Back to Journals » International Journal of Nanomedicine » Volume 17
Polysaccharide Electrospun Nanofibers for Wound Healing Applications
Authors Tan G, Wang L, Pan W , Chen K
Received 6 May 2022
Accepted for publication 23 July 2022
Published 6 September 2022 Volume 2022:17 Pages 3913—3931
DOI https://doi.org/10.2147/IJN.S371900
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Yan Shen
Guoxin Tan,1 Lijie Wang,2 Weisan Pan,3 Kai Chen4
1School of Pharmacy, Hainan University, Haikou, 570228, People’s Republic of China; 2School of Pharmacy, Shenyang Medical College, Shenyang, 110034, People’s Republic of China; 3School of Pharmacy, Shenyang Pharmaceutical University, Shenyang, 110016, People’s Republic of China; 4Hainan Provincial Key Laboratory for Research and Development of Tropical Herbs, School of Pharmacy, Hainan Medical University, Haikou, 571199, People’s Republic of China
Correspondence: Kai Chen, Hainan Provincial Key Laboratory for Research and Development of Tropical Herbs, School of Pharmacy, Hainan Medical University, Haikou, 571199, People’s Republic of China, Tel/Fax +86 898-66890907, Email [email protected]
Abstract: As a type of biological macromolecule, natural polysaccharides have been widely used in wound healing due to their low toxicity, good biocompatibility, degradability and reproducibility. Electrospinning is a versatile and simple technique for producing continuous nanoscale fibers from a variety of natural and synthetic polymers. The application of electrospun nanofibers as wound dressings has made great progress and they are considered one of the most effective wound dressings. This paper reviews the preparation of polysaccharide nanofibers by electrospinning and their application prospects in the field of wound healing. A variety of polysaccharide nanofibers, including chitosan, starch, alginate, and hyaluronic acid are introduced. The preparation strategy of polysaccharide electrospun nanofibers and their functions in promoting wound healing are summarized. In addition, the future prospects and challenges for the preparation of polysaccharide nanofibers by electrospinning are also discussed.
Keywords: polysaccharide, electrospun nanofibers, wound healing, preparation strategy, function
Introduction
An important application of electrospun nanofibrous membranes in the biomedical field is wound dressing.1,2 Electrospun nanofiber dressings exhibit high porosity, which allows proper oxygen, moisture, and nutrient exchange without causing wound dehydration and enables effective control of the moist microenvironment of the wound.3,4 The small pore size of the nanofibers can effectively inhibit the penetration of microorganisms from the external environment, and the high specific surface area can efficiently release loaded drugs. Nanofibers can form similar natural external matrix structures, provide sites for cell adhesion and proliferation, and modulate cellular responses.5 In addition, various active ingredients and drugs affecting wound healing, including antibiotics, growth factors, vitamins, herbal extracts and even cells have been loaded into nanofibers for controlled release to enhance the desired wound healing properties.6–8
At present, a wide range of materials can be used for electrospinning technology.9 The most common and earliest applications of electrospinning technology use various polymers, including various synthetic polymers and natural polymers.10,11 Synthetic polymers such as polylactic acid (PLA), polycaprolactone (PCL), polyvinyl alcohol (PVA), polyurethane (PU) and other materials have controllable mechanical strength and physical properties, and are easy to process.12 Natural polymers have become a better choice for wound dressing materials prepared by electrospinning as synthetic polymers lack biological properties.13,14 Polysaccharides from natural polymers are widely used as materials for electrospun wound dressings due to their low toxicity, good biocompatibility, degradability and reproducibility. Natural polysaccharides are biopolymers that widely exist in various organisms and are connected by monosaccharides through glycosidic bonds. Functional groups such as primary hydroxyl, secondary hydroxyl, amino and carboxyl groups on the macromolecules of natural polysaccharides show various chemical properties and strong chemical reactivity. Some of the molecular structures of polysaccharides such as chitosan, starch, alginate and hyaluronic acid are similar to glycosaminoglycans in the extracellular environment and thus have received extensive attention in the field of wound therapy.15,16 Figure 1 shows the chemical structure, bioactive groups, monosaccharide units and sites that can be used for biological modification of conventional polysaccharides (chitosan, starch, alginate and hyaluronic acid). A variety of polysaccharides can promote cell growth and proliferation, accelerate skin tissue regeneration and repair, and have good compatibility and biodegradability with body tissues.17 Therefore, polysaccharides have natural advantages as electrospun wound dressing materials.18,19
