Back to Journals » Journal of Inflammation Research » Volume 16
Possible Future Avenues for Rheumatoid Arthritis Therapeutics: Hippo Pathway
Authors Wang T, Wang Z, Qi W, Jiang G, Wang G
Received 21 January 2023
Accepted for publication 14 March 2023
Published 24 March 2023 Volume 2023:16 Pages 1283—1296
DOI https://doi.org/10.2147/JIR.S403925
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Tao Wang,1,2 Zhandong Wang,1 Wenxia Qi,1 Ganggang Jiang,1 Gang Wang2
1Gansu University of Chinese Medicine, Lanzhou, 730020, People’s Republic of China; 2Affiliated Hospital of Gansu University of Chinese Medicine, Lanzhou, 730020, People’s Republic of China
Correspondence: Gang Wang, Email [email protected]
Abstract: Rheumatoid arthritis (RA) is a persistent systemic autoimmune disease with the hallmarks of swelling of the joint, joint tenderness, and progressive joint destruction, which may cause synovial inflammation and pannus as a basic pathological change, resulting in joint malformations and serious disorders. At present, the precise etiology and mechanism of pathogenesis of RA are unknown. The imbalance of immune homeostasis is the origin of RA. Hippo pathway is widely expressed in a range of cell lineages and plays a fundamental role in maintaining the immune steady state and may be involved in the pathogenic mechanism of RA. This study reviews the progress of Hippo pathway and its main members in the pathogenesis of RA from three aspects: regulating the maintenance of autoimmune homeostasis, promoting the pathogenicity of synovial fibroblasts and regulating the differentiation of osteoclasts. The study also presents a new way to recognize the pathogenesis of rheumatoid arthritis, which is favorable for finding a new way for treating the rheumatoid arthritis.
Keywords: Hippo pathway, rheumatoid arthritis, pathogenesis, immune cell
Introduction
Rheumatoid arthritis (RA) is an autoimmune illness marked by synovitis and vasculitis. This eventually leads to joint cartilage and bone destruction. Production of RA is closely related to immune disorders, immune cell metastasis activation, and immune cell subgroup imbalance.1 The etiology of rheumatoid arthritis is not clear, which may be related to many factors, such as immune factors, genetic factors, environmental factors, infection factors, and so on.2 Clinical features of RA are chronic inflammation, synovial proliferation, and bone erosion. The main pathological features are synovitis and inflammatory factor infiltration, abnormal “tumor like” proliferation of synovial cells in neovascularization, and irreversible destruction of cartilage and bone. The exact etiology of RA is much unknown, and the disorder of immune balance is the basis for RA. At present, the Hippo pathway is highly conserved in evolution, and it is gradually found that the main members of the Hippo pathway play a vital role in the process of immune regulation.3,4 The canonical Hippo pathway regulates cell proliferation and death and maintains the homeostasis of tissues and organs by inhibiting the function of Yes-associated protein (YAP), while the non-canonical Hippo pathway has a core of Ste20-like kinases 1/2 (MST1/2) to regulate the immune response and maintain the homeostasis of the immune system.5–7 Under normal physiological conditions, Hippo pathway inhibits the differentiation of proinflammatory T helper type 17 (Th17) and promotes the differentiation of immunosuppressive regulatory T cells (Treg), which is essential for maintaining immune tolerance and homeostasis. When Hippo pathway is dysregulated, patients are prone to autoimmune diseases.8 Conditional knockout of MST1/2 in mice hematopoietic stem cells and inhibition of Hippo pathway can lead to severe immunodeficiency in mice, which is prone to severe pathogen infection and autoimmune diseases, such as inflammatory bowel disease, rheumatoid arthritis, Sjogren ‘s syndrome, etc.9 RA is an autoimmune disease, and Th17 and Treg cells are unbalanced in the whole process of the disease.10 This suggests that Hippo signaling may be involved in the pathogenesis of RA. The purpose of this study is to summarize the essential roles of core effector molecules and abnormal expression of upstream and downstream targets in the Hippo pathway in the pathogenesis of RA. It provides a new concept to understand the etiology of RA.
Hippo Pathway Overview
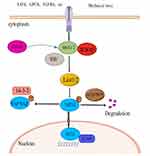
Hippo pathway is an evolutionarily conserved growth inhibitory signaling pathway that plays a central role in the legislation of cell proliferation and differentiation. The central component of Hippo pathway consists of a battery of kinases (Hippo (Hpo), warts (Wts), Salvador (Sav), MOB as tumor suppressor (Mats) and a transcriptional cofactor Yorkie (Yki). Homology of these components in mammals is Ste20-like kinase 1/2 (MST1/2), large tumor suppressor 1/2 (LATS1/2), Salvador 1 protein (SAV1, also called WW45), MOB kinase activators 1A and 1B (MOB1A/B) and Yes-associated protein/transcriptional coactivator with PDZ-binding motif (YAP/TAZ), respectively.11–17 In the canonical Hippo pathway, MST1/2 and LATS1/2, as the nuclear kinases of Hippo pathway, have interaction with the framework protein WW45 to phosphorylate and spark off the downstream kinase LATS1/2. At the same time, LATS1/2 further phosphorylates YAP/TAZ to bind to 14-3-3 protein and retain it in the cytoplasm, consequently arresting YAP/TAZ from coming into the nucleus and binding to transcriptional enhanced associate domain (TEAD) family transcription factor TEAD1-4 (homologous to Drosophila Sd), ultimately inhibiting cell proliferation and promoting cell apoptosis.18,19 Inhibition of the Hippo signaling pathway or the lack of its core member will lead to the increase of YAP/TAZ invasion into the nucleus, thus activating the transcription factor TEADs to transcribe its downstream target genes, promoting cell proliferation and inhibiting apoptosis. In mice, systematic double knock decrease in MST1 and MST2 causes early embryonic death.20 Systematic single knockdown of MST1 or MST1/2 does not induce tissue overgrowth or tumorigenesis because of the high homology and functional compensation of MST1 and MST1/2.21 The conditional double knockdown of MST1 and MST1/2 in liver, small intestine, pancreas or heart may result in malfunction of Hippo pathways such as cell proliferation and organ growth,22–27 and activation of YAP is the root cause of this phenomenon. Therefore, further knockout of YAP alleles in the conditional knockout mouse knocking out MST1/2 or other upstream genes can alleviate or completely rescue the organ proliferation and tumorigenesis caused by gene inactivation upstream of Hippo pathway.28–30 In short, the canonical Hippo pathway regulates cell proliferation and death by suppressing the function of YAP primarily to maintain the steady state of tissues and organs.
