Back to Journals » Therapeutics and Clinical Risk Management » Volume 18
Poor Sensorium at the Time of Intubation Predicts Polymicrobial Ventilator Associated Pneumonia
Authors Natarajan R, Ramanathan V , Sistla S
Received 7 September 2021
Accepted for publication 28 January 2022
Published 17 February 2022 Volume 2022:18 Pages 125—133
DOI https://doi.org/10.2147/TCRM.S337341
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Garry Walsh
Ramachandran Natarajan,1 Venkateswaran Ramanathan,1 Sujatha Sistla2
1Department of Medicine, Jawaharlal Institute of Postgraduate Medical Education and Research, Puducherry, India; 2Department of Microbiology, Jawaharlal Institute of Postgraduate Medical Education and Research, Puducherry, India
Correspondence: Venkateswaran Ramanathan, Department of Medicine, Jawaharlal Institute of Postgraduate Medical Education and Research, Dhanvantri Nagar, Puducherry, 605006, India, Email [email protected]
Background: Ventilator-associated pneumonia (VAP) is the most common nosocomial infection in the intensive care unit and is associated with a high mortality rate.
Aim: The study was conducted to estimate the frequency, outcomes, and predictors of polymicrobial VAP.
Methods: A prospective observational study was conducted in mechanically ventilated adult patients in the medical intensive care unit in a tertiary care hospital in India from July 2016 to July 2018 with a 30-day follow-up period. The patients were grouped into monomicrobial and polymicrobial VAP. We compared the 30-day outcome parameters such as discharge from hospital, in-hospital stay, death, and complications such as catheter associated urinary tract infection (CAUTI), central line associated blood stream infection (CRBSI), bacteremia and collapse of lung. The predictors of polymicrobial VAP were identified by multiple logistic regression.
Results: Out of 301 patients clinically diagnosed with VAP, 151 patients were excluded, and the remaining 150 developed 186 episodes of VAP during the study period. The incidence of polymicrobial VAP was 62.9%. Out of 150 patients, 51 patients had monomicrobial VAP, and 99 had polymicrobial VAP. On univariate analysis, diabetes mellitus and poor sensorium (Glasgow Coma Scale [GCS] score < 8) during endotracheal intubation; 30-day outcome, mean days of mechanical ventilation after VAP diagnosis and days in ICU; and CAUTI were significantly associated with polymicrobial VAP. On multivariable logistic regression, poor sensorium (GCS score < 8) at the time of endotracheal intubation was an independent predictor of polymicrobial VAP.
Conclusion: The incidence of polymicrobial VAP is high in the medical ICU and is associated with increased duration of mechanical ventilation, hospital stay, and incidence of CAUTI. Poor GCS score was the single independent predictor of polymicrobial VAP.
Keywords: monomicrobial VAP, nosocomial infection
Introduction
Ventilator-Associated Pneumonia (VAP) is a common nosocomial infection in the Intensive Care Unit (ICU) associated with high attributable mortality and cost.1–3 Unlike Community-Acquired Pneumonia (CAP) that is mainly caused by Gram-positive organisms, VAP is usually caused by Gram-negative organisms that are often multi-drug resistant, especially in Asian countries.4 Hence, high-end antibiotics are used in the empirical therapy for VAP. In ICU, about 50% of high-end antibiotics are used in the treatment of VAP.5 In low- and middle-income countries, Healthcare-Associated Infection (HCAI) rates are higher than the high-income countries, probably due to lack of infection prevention bundles or poor implementation of the same.6
In contrast to most nosocomial infections, VAP can be polymicrobial as well. Studies regarding polymicrobial VAP (pmVAP) are scarce, and the frequency of pmVAP is not known. In general, pmVAP is predominantly caused by Gram negative organisms like Acinetobacter baumannii, Pseudomonas aeruginosa and Klebsiella pneumoniae.7,8 Though it is well established that VAP caused by Methicillin-resistant Staphylococcus aureus (MRSA) is less common in India as compared to Western countries,9 the patterns of pmVAP is not clear. Patients with VAP caused by more than one organism may be systematically different from those caused by a single organism in both their baseline characteristics and outcome. The usual indications for admission in ICU include circulatory shock, cardiac arrest, respiratory failure and airway protection. Poor sensorium (GCS score <8), often secondary to cerebral disorders and encephalopathy, is one of the common causes that requires endotracheal intubation (for airway protection) and mechanical ventilation.
