Back to Journals » Clinical Ophthalmology » Volume 17
Posterior Vitreous Detachment and Its Role in the Evolution of Dry to Wet Age Related Macular Degeneration
Authors Bakaliou A , Georgakopoulos C , Tsilimbaris M, Farmakakis N
Received 31 December 2022
Accepted for publication 3 March 2023
Published 17 March 2023 Volume 2023:17 Pages 879—885
DOI https://doi.org/10.2147/OPTH.S403242
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Anastasia Bakaliou,1 Constantine Georgakopoulos,1 Miltiadis Tsilimbaris,2 Nikolaos Farmakakis1
1Ophthalmology Department, Medical School of Patras, University of Patras, Patras, Greece; 2Ophthalmology Department, Medical School of Crete, University of Crete, Heraklion, Greece
Correspondence: Constantine Georgakopoulos, Email [email protected]
Purpose: To examine the state of the posterior vitreous in eyes with exudative age-related macular degeneration, AMD, non-exudative AMD and in normal eyes.
Study: This is a prospective, cross-sectional study.
Methods: B-scan ultrasonography and Optical Coherence Tomography, OCT were performed in 165 patients older than 65 years with any AMD and in 22 patients older than 65 years with normal eyes in order to diagnose the eyes with complete posterior vitreous detachment, PVD and the eyes with persistent central vitreomacular adhesion, VMA. All patients were selected from the outpatient clinic of the Ophthalmology Department in the University Hospital of Patras. Fundus Fluoroangiography, FFA was used in order to determine the development of exudative AMD from non-exudative AMD. Follow up time was 48 months.
Results: 16/171 eyes with exudative AMD (9.36%) had complete PVD, and the rest 155/171 (90.64%) had central VMA. Eleven of 138 eyes with non-exudative AMD (7.97%) had complete PVD and the remaining 127 eyes (92.03%) had central VMA. During the 48 months of the study, 28 eyes, all with central VMA progressed to exudative AMD.
Conclusion: Vitreomacular adhesion is associated with both exudative and non-exudative AMD. Progression of the non-exudative eyes to exudative AMD seems to be lower in eyes with complete PVD. On the other hand, the progression of normal eyes to exudative AMD appears to be independent of the posterior vitreous status. Larger and longer studies need to replicate these findings and support the potential of a protective role of complete posterior vitreous detachment in the evolution of the disease.
Keywords: exudative age-related macular degeneration, vitreomacular adhesion, PVD, complete posterior vitreous detachment
A Letter to the Editor has been published for this article.
Introduction
Genetic factors, environmental factors, inflammation, oxidative stress, ischemia and the normal aging process have already been proposed as causative factors of exudative age-related macular degeneration (AMD).1 Although the pathogenesis of AMD is undoubtedly an interaction of all the above factors, significant evidence has emerged implicating inflammation contribute to the development of AMD. RPE releases a large number of inflammatory mediators (eg cytokines), contributing to an inflammatory cascade. When the long-term struggle between pro-inflammatory and anti-inflammatory responses eventually loses balance, AMD occurs. On the other hand, previous studies have found higher incidence of persistent central vitreomacular adhesion, VMA in AMD2,3 with no discrimination between exudative and non-exudative form, the latter is noted in the most recent observations.4 The role of the vitreoretinal interface has to be examined extensively in the context of AMD as it has been studied thoroughly for other macular diseases5 so that we may raise the assumption that persistent vitreomacular adhesion exposes the retina to this low grade inflammation and deteriorates dry to wet AMD. The purpose of this study is to examine the state of the posterior vitreous in eyes with exudative age-related macular degeneration, non-exudative AMD and in normal eyes and to correlate the evolution to wet AMD with the presence of central VMA.
Methods
This is a prospective, cross-sectional study which included 165 patients 65 years of age or older with AMD and 22 patients older than 65 years with normal macula in both eyes (Table 1). All the evidence was gathered from the outpatient clinic of the Ophthalmology Department in the University Hospital of Patras. Informed consent was obtained from the study participants. This study was mainly focused on the eyes that deteriorated to the active exudative type during the research (normal or dry eyes) and the status of the posterior vitreous.6 The study excluded patients who had undergone intravitreal injections with anti-VEGF agents in either eye or any kind of ophthalmic surgery. Additional exclusion criteria included diabetic retinopathy, any maculopathy and myopia more than 2 dioptres.
 |
Table 1 Sample Demographics |
All the eyes underwent full ophthalmologic examination at the initial visit and B Ultrasonography, OCT and posterior segment biomicroscopy was performed to confirm the status of the posterior vitreous, complete PVD or VMA.7
The eyes under examination were divided in the following groups (Figure 1).