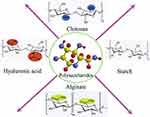 |
Figure 1 The chemical structure, bioactive groups, monosaccharide units and sites that can be used for biological modification of conventional polysaccharides (chitosan, starch, alginate and hyaluronic acid). Notes: Reprinted from: Rahimi M, Noruzi EB, Sheykhsaran E, et al. Carbohydrate polymer-based silver nanocomposites: Recent progress in the antimicrobial wound dressings. Carbohydr Polym. 2020;231:115696. doi:10.1016/j.carbpol.2019.115696.20 © 2019 Elsevier Ltd. All rights reserved. With permission from Elsevier. |
This paper introduces a variety of polysaccharide electrospun nanofibers, including chitosan, starch, alginate and hyaluronic acid, and discusses their application prospects in the field of wound healing. The preparation strategy of polysaccharide electrospun nanofibers and their functions in promoting wound healing are summarized in Figure 2. In addition, the future prospects and challenges for the preparation of polysaccharide nanofibers by electrospinning are also discussed.
 |
Figure 2 Schematic illustration of the structure characteristics and the function of promoting wound healing of polysaccharide electrospun nanofibers as wound dressings. |
Electrospinning Technique
Electrospinning technology is a common method used to prepare continuous nanofibers and has the advantages of simple operation, low cost, wide raw material sources and controllable process parameters.21 Electrospinning equipment mainly includes a solution propulsion device, a high voltage power supply and a collector.22 The process and principle of preparing nanofibers by electrospinning are as follows: First, a high-voltage electric field is formed between the spinning nozzle and the collector using a high-voltage power supply. The surface of the spinning liquid droplet at the top of the spinning nozzle is subject to the electrostatic force of the external high-voltage electric field as well as its own surface tension.23,24 The electrostatic force promotes the stretching and formation of fibers by the droplet, while the surface tension provides the resistance to the deformation of the droplet. When the applied electrostatic force is large enough to overcome the surface tension of the spinning solution, the droplets will be continuously stretched under the action of a high-voltage electric field to form nanofibers with extremely large specific surface areas.25 The solvent in the spinning fluid between the spinning nozzle and the collector is continuously volatilized, resulting in the formation of solidified polymer nanofibers.26 After a period of continuous spinning, a certain thickness of nanofiber membrane is obtained. The process is shown in Figure 3.
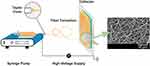 |
Figure 3 Illustration of a conventional electrospinning apparatus used in the production of nanofibers. Notes: Reprinted from: Graca MFP, de Melo-Diogo D, Correia IJ, Moreira AF. Electrospun Asymmetric Membranes as Promising Wound Dressings: A Review. Pharmaceutics. 2021;13(2):183. doi:10.3390/pharmaceutics13020183.27 Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0/.) |
The electrospinning process is controlled by many parameters, including solution parameters, process parameters and environmental parameters.28 Solution parameters include the viscosity, conductivity, molecular weight, and surface tension of the solution.29 Process parameters include the voltage, spinning distance, and the flow rate of solution.30 Each of these variables affects the diameter and shape of the fiber. By adjusting these parameters, fibers of the desired diameter and shape can be obtained. In addition to these variables, environmental parameters (humidity and temperature) also play an important role in the morphology and diameter of electrospun nanofibers.31
Potential Polysaccharide Electrospun Nanofibers for Wound Healing
Natural polysaccharides are very important natural macromolecules that exist widely in animals, plants, algae and other organisms. Polysaccharide electrospun nanofibers have great potential in wound treatment because of their excellent biological properties.32,33 In this paper, several common natural polysaccharides such as chitosan, starch, alginate, hyaluronic acid and their properties in wound healing by electrospinning are summarized (Table 1).