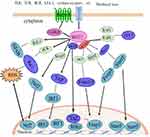
Hippo pathway performs a principal function in preserving the immune homeostasis, but unlike the canonical Hippo pathway that regulates the steady-state of tissue and organ size, the non-canonical Hippo pathway, with the core of kinase MST1/2, regulates the function of the immune system.7 In the immune system, kinase MST1/2 is involved in the interaction with other signaling pathways that modulate the immune function, including integrin signaling, cytokine receptor signaling, T cell receptor (TCR) and B cell receptor (BCR) signaling, and mitogen-activated protein kinase (MAPK) signaling, and antiviral signaling pathways that regulate immune responses, which are collectively known as non-canonical Hippo pathway.31 Numerous studies have revealed that major members of the Hippo pathway can perform diverse non-canonical biological regulatory functions by phosphorylating non-Hippo pathway molecules or interacting with other signaling pathways. For example, MST1/2 can regulate the biological function of lymphocytes independently of YAP/TAZ and LATS1/2.32 The MST1/2 kinase is capable of directly phosphorylating forkhead box O1 (Foxo1)/forkhead box O3 (Foxo3), blocking the binding of forkhead box O1/3 (foxo1/3) and 14-3-3 proteins. The 14-3-3 proteins are a family of conserved regulatory molecules expressed in all eukaryotic cells. A striking feature of the 14-3-3 proteins is their ability to bind a multitude of functionally diverse signaling proteins, including kinases, phosphatases, and transmembrane receptors) and promoting Foxo1/3 to the cell nucleus.33 MST1/2 also mediates toll-like receptor signal conduction in drosophila and mammals.34,35 YAP/TAZ antagonized the innate immune response by combining the TANK binding kinase 1 (TANK1) with Interferon regulatory factor 3 (IRF-3) and suppressing its expression.36,37 Other research has confirmed that Hippo pathway is related to other important signaling pathways involved in immunomodulation, including mitogen-activated protein kinase, forkhead box O and p53 signaling pathways.38,39 In recent years, control of non-canonical Hippo pathways in the immune system has been focused on this field of research.
Major members of Hippo pathways such as MST1/2, Mps One Binder (MOB), NOR-E1B (the Ras effector genes) are highly expressed in numerous immune-related tissues for instance mouse bone marrow, thymus, spleen and lymph nodes.40 The key kinases of the Hippo pathway, MST1/2 and its downstream TAZ and YAP, have been most extensively studied in the immune system. They participated in the regulation in regard to the proliferation and migration of T cells and B cells, the differentiation of Naïve T cells, and regulation of macrophage antimicrobial and antiviral functions.41 Hippo signal paths are highly conservative in evolution and interact with multiple signal transduction pathways and have a regulatory function in multiple organs, which have become a hot spot of international concern and are of foremost importance in autoimmune diseases.42 Since the pathogenesis of RA is now not yet totally elucidated, it is indispensable to research the function of the Hippo pathway in rheumatoid arthritis (Figures 1 and 2).
Hippo Pathway Participating in RA Pathogenesis by Regulating Th17/Treg
Immunomodulatory Functions of Hippo Pathway
The immunity system is an essential protection device for the body to recognize and eliminate the dissenters, and it is very important to maintain the homeostasis of the body’s internal environment. If the immune response is already too strong or too weak, the stability of the body becomes unbalanced, leading to sickness. The immune cell can sense the environment via the associated receptor, activate the corresponding signal transduction pathway, and produce downstream effective molecules. It carries out an immune response through a possible feedback regulation mechanism and maintains an immune homeostasis. The disorder of the immune system can cause various diseases, such as immunodeficiency, which makes the body’s ability to resist pathogens, reduce the ability to identify their mutant cells decrease, and even cause more pathogen infection or tumorigenesis. Excessive activation of the immune system causes the immune system to attack the body itself and cause autoimmune diseases. Therefore, maintaining the steady state of the immune system is extremely vital for the health of the body. A growing number of research have printed that Hippo pathway performs a quintessential function in maintaining the steady state of the immune system. By knocking out MST1/2 in mouse bone marrow hematopoietic stem cells, mice are susceptible to severe immune infection and autoimmune diseases.9 The research groups of Professor Basile from France and Professor Klein from Germany are a kind of familial hereditary immunodeficiency patient carrying MST1 inactivating mutation or gene deletion.43,44 These patients are highly susceptible to bacterial or viral infections, mucosal and skin Candida infections, suppurative granulation or skin abscesses, accompanied by lymphocyte and neutropenia and a variety of autoimmune diseases. All these data strongly indicate that the Hippo pathway performs an indispensable position in immunomodulation.
Hippo Pathway Regulates Th17 and Treg Differentiation
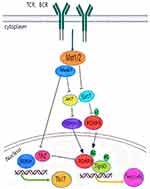
Members of Hippo pathway are closely related to T cell formation and development. Compared with wild-type mice, MST1-null mice exhibited granulocytopenia, decreased total T cell and B cell numbers, and significantly decreased Naïve T cell numbers in peripheral blood. However, the proportion of effector and memory T cells in liver and lung increased.32,45 MST1 kinase regulates T and B lymphocyte, peculiarly for T cell development, migration, homing and maintenance of autoimmune tolerance and immune homeostasis. MST1/2 performs an important function in regulating the manufacturing and feature of regulatory T cells (Tregs) and several helper T cells.