With this background, we undertook this study to determine the proportion, pattern of organisms, and the predictors of pmVAP among patients admitted in the Medical ICU. Also, we compared the outcomes of patients with pmVAP with monomicrobial VAP (mmVAP).
Methods
Study Design
This prospective observational study was conducted in the Medical Intensive Care Unit (MICU) of a tertiary care center in Southern India between July 2016 and July 2018.
Study Participants
Patients of age 18 years or more, mechanically ventilated for more than 48 hours and those clinically diagnosed with Ventilator-Associated Pneumonia (VAP) as per modified Clinical Pulmonary Infection Score (mCPIS)10 were included in the study after obtaining signatures in the written informed consent from the Legally Authorised Representative and participants after their recovery. The study was done as per the guidelines outlined in the Declaration of Helsinki and after approval by the Institute Ethics Committee at Jawaharlal Institute Post-Graduate Medical Education and Research, Puducherry (Certificate No.: JIP/IEC/2016/28/928). Patients in whom the mechanical ventilation was initiated before admission, antibiotic use in the four weeks prior to mechanical ventilation, newly diagnosed pneumonia prior to ventilation, admission in MICU 48 hours after initiation of mechanical ventilation, and patients who are immunosuppressed due to HIV infection, cancer chemotherapy, neutropenia, cytotoxic therapy or post-transplantation were excluded (Figure 1).
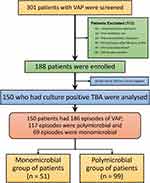 |
Figure 1 Patient enrolment flow diagram. Abbreviations: MV, mechanical ventilation; VAP, ventilator-associated pneumonia; TBA, tracheobronchial aspirate. |
Sample Size Estimation
Assuming a pmVAP incidence of 30%, with an 8% margin of error, with a level of significance at 0.05, the sample size estimated was 126 patients. Assuming a 15% failure to collect data, the sample size was corrected and rounded off to 150.
Study Procedure
Baseline clinical characteristics, including SAPS II score at admission, were recorded. Endotracheal Aspirate (ETA) was sent for microbiological culture at the time of clinical suspicion of VAP. ETA was collected and cultured as per standard recommendations.11 Patients were diagnosed to have “clinical VAP” if the initial mCPIS score at the time of suspicion or the CPIS score repeated after 72 hours was 6 or above. Patients whose ETA was culture positive for Potentially Pathogenic Microorganisms (PPM) with a semi-quantitative estimation of ETA culture of PPM≥105CFU/mL were considered to “microbiologically confirmed VAP”. Patients with clinically diagnosed VAP (CPIS score ≥6) but with negative ETA culture reports were excluded from the study. Subsequently, the patients were followed-up for 30 days for further episodes of VAP, outcome measures, and complications. Candida spp., Streptococcus viridans, Staphylococcus epidermidis, Neisseria spp., Enterococcus spp., Corynebacterium spp. were not considered as PPM.
Each episode of VAP was defined based on a duration of ten days from the diagnosis of VAP. Since the course of antibiotic therapy for VAP rarely exceeds ten days and to identify subsequent episodes of VAP, we defined the duration of each episode as ten days. A monomicrobial VAP episode was defined as a VAP episode in which not more than one PPM was isolated in the single ETA or multiple ETA collected during the episode. A polymicrobial VAP episode was defined as a VAP episode during which more than one PPM was isolated either in the single ETA or in the multiple ETA collected during the episode. Patients were grouped into polymicrobial VAP if they developed at least one polymicrobial VAP episode during the 30-day follow-up period. Other patients were grouped into monomicrobial VAP. The denominator for calculation of incidence of polymicrobial VAP was the total number of VAP episodes within 30 days from day of diagnosis of the first episode of VAP among study population. The numerator was the total number of polymicrobial VAP episodes within 30 days from day of diagnosis of the first episode of VAP among study population.