- A: 138 eyes with non- exudative AMD (drusen or pigment changes)
- B: 171 eyes with active exudative AMD
- C: 21 eyes with normal macula
 |
Figure 1 Percentage of normal eyes, eyes with exudative and dry AMD. |
The patients were frequently examined at the 1st, 3rd, 6th, 12th, 24th, 36th and 48th month. The same evaluation was performed at the subsequent follow-up visits and the diagnosis of transition of dry or normal to wet AMD was fluoroangiographically confirmed with the Heidelberg Spectralis HRA + OCT.
For the statistical analysis in the study, quantitative variables were expressed as mean and standard deviation, SD. Qualitative variables were expressed as absolute and relative frequencies. For the comparisons of proportions Fisher’s exact tests were used. All p values reported are two-tailed. Statistical significance was set at 0.05 and analyses were conducted using SPSS (version 22.0).
Results
Of the 171 eyes with exudative AMD, 16 (9.36%) had a complete PVD and in the rest 155 (90.64%) persistent central VMA was observed.
Of the 138 eyes with non-exudative AMD, 11 (7.97%) had complete PVD and in the rest 127 (92.03%) persistent central VMA was observed.
Finally, in the 21 eyes with normal macula in the 3rd group, 10 (47.62%) had complete PVD and the rest 11 (52.38%) had persistent central VMA. Vitreomacular traction was not recognized in any of the eyes. Table 2 contains the clinical characteristics of the eyes.
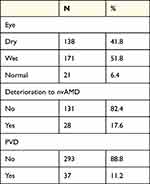 |
Table 2 Clinical Characteristics |
During the study 28 eyes turned into the exudative form of AMD. 26 eyes of them belonged to the non-exudative AMD group with persistent central VMA and the other 2 were normal eyes with persistent central VMA as well. In conclusion, 100% of the eyes that deteriorated to the exudative type of AMD appeared with a persistent adhesion in the central macula surrounded by a detached vitreous cortex (Figure 2). 84% of the eyes that did not deteriorate had persistent central VMA and the remaining 16% had complete PVD which applies to the observation period only as these eyes continued with the potential to deteriorate.
 |
Figure 2 Percentage of the eyes that were PVD or non-PVD regarding to deterioration. |
Statistical Analysis
The eyes included in the above groups were compared with respect to the presence of a complete PVD or partial PVD. The initial hypothesis that the evolution to the exudative type of AMD is independent of the state of the posterior vitreous was examined. The correlation of the data that is of interest can be seen in Table 3. Statistical significance has been set to 0.05 and the analysis has been conducted with SPSS (version 22.0).
 |
Table 3 Percentage of the Eyes That Were PVD or Non- PVD Regarding to Deterioration |
During the study, 28 eyes deteriorated and 100% of them belong to the category of non-PVD with persistent central VMA and the above correlation is statistically significant (p < 0.05). In conclusion, the development of exudative AMD is not independent of the status of the posterior vitreous, on the contrary, in the total of the eyes that deteriorated the posterior vitreous had been partially detached with remaining VMA. In Table 4 the deterioration of the eyes during time is presented. 53.6% of the eyes that deteriorated to the exudative type of AMD developed neovascularization during the first 12 months (Figure 3).
 |
Table 4 Deterioration in Time |
 |
Figure 3 Distribution of eyes converting to wet AMD. |
Discussion
The dominating theory regarding the pathogenesis of AMD supports that the disease is multifactorial under the effect of genetics and environment. Cigarette smoking, dietary elements, drusen and pigment changes8–11 are well known risk factors, while hereditary factors have been identified to be responsible for the development of active only exudative AMD.12–15 However, differences in grading of AMD in both eyes cannot be explained by environmental and genetic factors only. Taking into account the important role of the posterior vitreous cortex in other retinal disorders, it would be sensible to raise the assumption that in the case of AMD as well, the vitreoretinal interface has to be considered of great value in the deterioration of dry AMD to exudative AMD.
Through which mechanism the posterior vitreous can evolve (not trigger because the disease has already started) dry to neovascular AMD? Krebs et al4 in 2007 presented clear indications that partial PVD could be a potential risk factor for exudative AMD. Persistent VMA holds a positive relation to the appearance of early signs of AMD16 while it has been already proved that there is correlation between attached posterior vitreous and typical AMD.17 Additionally double prevalence of vitreomacular adhesion is observed in patients with typical exudative AMD.18 During the normal aging process of PVD, incomplete detachment of the posterior vitreous can lead to persistent VMA and promote exudative AMD via the following possible routes.