Hippo pathway members are involved in the legislation of Th17 and Treg differentiation in the body. MST1 is an intrinsic inhibitory molecule regulating T cell proliferation under the stimulation of antigen or anti-CD3 antibody, which is very essential for maintaining the variety of T cells in the organism.9,21 Recent studies have also shown that MST1/2 and its downstream TAZ molecules are able to restrain Th17 cells and enhance the differentiation of Treg cells. Mice with MST1 knockout or MST1/2 knockout in hematopoietic cell lines were more likely to develop autoimmune diseases, such as inflammatory bowel disease, particular and labial gland Sjögren’s syndrome.32,46 Among these mice, the differentiation and feature of Treg cells have been impaired, while the expression of Th17 cell-associated cytokines grew up in inflammatory tissues. Transplantation of bone marrow into recombination activation gene 1 (GAG1) may also cause Sjögren’s syndrome and enteritis, and supplementation with wild-type Treg cells inhibits the progression of these autoimmune diseases. MST1 can promote the differentiation of Treg cells and prevent autoimmunity and tissue damage. MST1 kinase is capable of enhancing its stability by directly phosphocreatine the transcription factor Foxo1/3. On the other hand, MST1 suppresses activation of AKT (also called Protein Kinase B, PKB) triggered by TCR activation. Threonine 42 (T42) suppresses AKT phosphorylation of FoxO1 and further enhances the stability of FoxO1/3 to encourage the development of Treg.47 MST1 deficiency can lead to the defect of differentiation and development of CD4+naïve T cells into Treg cells, which are induced by transforming growth factor β (TGF-β). Additionally, MST1 deficiency has an effect on the suppressive characteristics of Treg cells.47,48 Compared with wild-type mice, the CD4+Treg in thymus and spleen of mice were obviously decreased and the regulatory function was defective in MST1−/− mice.49
TAZ downstream of Hippo pathway performs an essential regulatory function in differentiation of Treg and Th17 cells.50 The expression degree of TAZ protein used to be substantially upregulated at some point of the differentiation of naive CD4+T cells into Th17 cells in both mouse or hand human. Studies revealed that51 TAZ affects the process of these diseases by encouraging the development and differentiation of Th17 and suppressing the differentiation and function of Treg. TAZ can activate Retinoic acid-related orphan receptor γt (RORγt) activity directly by binding to RORγt and can also form a complex with Foxp3 to relieve its inhibitory effect on Retinoic acid-related orphan receptor γt (RORγt), thus promoting the differentiation of Th17 cells. TAY can additionally bind to Tat-interactive Protein-60KDA (Tip60) to minimize the balance, leading to the disintegration of Foxp3, thereby weakening the development of Treg cells. In conclusion, Hippo pathway performs a crucial regulatory position in differentiation of Th17 and Treg cells, and its core molecules MST1/2 and TAZ play a key role (Figure 3).
Hippo Pathway is Involved in the Pathogenesis of RA
The large number of immune cells present in RA synovial tissue is mainly CD4+ T cells. However, there were more Th17 cells and fewer Treg cells in CD4+T cells, indicating an imbalance of Th17/Treg cells.52 The number and functional imbalance of Th17 and Treg cells are closely related to the occurrence of RA. Th17 cells secrete IL-21, IL23, IL-17A and IL17F, which promote inflammatory response, while Treg cells secrete IL-10 and IL-35 to suppress inflammatory response and maintain autoimmune tolerance.53 Th17/Treg imbalance exists in the whole process of RA disease, and eventually leads to synovial inflammation, joint destruction, bone erosion, etc.10 Activation of Hippo pathway can not only promote and enhance the differentiation and immunomodulatory function of Treg cells but also inhibit the development and differentiation of Th17 cells.49 Hippo pathway is vital to homeostatic maintenance of immune tolerance and inhibition of inflammation. MST1 kinase, a key protein in the Hippo pathway, encourages the development and advancement of Treg cells. When the MST1/2 gene was knocked out, the mice were susceptible to autoimmune diseases, Sjögren’s syndrome, inflammatory bowel disease, and rheumatoid arthritis54. Studies have found that when MST1 is knocked out, the normal reciprocity of Treg cells with antigen presenting cell (APC) and dendritic cells (DC) is impeded, and the insufficient connection between the two results in the failure of down-regulation of co-activating molecules (CD86) on DC, damaging the inhibitory function of Treg cells.55 Some studies have further shown that when Treg cells are induced and differentiated in vitro experiment, MST1 deficiency affects the process of TGF-ß-induced development and differentiation of CD4+Naïve T cells, resulting in Treg cell defect.56 TAZ, a crucial transcriptional coactivator in the Hippo pathway, boosts the differentiation of Th17 cells, which are carefully associated with the prevalence and development of some autoimmune diseases mediated by Th17 cells. The demonstration of TAZ in peripheral blood T cells from sufferers with some autoimmune diseases, Sjögren’s syndrome and rheumatoid arthritis, positively correlated with the demonstration of RORγt, a core transcription factor of Th17 cells; the precise knockdown of TAZ in mouse T cells alleviated the development of RA.8 MST1/2 and TAZ play a vital role in regulating the differentiation of naïve CD4+T cells into Th17 and Treg, which also proves the significance of Hippo pathway in the regulation of immune system homeostasis. When its expression is reduced, autoimmune imbalance will occur, which may be converted into the pathogenesis of RA. Studies have found that MST1 can modulate the susceptivity of collagen II-induced arthritis (CIA), decrease the release of proinflammatory cytokine, and help decrease the inflammatory immune response. The incidence and susceptivity of the arthritis in MST1−/− mice have been notably decreased, MST1 deficiency drastically decreased the histological symptoms of arthritis, consisting of synovitis and destruction of articular cartilage and bone,57 which are consistent with the basic pathological changes of RA. TAZ performs a primary position in the pathogenesis of RA. TAZ may additionally take part in the pathogenesis of RA with the aid of regulating the differentiation of Th17 cells. Chen Xing58 found that TAZ was highly expressed in the CIA model and RA patients. The severity of disease was notably lowered in the CIA model with conditional knockdown of TAZ, indicating that the decrease of TAZ could holdback the development of RA. Lin L et al treated mice with Acacetin in the CIA model and found that Acacetin could decrease TAZ expression, hinder the differentiation of Th17 cells, and restrict the proliferation of T cells.59
In conclusion, core molecules of Hippo pathway, MST1/2 and TAZ, maintain autoimmune homeostasis by regulating the differentiation of Th17/Treg cells. When the demonstration of MST1/2 and TAZ is abnormal, autoimmune imbalance will occur, which may be converted into the pathogenesis of RA. However, the exact pathogenesis of MST1/2 and TAZ in RA is still poorly understood and requires further study (Figure 4).
Promote the Pathogenicity of Synovial Fibroblasts
Fibroblast-like synoviocytes (FLSs) play a foremost role in keeping synovitis and aggressive destruction of joint in RA. As one of the principal cell types in the synovial tissue of RA, they are the key effector cells in the initiation and development of RA and are often considered as potential therapeutic targets for RA. The activated FLSs in RA have tumor cell-like characteristics, continuous proliferation, and resistance to apoptosis.60 Under the action of pro-inflammatory cytokines, RA-FLSs can produce chemokines and matrix metalloproteinase (MMP) to promote inflammatory response and bone destruction.61 They are the key cells of RA tissue damage, which interact with cytokines and promote the occurrence of RA.