Statistical Analysis
The data were compiled using Microsoft Excel spreadsheet and analyzed using IBM SPSS Statistics for Windows, version 19.0 (IBM Corp., Armonk, NY, USA). Descriptive and inferential statistics were used to analyze the data. P-value <0.05 was considered statistically significant. The 30-day outcomes between polymicrobial and monomicrobial VAP were compared using Chi-square test for linear trend. To compare continuous normally distributed data, the two-tailed unpaired t-test was used. The strength of association was measured as odds ratios. Unadjusted odds ratio with confidence intervals were reported in univariate analyses. The predictors were explored using multivariable logistic regression. Backward stepwise elimination was used to identify the predictors that were significant enough to be included in a predictive model with P ≤ 0.25 for inclusion and P ≤ 0.1 for retention.
Results
During the study period, 1438 admissions were made in MICU. Among these patients, 301 patients who developed VAP were screened, and 150 patients were included in the study. These 150 patients developed 186 VAP episodes, out of which 117 were polymicrobial episodes, and 69 were monomicrobial episodes. The baseline clinical characteristics of those with pmVAP were comparable with mmVAP except for the significantly higher proportion of patients with diabetes in the mmVAP group and poor sensorium (Glasgow Coma Scale [GCS] score less than 8) at the time of endotracheal intubation in the pmVAP group (Table 1). There was no difference in the mean duration of mechanical ventilation before VAP between monomicrobial and polymicrobial group (4.8days vs 5.8 days; P=0.41).
 |
Table 1 Baseline Clinical Characteristics of the Study Participants Including Risk Factor Analysis |
Out of 150 patients, 31 patients had more than one VAP episode, of which 26 had two episodes, and 5 had three VAP episodes during the 30-day study period (Figure 2). Polymicrobial VAP episodes consistently outnumbered mmVAP episodes in both initial and recurrent episodes. The proportion of pmVAP during the study period was 62.9% (95% CI, 55.9 to 69.9). According to the CDC surveillance criteria, the VAP rate among the study population during the study period, was 40 per 1000 ventilator days. In comparison to NHSN standards, our ICU had a higher incidence of VAP.
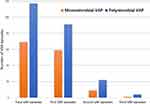 |
Figure 2 Distribution of monomicrobial and polymicrobial VAP episodes. Abbreviation: VAP, ventilator-associated pneumonia. |
Gram-negative bacilli were the predominant organisms isolated. Pseudomonas aeruginosa, Acinetobacter baumannii, and Klebsiella pneumoniae were the three most common pathogens among monomicrobial and polymicrobial VAP episodes (Table 2). Escherichia coli, Enterobacter spp, Acinetobacter lwoffii, and other Non-fermenting Gram-negative Bacilli (NFGNB) were isolated only from polymicrobial VAP episodes. MSSA and MRSA were almost equally isolated in both monomicrobial and polymicrobial groups.
 |
Table 2 Distribution of Organisms Among Episodes of Monomicrobial and Polymicrobial Ventilator-Associated Pneumonia (VAP) |
Among the different combinations of organisms causing pmVAP, the combination of Non-fermenters (Acinetobacter baumannii, Pseudomonas aeruginosa, Acinetobacter lwoffii, and other non-fermenting Gram-negative bacilli) with Enterobacteriaceae (Klebsiella pneumoniae, Escherichia coli and Enterobacter spp) constituted 87.2% (102 out of 117 episodes). Gram-positive organisms were present in 12.8% of the combinations (Table 3).
 |
Table 3 Frequency of Different Microbial Pathogen Combinations in Polymicrobial VAP |
Out of 150 patients, 99 had at least one polymicrobial episode during the study period and were grouped into polymicrobial VAP. The remaining 51 patients had one or more monomicrobial episodes of VAP. The 30-day outcomes between the mmVAP and pmVAP groups were statistically significant (P=0.02 by Chi-squared analysis for linear trend). Patients with pmVAP had a significantly longer duration of mechanical ventilation (6.5 vs 10.7 days; P<0.001), ICU stay (13.5 vs 17.8 days; P=0.008), and hospital stay (19.5 vs 26.3 days; P=0.002) than mmVAP group. Among the complications, patients with pmVAP had a significantly higher (9.8% vs 26%; P=0.01) Catheter-Associated Urinary Tract Infection (CAUTI) than mmVAP (Table 4).