Anomalous PVD with VMA can cause low grade inflammation in the macula.19–21 Moreover, the presence of attached posterior cortex could prevent the normal diffusion of oxygen and nutrients causing relative ischemia, VEGF production and choroidal neovascularization, CNV. Adhesion of the viscous posterior vitreous cortex to the retina maintains a barrier to diffusion and convection currents in the vitreous cavity according to the laws of Fick’s, Stokes-Einstein and Hagen-Poiseuille. If the vitreous is detached from the surface of the retina, the low viscosity fluid transports oxygen and nutrients towards an ischemic area of the retina, and cytokines away from the retina, at a faster rate than through attached vitreous gel. Vitreoretinal adhesion can exacerbate retinal hypoxia.22 In addition, the presence of attached posterior cortex exposes macula to cytokines or free radicals in the vitreous resulting in the development of CNV.23 The above mentioned ischemia results in the production of VEGF-A and the promotion of Ang-2 which is normally in low levels. The complex of Αng-2 with the receptor Τie2 causes destabilization of the retinal vessels’ wall and potential neovascularization.24–26
In the present study the total of the eyes that evolved to neovascular AMD had sustained partial PVD. At the same time, the total amount of the dry eyes that remained stable had complete PVD. Large scale clinical trials have to be conducted regarding the relationship of incomplete PVD and the development of neovascular AMD. There are more known risk factors that need to be co-examined such as genetics, smoking and antioxidants. However, a correlation has risen from our research between VMA and active exudative AMD.
Conclusion
In conclusion, VMA is not the cause of neovascular AMD, the traction forces deteriorate the disease. As a result, in everyday clinical practice it is currently necessary to register and follow up the state of the posterior vitreous for the treatment and the prognosis of the patient. It could be supported that since VMA is indicated as a potential risk factor for the development of exudative active AMD, complete PVD could act as a protective factor reasonably raising the concept of protective pharmaceutic vitreolysis or even vitrectomy in high risk patients for exudative AMD.27–29
Ethics
This study was approved by the University of Patras and adheres to the guidelines of the Declaration of Helsinki.
Funding
There is no funding to report.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Spaide RF, Armstrong D, Browne R. Choroidal neovascularization in age related macular degeneration - what is the cause? Retina. 2003;23:595–614. doi:10.1097/00006982-200310000-00001
2. Ondes F, Yilmaz G, Acar MA, Unlü N, Kocaoğlan H, Arsan AK. Role of the vitreous in age-related macular degeneration. Jpn J Ophthalmol. 2000;91–93. doi:10.1016/S0021-5155(99)00174-4
3. Weber-Krause B, Eckardt C. Incidence of posterior vitreous detachment in the elderly. Ophthalmologe. 1997;94:619–623. doi:10.1007/s003470050170
4. Krebs I, Brannath W, Glittenberg C, Zeiler F, Sebag J, Binder S. Posterior macular adhesion: a potential risk factor for exudative age-related macular degeneration? Am J Ophthalmol. 2007;135:351–355. doi:10.1016/s0002-9394(02)01944-x
5. Johnson MW. Perifoveal vitreous detachment and its macular complications. Trans Am Ophthalmol Soc. 2005;103:537–567.
6. Kim YC, Harasawa M, Villanueva GS, et al. Enhanced high-density line spectral-domain optical coherence tomography imaging of the vitreoretinal interface: description of selected cases. Semin Ophthalmol. 2016;31:559–566. doi:10.3109/08820538.2015.1009560
7. Schmidt-Erfurth U, Waldstein SM, Klimscha S, et al. Prediction of individual disease conversion in early AMD using artificial intelligence. Invest Ophthalmol Vis Sci. 2018;59(8):3199–3208.
8. Clemons TE, Milton RC, Klein R, et al; Age-Related Eye Disease Study Research Group. Risk factors for the incidence of advanced age-related macular degeneration in the Age-Related Eye Disease Study (AREDS): AREDS report no. 19. Ophthalmology. 2005;112:533–539.
9. Chakravarthy U, Augood C, Bentham GC, et al. Cigarette smoking and age-related macular degeneration in the EUREYE study. Ophthalmology. 2007;114(6):1157–1163. doi:10.1016/j.ophtha.2006.09.022
10. Francis PJ, George S, Schultz Francis DW, et al. The LOC387715 gene, smoking, body mass index, environmental association with advanced age-related macular degeneration. Hum Hered. 2007;63:212–218.
11. Age-Related Eye Disease Study Research Group. Risk factors associated with age- related macular degeneration. A case-control study in the age-related eye disease study: age- Related Eye Disease Study (AREDS) Report No. 3. Ophthalmology. 2000;107(12):2224–2232.