The Hippo-YAP Pathway is “Turned Off” in RA-FLS
The hippo passage is one of the vital pathways to adjust cell growth, proliferation and organ development, while YAP/TAZ activity is the key to cell proliferation during organ growth, tissue renewal, and regeneration.62 Studying synovium of intraarticular fracture or osteoarthritis patient, Anke J. Roelofs et al63 found that YAP was upregulated in the hyperplastic synovium of injured mouse knees and analyzed synovium from patients with intra-articular fractures or osteoarthritis, and found that YAP was detected in areas of the synovial intima with an activated, hyperplastic appearance. However, YAP was hardly detected in non-proliferative synovial lining in the same histological sections or in normal knee synovium. When Hippo pathway is “turned on”, cell proliferation and transcription of genes that promote survival are repressed. MST1/2 bound to Salvador is phosphorescent, activating Lats12, and Lats12 directly phosphocreatines YAP/TAZ, which is inactivated and degraded after binding to 14-3-3 proteins in the cytoplasm.64 When Hippo pathway is “turned off”, YAP/TAZ enters the nucleus, forming the YAP-TEAD complex in the nucleus and regulating transcription factors to initiate the transcription genes that promote survival and proliferation.65 The nuclear translocation of YAP was significantly increased in FLS of RA patients and in FLS of CIA rats by immunofluorescence staining.66 The FLS of CIA rats were treated with verteporfin (VP), a YAP-TEAD binding inhibitor, and the effect of VP on YAP nuclear translocation was detected by immunofluorescence. The results showed that VP significantly inhibited the total expression of YAP in the CIA-FLS. Inhibition of YAP-TEAD binding reduced the nuclear translocation of YAP in the FLS of CIA rats.67 This suggests that YAP lays on the cytoplasm of FLSs in normal synovial tissues and has low expression, while YAP is located in the nucleus of FLSs and has high demonstration in synovial tissues of RA or CIA. Therefore, YAP, the effector molecule of Hippo pathway, is abnormally reflected in RA patients. It was also found that YAP and TAZ, important members of Hippo pathway in RA-FLSs, were highly expressed. In the inflammatory state of RA or CIA, Hippo pathway is turned off in FLS, and YAP expression is increased and translocated into the nucleus, which may help promote abnormal growth and migration of FLS.68
YAP/TAZ Drives the Invasiveness of RA-FLS
YAP/TAZ is associated with tissue-size homeostasis when Hippo pathway is “Turned off”. When hypo-phosphorylated YAP/TAZ is transported to the nucleus, it binds to and activates the TEAD family transcription factors, promoting target gene to be transcribed,69 whereas YAP/TAZ mediated transcription by driving cell proliferation, survival, as well as cell plasticity and slimness, and is therefore critical for tissue development and regeneration.70 YAP/TAZ has transcriptional activity in RA-FLS and affects the severity of RA by driving the aggressive phenotype of fibroblast-like synovial cells and fibroinflammatory responses. Inhibition of YAP/TAZ transcriptional activity by vasopressin can reduce the invasive phenotype of RA-FLS. It reduces apoptosis resistance, proliferation, invasion and inflammatory response.71 Bottini et al also confirmed this view. They found that72 YAP promotes the invasion of synovial fibroblasts in K/BxN serum metastatic arthritis mice. When the YAP inhibitor verteporfin was used, it reduced the expression of TNF-α induced matrix metalloproteinase-1 and interleukin-6, confined the invasiveness of RA-FLSs, and improved the severity of arthritis. Using slow virus mediated gene knockout technology, Wei Zhou et al73 knocked out YAP or TAZ in RA-FLS model to assess whether YAP or TAZ knockout influences the invasiveness in RA-FLS. As the result, it was confirmed that YAP or TAZ knockout RA-FLS significantly reduced the wound closure in the wound healing test in comparison with the stabilized expression Short hairpin RNA (shRNA) cell, and it significantly reduced the number of migration and invasion cell in the Transwell test. This indicates that YAP or TAZ knockout suppresses RA-FLS migration and invasion. The reason is that the demonstration of N-cadherin is decreased, and at the same time, the E-cadherin and β-catenin is increased in RA-FLSs. YAP also plays an important role in synovial intimal hyperplasia after joint surface injury. Therefore, the Hippo-YAP pathway is “turned off” in RA-FLS, activating YAP/TAZ transcriptional activity. Transcriptional activity of YAP/TAZ promotes the invasiveness of rheumatoid arthritis synovial fibroblasts. Loss of YAP or TAZ results in suppression of RA-FLS’ ability to invade aggressively, constituting a factor in the development of RA. Nevertheless, the factors that turn off the signal and the mechanism by which it is regulated are unclear.
Regulation of Osteoclasts by YAP/TAZ
Osteoclasts and osteoblasts play a major role in the regulation of the balance of the skeletal system. Under normal physiological conditions, osteoclasts and osteoblasts balance with each other to maintain bone homeostasis. Bone destruction in RA is mainly mediated by osteoclasts, and persistent joint inflammation enhances the differentiation and activity of osteoclasts and impairs the differentiation and function of osteoblasts, leading to imbalance of bone homeostasis and bone destruction.74 Within osteoclasts, RANKL interacts with cytokines to phosphorylate downstream PKB/AKT in the cytoplasm and promotes the formation of osteoclasts.75 The imbalance in the process of osteoclast formation activation is the main cause of bone destruction. Hippo pathway also performs an extremely vital function in regulation of bone balance.
Regulation of Osteoclasts by YAP/TAZ
YAP, an effector molecule in Hippo pathway, induces AKT phosphorylation, suggesting that YAP can promote osteoclast formation, which is blocked when the activity of YAP is checked using verteporfin.76 When the manifestation of connective tissue growth factor (CTGF), the target gene of YAP, is down-regulated, the differentiation process of osteoclast precursor cells into mature osteoclasts will be destroyed.77 However, TAZ curbs osteoclast differentiation. It was found that when TAZ gene knockout induced increased osteoclast formation in mice, the over expression of TAZ blocked RANKL-induced osteoclast formation, which was confirmed by osteoclast formation assay in vitro. However, silencing of TAZ reduces its formation because TAZ binds to transforming growth factor-β-activated kinase 1 (TAK1) and mutually restrains Nuclear factor kappa B (NF-κB) signaling, restricting osteoclast differentiation.78 The specific role of YAP/TAZ in bone destruction of RA has not been reported.