 |
Table 4 Comparison of Outcome Parameters Between Monomicrobial and Polymicrobial Groups |
To adjust for confounding, twelve predictors were analyzed using multivariable logistic regression. A GCS score of less than 8 at the time of endotracheal intubation was found to be the single most independent predictor (odds ratio, 2.70; 95% CI, 1.13 to 6.48) of polymicrobial VAP after adjustment for confounders (Table 1). Only four predictors (GCS <8 at intubation, diabetes, duration of mechanical ventilation before VAP and male gender) were found to be significant enough to be included in a predictive model after backward stepwise elimination with P ≤ 0.25 for inclusion and P ≤ 0.1 for retention.
Discussion
In resource-limited settings, pmVAP is very common, but there is a paucity of data in literature. Hence, we undertook this study to determine the prevalence and pattern of polymicrobial VAP.
The mean (SD) age of the patients in our study population was 45.1216 years, which is much lower than the previous studies.8,12 Males constituted 67% of the study population, and it was similar to previous studies. Emergency intubation was done in 85% of our cases. Among the risk factors, diabetes mellitus was the most common comorbidity present. Risk factors like DM, GCS <8, and reintubation were present in about 25% of the population. Chronic kidney disease and corticosteroid use were less than 10% in our study population. The presence of pneumonia at recruitment may lead to misclassification and the diagnosis of VAP challenging. Hence, patients with pneumonia were excluded. Patients who received antibiotics up to 4 weeks prior to recruitment were excluded since it may affect the estimation of the incidence of polymicrobial VAP.
Between the groups, diabetes was present in 19% of the polymicrobial group, whereas it was higher (35%) among the monomicrobial group, and the difference was statistically significant. It was contradictory to the general belief that more infections prevail among diabetics than non-diabetics. A closer look at the study data revealed that the main reason for the lower incidence of polymicrobial VAP in diabetic patients as compared to monomicrobial VAP was early extubation. Since the metabolic derangements like diabetic ketoacidosis and hyperglycemic hyperosmolar states in these patients were rapidly corrected, these patients were liberated from ventilator early and were thus less likely to develop polymicrobial VAP as compared to monomicrobial VAP.
The proportion of polymicrobial VAP episodes among microbiologically confirmed VAP episodes was about 60%. For our study purpose, VAP diagnosis was made using mCPIS criteria instead of the CDC surveillance criteria. Besides, the study has included only those VAP episodes with microbiological evidence of infection with PPM. It has excluded VAP episodes that are presumptively treated as VAP but culture negative. The proportion of polymicrobial VAP will be reduced if all patients diagnosed with mCPIS are included in the denominator. The proportion is an overestimate since only microbiologically proven VAP are included in the denominator, and many patients with VAP are excluded for various reasons.
Also, the prevalence of VAP as such varies according to the criteria used for diagnosing VAP. The bedside diagnosis of VAP remains elusive and is predominantly based on clinical suspicion.13 The modified Clinical Pulmonary Infection Score (mCPIS) score proved useful to make a presumptive diagnosis to initiate therapy.14 Unlike the CPIS score, the revised CDC surveillance criteria have poor sensitivity and specificity for VAP diagnosis.15 Based on surveillance criteria definitions of CDC/NHSN/IDSA, the incidence of VAP in Asian countries is between 3.5 and 46/1000 days of MV, which is higher than NHSN.16 In comparison to NHSN standards, our ICU also had a higher incidence of VAP — 40 per 1000 ventilator days as per CDC surveillance criteria.15
The incidence of polymicrobial VAP is higher than monomicrobial VAP, probably because we excluded clinically suspected culture-negative VAP and analyzed only those with VAP wherein organisms were isolated. Other factors like poor Patient:Nurse ratio and poor implementation of VAP bundled care practices might be responsible. A study analyzing the effects of the VAP prevention bundle in Scotland found a significant reduction in VAP rates after the intervention.17 Bundled care to prevent VAP components like 30 degrees head end elevation and sedation interval are in practice. Sicker patients (higher SAPS-II) are admitted to our ICU. The prevalence of pmVAP had ranged from 10% to 70%. Unlike previous studies, we not only looked at the first episode, but all the episodes during the 30-day follow-up period.8,12 The proportion of pmVAP was 60% in the first, 70% in the second, and 80% in the third episodes. As expected, the prevalence of polymicrobial VAP increased with the duration of mechanical ventilation. In contrast to other studies,8,12 A. baumannii was isolated at a much higher rate in our study’s polymicrobial group.
