clonidine may be limited.
Within the constraints of these limitations, our preliminary data demonstrate the safety of adding clonidine to an epidural infusion of 2-chloroprocaine in infants less than 12 months of age with a large number (n=19) that were ≤3 months of age. The description of our clinical practice outlines dosing suggestions regarding both the 2-chloroprocaine infusion rates as well as the clonidine concentration. Future prospective studies are needed to compare 2-chloroprocaine with clonidine to 2-chloroprocaine alone to clearly determine if the combination is superior. Additionally, determination of the optimal clonidine concentration is currently speculative and based on limited pediatric data.
Disclosure
The authors have no conflicts of interest or funding sources to disclose.
References
1. Maitra S, Baidya DK, Pawar DK, Arora MK, Khanna P. Epidural anesthesia and analgesia in the neonate: a review of current evidences. J Anesth. 2014;28:768–779.
2. Murrell D, Gibson PR, Cohen RC. Continuous epidural analgesia in newborn infants undergoing major surgery. J Pediatr Surg. 1993;28(4):548–552. doi:10.1016/0022-3468(93)90614-Q
3. Ecoffey C, Dubousset AM, Samii K. Lumbar and thoracic epidural anesthesia for urologic and upper abdominal surgery in infants and children. Anesthesiology. 1986;65:87–90.
4. Muhly WT, Gurnaney HG, Kraemer FW, Ganesh A, Maxwell LG. A retrospective comparison of ropivacaine and 2-chloroprocaine continuous thoracic epidural analgesia for management of post thoracotomy pain in infants. Pediatr Anaesth. 2015;25(11):1162–1167. doi:10.1111/pan.12745
5. Berde CB. Convulsions associated with pediatric regional anesthesia. Anesth Analg. 1992;75:164–166.
6. Larsson BA, Lönnqvist PA, Olsson GL. Plasma concentrations of bupivacaine in neonates after continuous epidural infusion. Anesth Analg. 1997;84:501–505.
7. Peutrell JM, Holder K, Gregory M. Plasma bupivacaine concentrations associated with continuous extradural infusions in babies. Br J Anaesth. 1997;78(2):160–162. doi:10.1093/bja/78.2.160
8. Dontukurthy S, Tobias JD. Updates on local anesthetic systemic toxicity, prevention and treatment during regional anesthesia in infants and young children. J Pediatr Pharmacol Ther. In press 2020.
9. Veneziano G, Tobias JD. Chloroprocaine for epidural anesthesia in infants and children. Paediatr Anaesth. 2017;27(6):581–590. doi:10.1111/pan.13134
10. Veneziano G, Iliev P, Tripi J, et al. Continuous chloroprocaine infusion for thoracic and caudal epidurals as a postoperative analgesia modality in neonates, infants, and children. Paediatr Anaesth. 2016;26(1):84–91. doi:10.1111/pan.12807
11. Ross EL, Reiter PD, Murphy ME, Bielsky AR. Evaluation of prolonged epidural chloroprocaine for postoperative analgesia in infants. J Clin Anesth. 2015;27:463–469.
12. Zsigmond EK, Downs R. Plasma cholinesterase activity in newborns and infants. Can Anaesth Soc J. 1971;18(3):278–285. doi:10.1007/BF03025463
13. Kuhnert BR, Kuhnert PM, Philipson EH, Syracuse CD, Kaine CJ, Yun CH. The half-life of 2-chloroprocaine. Anesth Analg. 1986;65(3):273–278. doi:10.1213/00000539-198603000-00009
14. Kaur M. Adjuvants to local anesthetics: a combination wisdom. Anesth Essays Res. 2010;4(2):122–123. doi:10.4103/0259-1162.73523
15. Swain A, Nag DS, Sahu S, Samaddar DP. Adjuvants to local anesthetics: current understanding and future trends. World J Clin Cases. 2017;5(8):307–323. doi:10.12998/wjcc.v5.i8.307
16. Yang Y, Yu LY, Zhang WS. Clonidine versus other adjuncts added to local anesthetics for pediatric neuraxial blocks: a systematic review and meta-analysis. J Pain Res. 2018;11:1027–1036. doi:10.2147/JPR.S158264
17. Gammaitoni AR, Fine P, Alvarez N, McPherson ML, Bergmark S. Clinical application of opioid equianalgesic data. Clin J Pain. 2003;19(5):286–297. doi:10.1097/00002508-200309000-00002
18. Hummel P, Puchalski M, Creech SD, Weiss MG. Clinical reliability and validity of the N-PASS: neonatal pain, agitation and sedation scale with prolonged pain. J Perinatol. 2008;28(1):55–60. doi:10.1038/sj.jp.7211861
19. Merkel SI, Voepel-Lewis T, Shayevitz JR, Malviya S. The FLACC: a behavioral scale for scoring postoperative pain in young children. Pediatr Nurs. 1997;23:293–297.