Regulation of Osteoclasts by MST1/2
The precursor cells of osteoclasts came from hematopoietic stem cells in bone marrow. The core members of Hippo pathway can affect the differentiation of hematopoietic stem cells, and further affect the differentiation and development of osteoclasts. MST1/2 kinase, an upstream molecule in Hippo pathway, may affect the gene locus of hematopoietic stem cells and the development of osteoclast precursor cells.79 The model of MST1/2 gene knockout mice was established. It was found80 that mice with MST1/2 deficiency could develop the osteoporosis phenotype, and the number of osteoclasts increased in vivo. MST1/2 was considered to be related to the proliferation and apoptosis of mouse osteoclasts. Embryonic mice lacking MST1 or MST1/2 genes (MST1−/−or MST1/2−/−) have a decreased proliferation capacity of haematopoietic stem cells (HSCS) because the generation of HSCS derived osteoclast precursor cells may be obstructed. Meanwhile, a large number of apoptosis cells can be observed in MST1−/− and MST1/2−/− mice at the embryonic stage, showing that MST1/2 performs a main function maintaining cell number and supporting cell life activities.81 In the absence of MST1/2 kinase in osteoclasts, the NF-κB pathway is further activated, which subsequently leads to increased osteoclast formation, and the expression of RANKL induced activated T-nuclear factor 1 and tartrate-resistant acid phosphatase is increased. On the contrary, when MST1/2 is overexpressed in osteoclasts, it can suppress the RANKL caused osteoclast differentiation.79 Studies82 have observed that the degree of expression of MST1 kinase in serum of RA sufferers used to be drastically decreased than that of sufferers with systemic lupus erythematosus (SLE) and health controls. At the same time, there was a remarkable negative correlation between the expression level of MST1 kinase and the degree of bone erosion. Most SLE patients do not have bone destruction in joints, while RA patients are characterized by synovitis and bone erosion.83 This suggests that MST1 kinase is a protective factor of bone erosion in RA patients.84 MST1/2 has a regulatory effect on osteoclasts. However, the concrete mechanism is unknown.
Hippo Pathway is the Direction of Treatment for RA
The Treatment Dilemma of RA
As a chronic disease, RA has no radical treatment at present. It can only be alleviated by drugs, which hinders the development of the disease. Although the rapid development of modern science and technology, the vigorous development of molecular biology technology, and the mature application of chemical medicine and pharmacognosy technology bring infinite hope for the development of new drugs for the treatment of RA, there are still limited drugs to choose at present, far from meeting the needs of patients. The predicament of RA remedy is that a huge range of sufferers have no response or low response to present pills. For example, the Rheumatology 70% response criteria (ACR70) response charge to methotrexate is commonly round 20–40%. The ACR70 response rate to TNF inhibitors, IL-6 receptor inhibitors, t-cell co-stimulator blockers, and b-cell inhibitors is about 30–40% in combination with methotrexate therapy.85 However, this does no longer rule out the 20–25% of sufferers who reply to methotrexate alone. In addition, extraordinary organic dealers have comparable drug resistance, suggesting that they may additionally intervene RA in the equal way.86,87 Another dilemma in the treatment of RA is manifested in the extreme scarcity of drugs that inhibit joint destruction. Early researches have proven that RA joint destruction is positively correlated with neighborhood inflammatory activity, and inhibition of inflammatory response is really helpful to the method of joint destruction.88 However, some researches in current years have proven that joint destruction is nonetheless progressing even when the ailment undertaking of RA is very low. When clinicians used TNF-α inhibitors to deal with RA, they observed that in some patients, the destruction of joints was once inhibited when the manipulation of irritation used to be no longer perfect.89–91 The reality that the joint inflammatory response and joint destruction are now not usually constant suggests that there may additionally be mechanisms unbiased of irritation in RA joint destruction, whereas there are exceedingly few tablets (for example, Iguratimod) that can inhibit joint destruction in RA.
Hippo Pathway is a Potential Target for the Treatment of RA
Currently, there is no cure for RA,92 however, with the deepening of research, it has progressively developed from non-steroidal anti-inflammatory tablets and anti-rheumatic pills to focused pills organic agents, which can greater efficaciously manipulate the situation of RA sufferers and significantly enhance the nice of existence of patients.93 In latest years, the lookup on RA generally targeted on the interleukin household (such as IL-17, IL-6), TNF-α and different downstream cytokines.94 It is now not very clear how the upstream of every channel regulates the prevalence and improvement of RA. At present, extensively used biologics in the medical institution additionally act on inflammatory elements downstream of the pathway, and the incapability to inhibit the improvement of RA disorder from upstream renders some sufferers much less responsive to present biologics; therefore, there is nonetheless a pressing want to discover new targets.95 The core effector molecules in the Hippo pathway have immunomodulatory functions, participate in the pathogenicity of synovial fibroblasts, and regulate osteoclast differentiation. Therefore, the Hippo pathway is an achievable goal for the cure of rheumatoid Arthritis (Table 1).
 |
Table 1 Hippo Pathway is Involved in the Pathogenesis of RA |
Conclusion and Perspective
The regulatory function of multiple members of Hippo pathway in the immune system has gradually become a research hotspot, especially the regulatory role of Hippo pathway plays in the development and differentiation of immune cells, autoimmune diseases, and the response to the invasion of viruses and bacteria. Hippo pathway exists in different cells, tissues, and organs of human body. It plays an important role not only in regulating the size, growth and development of organs but also in maintaining the stability of immune system and regulating the differentiation of synovial fibroblasts and osteoclasts. The Hippo pathway may be involved in the pathogenesis of RA. Autoimmune imbalance occurs when the expression of MST1/2, the key molecule of Hippo pathway, is decreased, which may be converted into the pathogenesis of RA. Transcription activity of YAP/TAZ promotes the invasiveness of rheumatoid arthritis synovial fibroblasts, which assists in the achievement of the occurrence of RA. Regulation of osteoclasts by MST1 kinase may be a protective factor for bone erosion in RA patients. Therefore, it may be future therapeutic targets for RA to promote the expression of MST1/2 and inhibit the expression of YAP/TAZ. However, the connection between the key molecules of the Hippo pathway and the precise pathogenesis of RA remains unclear. Supplementary studies on the precise mechanisms of the core effector molecules, upstream and downstream regulators of the Hippo pathway in the pathogenesis of RA would be of enormous significance for the research of new targets for RA in the future.
Data Sharing Statement
The data are included in the article as table.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work received support from the National Natural Science Foundation of China (81860858 and 81960832), Youth Science and technology Fund of Science and Technology Department of Gansu Province (21JR11RA158).