The number of days of mechanical ventilation after VAP diagnosis was significantly higher in polymicrobial VAP (10.7 days) than monomicrobial VAP (6.5 days). Similarly, the days of ICU stay were significantly higher in polymicrobial VAP. However, the association cannot be interpreted as causation because the risk of polymicrobial VAP increases with increased duration of ventilation as necessitated by the primary indication for mechanical ventilation. The trend in 30-day outcomes was significantly different in both groups. This is as expected since sicker patients tend to stay mechanically ventilated for a longer period and are at a higher risk of developing polymicrobial VAP. It is possible that polymicrobial VAP increases the risk of mortality. Among the complications studied, CAUTI was significantly high in the polymicrobial group and this could be due to sicker patients on urinary catheterization for a prolonged period. The association may also indicate the possibility of increased chances of infection due to poor implementation of infection control practices like infection prevention bundles in the ICU.
Among the risk factors and predictors for polymicrobial VAP, diabetes showed a negative association with polymicrobial VAP as compared to monomicrobial VAP in univariate analysis as well as in multivariable logistic regression. Among the twelve predictors, GCS <8 at the time of intubation was the only independent predictor of polymicrobial VAP (odds ratio, 2.70; 95% CI, 1.13 to 6.48). The poor sensorium probably facilitated the colonization of upper aerodigestive tract with VAP causing Gram-negative organisms and their micro-aspiration. However, the mean duration of mechanical ventilation prior to VAP onset was comparable.
In general, risk factors for polymicrobial infection among ICU patients include advanced age and cerebrovascular diseases.18 Our finding that the association of poor sensorium with polymicrobial VAP is similar. However, we did not find an association with age. Among patients with polymicrobial VAP, pleural effusion was found to be more frequent in an earlier study.12 No other risk factors for polymicrobial VAP have been previously identified.
Unlike previous studies8,12 we have looked at subsequent VAP episodes during the 30-day study period from the onset of the first episode of VAP, instead of just limiting to the first episode. The study enrolled participants prospectively, and we could achieve the sample size. We screened for patients using mCPIS score for VAP but analyzed patients only with microbiologically confirmed VAP. This would have resulted in an overestimation of the proportion of pmVAP, as many patients with culture-negative clinical VAP diagnosis were excluded. We used a semi-quantitative culture of the endotracheal aspirate collected using a mucus trap to implicate the organism as a cause of VAP. This method is commonly used for quantitative culture in developing countries and reflects the incidence of polymicrobial VAP in day-to-day practice. Since a protected specimen brush was not used in our study, a few patients with ventilator-associated tracheobronchitis might have been misclassified as VAP. The exclusion of immunocompromised patients susceptible to infection might have underestimated the overall proportion of polymicrobial VAP in this study. Future studies are required to determine the incidence and outcomes of polymicrobial VAP in immunocompromised patients.
To conclude, the proportion of polymicrobial VAP was quite high in our ICU, contributing to about 60% of microbiologically proven VAP in medical ICU. Polymicrobial VAP was associated with a poor outcome as compared to mmVAP. Non-fermenting Gram-negative bacilli were the predominant organisms causing VAP. Poor sensorium at the time of intubation was found to be an independent predictor of pmVAP.
Funding
No funding received.
Disclosure
None of the authors have potential conflicts of interest to be disclosed.