20. Agarwal R, Gutlove DP, Lockhart CH. Seizures occurring in pediatric patients receiving continuous infusion of bupivacaine. Anesth Analg. 1992;75(2):284–286. doi:10.1213/00000539-199208000-00023
21. McCloskey JJ, Haun SE, Deshpande JK. Bupivacaine toxicity secondary to continuous caudal epidural infusion in children. Anesth Analg. 1992;75:287–290.
22. Bösenberg AT, Thomas J, Cronje L, et al. Pharmacokinetics and efficacy of ropivacaine for continuous epidural infusion in neonates and infants. Pediatr Anesth. 2005;15(9):739–749. doi:10.1111/j.1460-9592.2004.01550.x
23. Unnerstall JR, Kopajtic TA, Kuhar MJ. Distribution of alpha-2 agonist binding sites in the rat and human central nervous system: analysis of some functional, anatomic correlates of the pharmacologic effects of clonidine and related adrenergic agents. Brain Res. 1984;319(1):69–101. doi:10.1016/0165-0173(84)90030-4
24. Eisenach JC, De Kock M, Klimscha W. Alpha(2)-adrenergic agonists for regional anesthesia. A clinical review of clonidine (1984–1995). Anesthesiology. 1996;85(3):655–674. doi:10.1097/00000542-199609000-00026
25. Gupta S, Raval D, Patel M, Patel N, Shah N. Addition of epidural clonidine enhances postoperative analgesia: a double-blind study in total knee- replacement surgeries. Anesth Essays Res. 2010;4(2):70–74. doi:10.4103/0259-1162.73510
26. Allen TK, Mishriky BM, Klinger RY, Habib AS. The impact of neuraxial clonidine on postoperative analgesia and perioperative adverse effects in women having elective Caesarean section-a systematic review and meta-analysis. Br J Anaesth. 2018;120(2):228–240. doi:10.1016/j.bja.2017.11.085
27. Jamali S, Monin S, Begon C, Dubousset AM, Ecoffey C. Clonidine in pediatric caudal anesthesia. Anesth Analg. 1994;78(4):663–666. doi:10.1213/00000539-199404000-00008
28. Schnabel A, Poepping DM, Pogatzki-Zahn EM, Zahn PK. Efficacy and safety of clonidine as additive for caudal regional anesthesia: a quantitative systematic review of randomized controlled trials. Paediatr Anaesth. 2011;21(12):1219–1230. doi:10.1111/j.1460-9592.2011.03715.x
29. Ansermino M, Basu R, Vandebeek C, et al. Nonopioid additives to local anaesthetics for caudal blockade in children: a systematic review. Paediatr Anaesth. 2003;13(7):561–573. doi:10.1046/j.1460-9592.2003.01048.x
30. De Negri P, Ivani G, Visconti C, et al. The dose-response relationship for clonidine added to a postoperative continuous epidural infusion of ropivacaine in children. Anesth Analg. 2001;93:71–76. doi:10.1097/00000539-200107000-00016
31. Klamt JG, Garcia LV, Stocche RM, Meinberg AC. Epidural infusion of clonidine or clonidine plus ropivacaine for postoperative analgesia in children undergoing major abdominal surgery. J Clin Anesth. 2003;15(7):510–514. doi:10.1016/j.jclinane.2003.02.005
32. Klamt JG, Santoni M, Garcia LV, Stocche RM. Perioperative analgesia with continuous epidural infusion of morphine combined with clonidine in children undergoing abdominal surgeries. Rev Bras Anestesiol. 2007;57(6):606–617. doi:10.1590/S0034-70942007000600003
33. Ivani G, Tonetti F. Postoperative analgesia in infants and children: new developments. Minerva Anestesiol. 2004;70:399–403.
34. Walker SM, Yaksh TL. Neuraxial analgesia in neonates and infants: a review of clinical and preclinical strategies for the development of safety and efficacy data. Anesth Analg. 2012;115:638–662.
35. Bailey PL, Sperry RJ, Johnson GK, et al. Respiratory effects of clonidine alone and combined with morphine, in humans. Anesthesiology. 1991;74(1):43–48. doi:10.1097/00000542-199101000-00008
36. Penon C, Ecoffey C, Cohen SE. Ventilatory response to carbon dioxide after epidural clonidine injection. Anesth Analg. 1991;72(6):761–991. doi:10.1213/00000539-199106000-00007
37. Ivani G, Bergendahl HT, Lampugnani E, et al. Plasma levels of clonidine following epidural bolus injection in children. Acta Anaesthesiol Scand. 1998;42(3):306–311. doi:10.1111/j.1399-6576.1998.tb04921.x
38. Fellmann C, Gerber AC, Weiss M. Apnoea in a former preterm infant after caudal bupivacaine with clonidine for inguinal herniorrhaphy. Paediatr Anaesth. 2002;12(7):637–640. doi:10.1046/j.1460-9592.2002.00924.x
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.