Disclosure
The authors declare that they have no conflicts of interest.
References
1. Lubberts E. Role of T lymphocytes in the development of rheumatoid arthritis. Implications for treatment. Curr Pharm Des. 2014;21(2):142–146. doi:10.2174/1381612820666140825122247
2. Feldmann M. Clinical research progress on the pathogenesis of rheumatoid arthritis. Clin Med. 2012;42(07):123–125.
3. Kojima F, Sekiya H, Hioki Y. Facilitation of colonic T cell immune responses is associated with an exacerbation of dextran sodium sulfate-induced colitis in mice lacking microsomal prostaglandin E synthase-1. Inflamm Regen. 2022;42:1. doi:10.1186/s41232-021-00188-1
4. Zaid T, Janse van Rensburg Helena J, Xiaolong Y. The Hippo Pathway: immunity and cancer. Cancers. 2018;10:4.
5. Yuting S, De J, Ziwei Z, et al. The critical role of the Hippo pathwayHippo pathway in kidney diseases. Front Pharmacol. 2022;13:1.
6. Yu FX, Zhao B, Guan KL. Hippo pathway in organ size control, tissue homeostasis, and cancer. Cell. 2015;163(4):811–828.
7. Hong L, Li X, Zhou D, et al. Role of Hippo signaling in regulating immunity. Cell Mol Immunol. 2018;15(12):1003–1009.
8. Geng J, Yu S, Zhao H, et al. The transcriptional coactivator TAZ regulates reciprocal differentiation of Th17 cells and Treg cells. Nat Immunol. 2017;18(7):800–812.
9. Mou F, Praskova M, Xia F, et al. The Mst1 and Mst2 kinases control activation of rho family GTPases and thymic egress of mature thymocytes. J Exp Med. 2012;209(4):741–759.
10. Samson M, Audia S, Janikashvili N, et al. Brief report: inhibition of interleukin-6 function corrects Th17 / Treg cell imbalance in patients with rheumatoid arthritis. Arthritis Rheumatism. 2012;64(8):2499–2503.
11. Creasy CL, Chernoff J. Cloning and characterization of a member of the MST subfamily of Ste20-like kinases. Gene. 1995;167(1–2):303–306.
12. Creasy CL, Chernoff J. Cloning and characterization of a human protein kinase with homology to Ste20. J Biol Chem. 1995;270(37):21695–21700.
13. Tao WF, Zhang S, Turenchalk GS, et al. Human homologue of the drosophila melanogaster lats tumour suppressor modulates CDC2 activity. Nat Genet. 1999;21(2):177–181.
14. Bichsel SJ, Tamaskovic R, Stegert MR, Hemmings BA. Mechanism of activation of NDR (nuclear Dbf2-related) protein kinase by the hMOB1 protein. J Biol Chem. 2004;279(34):35228–35235.
15. Tapon N, Harvey KF, Bell DW, et al. Salvador promotes both cell cycle exit and apoptosis in drosophila and is mutated in human cancer cell lines. Cell. 2002;110(4):467–478.
16. Sudol M. Yes-associated protein (YAP65) is a proline-rich phosphoprotein that binds to the SH3 domain of the YES proto-oncogene product. Oncogene. 1994;9(8):2145–2152.
17. Kanai F, Marignani PA, Sarbassova D, et al. TAZ: a novel transcriptional co-activator regulated by interactions with 14-3-3 and PDZ domain proteins. EMBO J. 2000;19(24):6778–6791.
18. Halder G, Johnson RL. Hippo signaling: growth control and beyon. Development. 2011;138(1):9–22.
19. Pan D. The Hippo pathway in development and cancer. Dev Cell. 2010;19(4)):491–505.
20. Zhou DW, Conrad C, Xia F, et al. Mst1 and Mst2 maintain hepatocyte quiescence and suppress hepatocellular carcinoma development through inactivation of the YAP1 oncogene. Cancer Cell. 2009;16(5):425–438.
21. Zhou DW, Medoff BD, Chen LF, et al. The Nore1b/Mst1 complex restrains antigen receptor-induced proliferation of naïve T cells. Proc Natl Acad Sci USA. 2008;105(51):20321–20326.
22. Zhou DW, Zhang YY, Wu HT, et al. Mst1 and Mst2 protein kinases restrain intestinal stem cell proliferation and colonic tumorigenesis by inhibition of yes-associated protein (YAP) overabundance. Proc Natl Acad Sci USA. 2011;108(49):E1312–E1320.
23. Lee KP, Lee JH, Kim TS, et al. The Hippo-Salvador pathway restrains hepatic oval cell proliferation, liver size, and liver tumorigenesis. Proc Natl Acad Sci USA. 2010;107(18):8248–8253.
24. Lu L, Li Y, Kim SM, et al. signaling is a potent in vivo growth and tumor suppressor pathway in the mammalian liver. Proc Natl Acad Sci USA. 2010;107(4):1437–1442.
25. Song H, Mak KK, Topol L, et al. Mammalian Mst1 and Mst2 kinases play essential roles in organ size control and tumor suppression. Proc Natl Acad Sci USA. 2010;107(4):1431–1436.
26. Heallen T, Zhang M, Wang J, et al. Hippo pathway inhibits wnt signaling to restrain cardiomyocyte proliferation and heart size. Science. 2011;332(6028):458–461.
27. Gao T, Zhou DW, Yang CH, et al. Hippo signaling regulates differentiation and maintenance in the exocrine pancreas. Gastroenterology. 2013;144(7):1543–1553.e1.
28. Zhou D, Zhang Y, Wu H, et al. Mst1 and Mst2 protein kinases restrain intestinal stem cell proliferation and colonic tumorigenesis by inhibition of Yes-associated protein (YAP) overabundance. Proc Natl Acad Sci USA. 2011;108(49):E1312–E1320.
29. Zhang N, Bai H, David KK, et al. The Merlin /NF2 tumor suppressor functions through the YAP oncoprotein to regulate tissue homeostasis in mammals. Dev Cell. 2010;19(1):27–38.
30. Zhao B, Wei X, Li W, et al. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 2007;21(21):2747–2761.
31. Hao Z, Yiran S, Jin-Jia Z, et al. Non-canonical Hippo pathway regulates immune homeostasis. J Xiamen Univ. 2021;61(03):377–386.
32. Zhou D, Medoff BD, Chen L, et al. The Nore1B /Mst1 complex restrains antigen receptor-induced proliferation of naïve T cells. Proc Natl Acad Sci USA. 2008;105(51):20321–20326.