References
1. de Pont AC. Attributable mortality of ventilator-associated pneumonia. Lancet Infect Dis. 2013;13(12):1014. doi:10.1016/S1473-3099(13)70300-1
2. Branch-Elliman W, Wright SB, Howell MD. Determining the ideal strategy for ventilator-associated pneumonia prevention. Cost-benefit analysis. Am J Respir Crit Care Med. 2015;192(1):57–63. doi:10.1164/rccm.201412-2316OC
3. Stone PW. Economic burden of healthcare-associated infections: an American perspective. Expert Rev Pharmacoecon Outcomes Res. 2009;9(5):417–422. doi:10.1586/erp.09.53
4. Chawla R. Epidemiology, etiology, and diagnosis of hospital-acquired pneumonia and ventilator-associated pneumonia in Asian countries. Am J Infect Control. 2008;36(4 Suppl):S93–100. doi:10.1016/j.ajic.2007.05.011
5. Bergmans DC, Bonten MJ, Gaillard CA, et al. Indications for antibiotic use in ICU patients: a one-year prospective surveillance. J Antimicrob Chemother. 1997;39(4):527–535. doi:10.1093/jac/39.4.527
6. Alp E, Cookson B, Erdem H, Rello J. Infection control bundles in intensive care: an international cross-sectional survey in low- and middle-income countries. J Hosp Infect. 2019;101(3):248–256. doi:10.1016/j.jhin.2018.07.022
7. Cilloniz C, Martin-Loeches I, Garcia-Vidal C, San Jose A, Torres A. Microbial etiology of pneumonia: epidemiology, diagnosis and resistance patterns. Int J Mol Sci. 2016;17(12):2120. doi:10.3390/ijms17122120
8. Combes A, Figliolini C, Trouillet JL, et al. Incidence and outcome of polymicrobial ventilator-associated pneumonia. Chest. 2002;121(5):1618–1623. doi:10.1378/chest.121.5.1618
9. Chaudhury A, Rani AS, Kalawat U, Sumant S, Verma A, Venkataramana B. Antibiotic resistance & pathogen profile in ventilator-associated pneumonia in a tertiary care hospital in India. Indian J Med Res. 2016;144(3):440–446. doi:10.4103/0971-5916.198679
10. Zilberberg MD, Shorr AF. Ventilator-associated pneumonia: the clinical pulmonary infection score as a surrogate for diagnostics and outcome. Clin Infect Dis. 2010;51(Suppl 1):S131–5. doi:10.1086/653062
11. Kalil AC, Metersky ML, Klompas M, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016;63(5):e61–e111. doi:10.1093/cid/ciw353
12. Ferrer M, Difrancesco LF, Liapikou A, et al. Polymicrobial intensive care unit-acquired pneumonia: prevalence, microbiology and outcome. Crit Care. 2015;19:450. doi:10.1186/s13054-015-1165-5
13. Douglas IS. New diagnostic methods for pneumonia in the ICU. Curr Opin Infect Dis. 2016;29(2):197–204. doi:10.1097/QCO.0000000000000249
14. Singh N, Rogers P, Atwood CW, Wagener MM, Yu VL. Short-course empiric antibiotic therapy for patients with pulmonary infiltrates in the intensive care unit. A proposed solution for indiscriminate antibiotic prescription. Am J Respir Crit Care Med. 2000;162(2 Pt 1):505–511. doi:10.1164/ajrccm.162.2.9909095
15. Center for Disease Control. Device associated module. Pneumonia (Ventilator-associated [VAP] and non-ventilator-associated Pneumonia [PNEU]) event. Available from: https://www.cdc.gov/nhsn/pdfs/pscmanual/6pscvapcurrent.pdf.
16. Ego A, Preiser JC, Vincent JL. Impact of diagnostic criteria on the incidence of ventilator-associated pneumonia. Chest. 2015;147(2):347–355. doi:10.1378/chest.14-0610
17. Morris AC, Hay AW, Swann DG, et al. Reducing ventilator-associated pneumonia in intensive care: impact of implementing a care bundle. Crit Care Med. 2011;39(10):2218–2224. doi:10.1097/CCM.0b013e3182227d52
18. Fengcai S, Di X, Qianpeng H, Hongke Z, Yiyu D. Microbial characteristics in culture-positive sepsis and risk factors of polymicrobial infection in ICU. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2015;27(9):718–723.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