12. Edwards A, Ritter R, Kenneth JA, Manning A, Panhuysen C, Farrer L. Complement factor H polymorphism and age- related macular degeneration. Science. 2005;308:421–424. doi:10.1126/science.1110189
13. Scott WK, Schmidt S, Hauser M, et al. Independent effects of complement factor H Y402H polymorphism and cigarette smoking on risk of age-related macular degeneration. Ophthalmology. 2007;114:1151–1156. doi:10.1016/j.ophtha.2006.08.054
14. Souied EH, Benlian P, Amouyel P, et al. The γ e4 allele of the apolipoprotein E gene as a potential protective factor for exudative age-related macular degeneration. Am J Ophthalmol. 1998;125(3):353–359. doi:10.1016/S0002-9394(99)80146-9
15. Churchill AJ, Carter JG, Lovell H, et al. VEGF polymorphisms are associated with neovascular age-related macular degeneration. Hum Mol Genet. 2006;15:2955–2961. doi:10.1093/hmg/ddl238
16. Schulze S, Neugebauer A, KrollSchulze P. Appearance of age-related macular degeneration in vitrectomized and nonvitrectomized eyes: an intraindividual case study. Acta Ophthalmol. 2012;90(3):244–247. doi:10.1111/j.1755-3768.2010.01929.x
17. Nomura Y, Ueta T, Iriyama A, et al. Vitreomacular interface in typical exudative age-related macular degeneration and polypoidal choroidal vasculopathy. Ophthalmology. 2011;118(5):853–859. doi:10.1016/j.ophtha.2010.09.001
18. Jackson T, Nicod E, Angelis A, et al. Vitreous attachment in age-related macular degeneration, diabetic macular edema, and retinal vein occlusion: a systematic review and meta-analysis. Retina. 2013;33(6):1099–1108. doi:10.1097/IAE.0b013e31828991d6
19. Anderson DH, Mullins R, Hageman G, et al. A role for local inflammation in the formation of drusen in the aging eye. Am J Ophthalmol. 2002;134(3):411–431. doi:10.1016/S0002-9394(02)01624-0
20. Donoso LA, Kim D, Frost A, Callahan A, Hageman G. The role of inflammation in the pathogenesis of age-related macular degeneration. Surv Ophthalmol. 2006;51:137–152. doi:10.1016/j.survophthal.2005.12.001
21. Zarbin MA. Current concepts in the pathogenesis of age-related macular degeneration. Arch Ophthalmol. 2004;122(4):598–614. doi:10.1001/archopht.122.4.598
22. Stefánsson E, Geirsdóttir A, Sigurdsson H. Metabolic physiology in age related macular degeneration. Prog Retin Eye Res. 2011;30(1):72–80. doi:10.1016/j.preteyeres.2010.09.003
23. Adamis AP, Shima DT. The role of vascular endothelial growth factor in ocular health and disease. Retina. 2005;25(2):111–118. doi:10.1097/00006982-200502000-00001
24. Ma L, Brelen M, Tsujikawa M, et al. Identification of ANGPT2 as a new gene for neovascular age-related macular degeneration and polypoidal choroidal vasculopathy in the Chinese and Japanese populations. Invest Ophthalmol Vis Sci. 2017;58(2):1076–1083. doi:10.1167/iovs.16-20575
25. Danny SN, Yip YW, Bakthavatsalam M, et al. Elevated angiopoietin 2 in aqueous of patients with neovascular age related macular degeneration correlates with disease severity at presentation. Sci Rep. 2017;7(1):45081
26. Ilim O, Akkin C, Oztas Z, et al. The role of posterior vitreous detachment and vitreomacular adhesion in patients with age-related macular degeneration. Ophthalmic Surg Lasers Imaging Retina. 2017;48(3):223–229. PMID: 28297034. doi:10.3928/23258160-20170301-05
27. Waldstein S, Wright J, Warburton J, et al. Predictive value of retinal morphology for visual acuity outcomes of different ranibizumab treatment regimens for neovascular AMD. Ophthalmology. 2016;123(1):60–69. doi:10.1016/j.ophtha.2015.09.013
28. Awasthi U, Grover R, Videkar C, et al. Comment on: the role of posterior vitreous detachment on the efficacy of anti-vascular endothelial growth factor intravitreal injection for treatment of neovascular age-related macular degeneration. Indian J Ophthalmol. 2019;67(10):1783. doi:10.4103/ijo.IJO_160_19
29. Munk M, Arendt P, Yu S, et al. The impact of the vitreomacular interface in neovascular age-related macular degeneration in a treat-and- extend regimen with exit strategy. Ophthalmol Retina. 2018;2:288–294. doi:10.1016/j.oret.2017.07.010
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