33. Yuan ZQ, Lehtinen MK, Merlo P, Villén J, Gygi S, Bonni A. Regulation of neuronal cell death by MST1-FOXO1 signaling. J Biol Chem. 2009;284(17):11285–11292.
34. Geng J, Sun X, Wang P, et al. Kinases Mst1 and Mst2 positively regulate phagocytic induction of reactive oxygen species and bactericidal activity. Nat Immunol. 2015;16(11):1142–1152.
35. Liu B, Zheng Y, Yin F, et al. Toll receptor-mediated hippo signaling controls innate immunity in Drosophila. Cell. 2016;164(3):406–419.
36. Wang S, Xie F, Chu F, et al. YAP antagonizes innate antiviral immunity and is targeted for lysosomal degradation through IKKε-mediated phosphorylation. Nat Immunol. 2017;18(7):733–743.
37. Zhang Q, Meng F, Chen S, et al. Hippo signalling governs cytosolic nucleic acid sensing through YAP / TAZ-mediated TBK1blockade. Nat Cell Biol. 2017;19(4):362–374.
38. Fallahi E, O’Driscoll NA, Matallanas D. The MST/Hippo Pathway and cell death: a non-canonical affair. Genes. 2016;7(6):28.
39. Furth N, Aylon Y, Oren M. p53 shades of Hippo. Cell Death Differ. 2018;25:81–92.
40. Zhou D, Medoff BD, Chen LF, et al. The Nore1B/Mst1 complex restrains antigen receptor-induced proliferation of naïve T cells. Proc Natl Acad Sci USA. 2008;105(51):20321–20326.
41. Geng J, Hong LX, Chen LF. Research progress of Hippo pathway related molecules involved in the regulation of immune cell function. Bioscience. 2017;29(9):908–915.
42. Yu-jie D, Jin-qiu L, Wen-ling L, et al. Reciprocal inhibition of YAP/TAZ and NF- κB regulates osteoarthritic cartilage degradation. Nat Commun. 2018;9(1):4564.
43. Abdollahpour H, Appaswamy G, Kotlarz D, et al. The phenotype of human STK4 deficiency. Blood. 2012;119(15):3450–3457.
44. Nehme NT, Schmid JP, Debeurme F, et al. MST1 mutations in autosomal recessive primary immunodeficiency characterized by defective naïve T-cell survival. Blood. 2012;119(15):3458–3468.
45. Dong Y, Du X, Ye J, et al. A cell intrinsic role for Mstl in regulating thymocyte egress. J Immunol. 2009;183(6):3865–3872.
46. Du X, Shi H, Li J, et al. Mst1/Mst2 regulate development and function of regulatory T cells through modulation of Foxo1 / Foxo3 stability in autoimmune disease. J Immunol. 2014;192(4):1525–15 35.
47. Du XR, Shi H, Li J, et al. Mst1/Mst2 regulate development and function of regulatory T cells through modulation of Foxo1/Foxo3 stability in autoimmune disease. J Immunol. 2014;192(4)::1525–1535.
48. Tomiyama T, Ueda Y, Katakai T, Kondo N, Okazaki K, Kinashi T. Antigen-specific suppression and immunological synapse formation by regulatory T cells require the Mst1 kinase. PLoS One. 2013;8(9):e73874.
49. Jun-Hong L, Hu-Yue-yue L, Lan-fen C. Research progress of Hippo pathway in regulating the maintenance of immune cell homeostasis. Chin J Immunol. 2019;35(15):1793–1801.
50. Geng J, Yu SJ, Zhao H, et al. The transcriptional coactivator TAZ regulates reciprocal differentiation of TH17 cells and Treg cells. Nat Immunol. 2017;18(7):800–812.
51. Xin L, Xiong C, Liang L. Research progress on the role of Th17 cells in the pathogenesis of rheumatoid arthritis. Chin Pharmacol Bull. 2020;36(3)::309–12.
52. Feng Z, Zongshun D, Lin Y, et al. Study on the molecular mechanism of “wind, cold and damp” affecting Th17/Treg imbalance and promoting rheumatoid arthritis syndrome. J Hum Univ Trad Chin Med. 2021;41(11):1657–1662.
53. Weiqi Z, Xu L, Cheng ZY, et al. Transcriptional and posttranslational regulation of Th17/Treg balance in health and disease. Eur J Immunol. 2021;51:9.
54. Geng J, Sun XF, Wang P, et al. Kinases Mst1 and Mst2 positively regulate phagocytic induction of reactive oxygen species and bactericidal activity. Nat Immunol. 2015;16(11):1142–1152.
55. Tomiyama T, Ueda Y, Katakai T, et al. Antigen-specific suppression and immunological synapse formation by regulatory T cells require the Mstl kinase. PLoS One. 2013;8(9):e73874.
56. Li XJ, Park ES, Park MH, et al. 3,3’-Diindolylmethane suppresses the growth of gastric cancer cells via activation of the Hippo signaling pathway. Oncol Rep. 2013;30(5):2419–2426.
57. Salojin KV, Hamman BD, Chang WC, et al. Genetic deletion of Mst1 alters T cell function and protects against autoimmunity. PLoS One. 2014;9:e98151.
58. Chen X. The Role and Clinical Significance of TAZ in the Pathogenesis of Rheumatoid Arthritis. Xiamen University; 2019.
59. Liu L, Ji Y, Zu B, et al. Acacetin regulated the reciprocal differentiation of Th17 cells and Treg cells and mitigated the symptoms of collagen-induced arthritis in mice. Scand J Immunol. 2018;88(4):el2712.
60. Wu J, Yang XH, Ma L, et al. Effects of miR-26a-5p on apoptosis of human rheumatoid arthritis fibroblast-like synoviocytes through JAK2/STAT3 signaling pathway. J Jilin Univ. 2021;47(2):460–468.
61. Bottini N, Firestein GS. Duality of fibroblast-like synoviocytes in RA: passive responders and imprinted aggressors. Nat Rev Rheumatol. 2013;9(1):24–33.
62. Piccolo S, Dupont S, Cordenonsi M. The biology of YAP/TAZ: hippo signaling and beyond. Physiol Rev. 2014;94(4):1287–1312.
63. Bottini A, Wu DJ, Ai R, et al. PTPN14 phosphatase andYAP promote TGFβ signalling in rheumatoid synoviocytes. Ann Rheum Dis. 2019;78(5):600–609.
64. Wang Z, Lu W, Zhang Y, et al. The Hippo Pathway and viral infections. Front Microbiol. 2019;10:3033.
65. Zhang S, Chen Q, Liu Q, et al. Hippo signaling suppresses cell ploidy and tumorigenesis through Skp2. Cancer Cell. 2017;31(5):669–684.e7.
66. Robin C, Estelle A, Guillaume C, et al. YAP/TAZ: key players for rheumatoid arthritis severity by driving fibroblast like synoviocytes phenotype and fibro-inflammatory response. Front Immunol. 2021;12:1.
67. Pai-Pi G. The Mechanism of GRK2 Shutting Down Hippo-YAP Signaling to Promote Abnormal Proliferation of Fibroblast-Like Synoviocytes. Anhui Medical University; 2021.
68. Sheng Q. Knockdown of YAP and TAZ Inhibit the Migration and Invasion of RA-FLS by Regulating Autophagy. Yangzhou University; 2019.
69. Totaro A, Panciera T, Piccolo S. YAP/TAZ upstream signals and downstream responses. Nat Cell Biol. 2018;20(8)::888–899.
70. He L, Pratt H, Gao M, et al. YAP and TAZ are transcriptional co-activators of AP-1 proteins and STAT3 during breast cellular transformation. Elife. 2021;10:e67312.
71. Caire R, Audoux E, Courbon G, et al. YAP/TAZ: key players for rheumatoid arthritis severity by driving fibroblast like synoviocytes phenotype and fibro-inflammatory response. Front Immunol. 2021;12:1.
72. Zhou W, Shen Q, Wang H, et al. Knockdown of YAP/TAZ inhibits the migration and invasion of fibroblast synovial cells in rheumatoid arthritis by regulating autophagy. J Immunol Res. 2020;2020:1.
73. Roelofs AJ, Zupan J, Riemen AHK, et al. Joint morphogenetic cells in the adult mammalian synovium. Nat Commun. 2017;8:15040.
74. Baum R, Gravallese EM. Impact of inflammation on the osteoblast in rheumatic diseases. Curr Osteo Poros Rep. 2014;12(1):9–16.
75. Okamoto K, Nakashima T, Shinohara M, et al. Osteoim munology: the conceptual framework unifying the immune and skeletal systems. Physiol Rev. 2017;97(4):1295–1349.
76. Zhao L, Guan H, Song C, et al. YAP1 is essential for osteoclastogenesis through a TEADs-dependent mechanism. Bone. 2018;110:177–186.
77. Aoyama E, Kubota S, Khattab HM, et al. CCN2 enhances RANKL-induced osteoclast differentiation via direct binding to RANK and OPG. Bone. 2015;73:242–248.
78. Wanlei Y, Xuanyuan L, Tan Z, et al. TAZ inhibits osteoclastogenesis by attenuating TAK1/NF-κB signaling. Bone Res. 2021;9(1):1–10.
79. Wan-lei Y, Wei-qi H, Qin A, et al. The emerging role of Hippo pathway in regulating osteoclast formation. J Cell Physiol. 2018;233(6):4606–4617.
80. Lee J, Youn BU, Kim K, et al. Mst2 controls bone homeostasis by regulating osteoclast and osteoblast differentiation. J Bone Miner Res. 2015;30(9):1597–1607.
81. Oh S, Lee D, Kim T, et al. Crucial role for Mst1 and Mst2 kinases in early embryonic development of the mouse. Mol Cell Biol. 2009;29(23):6309–6320.
82. Feng YC. Expression and Clinical Study of Serum MST1 Kinase Level in Rheumatoid Arthritis. Lanzhou: The Second Clinical Medical College of Lanzhou University; 2019.
83. Siyu S, Xiaodong F. Clinical research progress of joint pain in systemic lupus erythematosus. Chin J Pain Med. 2016;22:141–143.
84. Zhang SD, Wang YQ, Liu YW, et al. Research progress of traditional Chinese medicine in delaying the process of bone erosion in rheumatoid arthritis. Chin J Trad Chin Med. 2021;37(2):321–325.
85. Fu SD. Advances in new target drugs for rheumatoid arthritis. Chin J Drug Eval. 2012;39(1):44–47.
86. Schoels M, Aletaha D, Smolen JS, Wong JB. Comparative effectiveness and safety of biological treatment options after tumour necrosis factor alpha inhibitor failure in rheumatoid arthritis: systematic review and indirect pairwise meta-analysis. Ann Rheum Dis. 2012;71(8):1303–1308.
87. Smolen JS, Kay J, Matteson EL, et al. Insights into the efficacy of golimumab plus methotrexate in patients with active rheumatoid arthritis who discontinued prior anti-tumour necrosis factor therapy: post-hoc analyses from the GO-AFTER study. Ann Rheum Dis. 2014;73(10):1811–1818.
88. Ruihua L, Zengyu C, Haodong X, et al. Research progress on influencing factors of bone destruction in rheumatoid arthritis. Chinese Med Guide. 2022;19(35):36–39+65.
89. Aletaha D, Smolen JS. Joint damage in rheumatoid arthritis progresses in remission according to the disease activity score in 28 joints and is driven by residual swollen joints. Arthritis Rheum. 2011;63(12):3702–3711.
90. Binder NB, Puchner A, Niederreiter B, et al. Tumor necrosis factor-inhibiting therapy preferentially targets bone destruction but not synovial inflammation in a tumor necrosis factor-driven model of rheumatoid arthritis. Arthritis Rheum. 2013;65(3):608–617.
91. Smolen JS, Han C, Bala M, et al. Evidence of radiographic benefit of treatment with infliximab plus methotrexate in rheumatoid arthritis patients who had no clinical improvement: a detailed subanalysis of data from the anti-tumor necrosis factor trial in rheumatoid arthritis with concomitant therapy study. Arthritis Rheum. 2005;52(4):1020–1030.
92. Qianwen Y, Yusong W, Fen L. Research progress on the mechanism of drugs for the treatment of rheumatoid arthritis. Chin J Med Front. 2020;12(11):4–8.
93. Burmester GR, Pope JE. Novel treatment strategies in rheumatoid arthritis. Lancet. 2017;389(10086):2338–2348.
94. Papadaki G, Goutakoli P, Tiniakou I, et al. IL-6 signaling attenuates TNF-α production by plasmacytoid dendritic cells in rheumatoid arthritis. J Immunol. 2022;2022:1.
95. Lefeng C, Jianda M, Qianhua L, Yi D. Effect of compliance with biological agents on treatment target of rheumatoid arthritis. New Medi. 2019;50(05):313–318.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.