 |
Table 1 Natural Polysaccharide for Wound Dressing Prepared by Electrospinning |
Chitosan
Chitosan is a mucopolysaccharide macromolecule formed by the partial deacetylation of chitin, which is an alkaline natural polysaccharide substance that mainly exists in the exoskeletons of arthropods, including crustaceans.57 Due to deacetylation, the amino group on the molecular chain of chitosan is easily protonated and has a positive charge, which endows chitosan with broad antibacterial properties and hemostatic effects.58 Furthermore, chitosan possesses essential the properties of ideal wound dressings such as biocompatibility, biodegradability, and low toxicity.59 Therefore, chitosan has become an excellent choice for the preparation of wound dressing materials.38 Chitosan solution alone is not easy to electrospin due to the high viscosity of the solution. Hence, synthetic polymers such as polyvinyl oxide (PEO) or polyvinyl alcohol (PVA) are usually added to reduce the viscosity of the chitosan solution to achieve the desired spinning effect.60
Bayat et al prepared bromelain-loaded chitosan nanofibers by electrospinning.61 PEO was added to the spinning solution to improve the spinnability of chitosan. Chitosan nanofibers loaded with bromelain showed better physicochemical properties, release properties and lower cytotoxicity. In addition, the chitosan nanofibers with bromelain exhibited excellent wound healing activity.
The natural advantage of chitosan for wound healing is its antimicrobial activity. This activity is due to the interaction of the protonated amino groups (NH3+) of chitosan with the negatively charged bacterial cell membrane. A layer of polymer film is formed on the cell surface, which changes the permeability of the cell membrane and interferes with the normal metabolism of the bacterium, thereby inhibiting bacterial growth. Adeli et al prepared PVA/chitosan/starch nanofibers by electrospinning.62 Antibacterial tests showed that the nanofibers had good antibacterial activity against Staphylococcus aureus and Escherichia coli. Furthermore, the increased proportion of chitosan in the nanofibers increased antibacterial activity.
Chitosan is a potential hemostatic material. The hemostatic mechanism of chitosan is related to its positive charge, which can promote the aggregation of negatively charged red blood cells and increase the adhesion of platelets, thereby promoting blood coagulation. Deineka et al prepared porous chitosan electrospun nanofiber membranes.63 In vivo liver bleeding experiments showed that chitosan nanofibers had high hemostatic performance and a high degree of biodegradation at the later stage of surgery. The results show that chitosan nanofibers have great application potential in the treatment of bleeding wounds.
Starch
Starch is a natural biodegradable polysaccharide consisting mainly of dehydrated glucose units forming two distinct polymers, amylose and amylopectin.64 The intrinsic physicochemical properties of starch can affect the endogenous coagulation pathway. Starch can act as a natural adhesive to reversibly shrink epithelial cells and promote the repair of damaged cells. Starch is an attractive polymer for wound healing because of its wide availability, low cost, biocompatibility, biodegradability and promotion of wound healing.65,66
Waghmare et al successfully prepared electrospun starch nanofibers using 30:70% w/w starch and polyvinyl alcohol.43 This ratio of polymers yielded fibers with dimensions suitable for wound healing applications. Starch nanofibers can promote the growth and proliferation of skin cells, suggesting their potential to promote wound healing.
However, the high hydrophilicity and poor mechanical properties of starch hinder its application in wound healing. Mistry et al prepared electrospun nanofibers by combining starch with the hydrophobic synthetic polymer TPU and further cross-linked them using glutaraldehyde.42 The cross-linked starch nanofibers exhibited higher water stability and greater mechanical strength. The cytotoxicity results confirmed the biocompatibility of starch nanofibers. In vivo and histological evaluations indicated that starch nanofibers could enhance the speed of wound healing.
Alginate
Alginate is a natural anionic polysaccharide isolated from brown algae.67 It is widely used to promote wound healing due to its advantages of biocompatibility, biodegradability, low cytotoxicity and mucosal adhesion.68 In addition, calcium ions in alginate dressings exchange with sodium ions in wound secretions, causing alginate to gel at the wound site and provide the appropriate moist environment for wound healing. Therefore, the preparation of alginate into electrospun nanofibers has great potential for wound healing. However, the molecular weight of alginate is low, and it is easily forms a gel when the concentration is slightly higher, so it is difficult to electrospin pure sodium alginate.69 Researchers often blend it with synthetic polymers to prepare electrospun nanofibers.70 Lu et al blended alginate with PEO solution to improve the spinnability of alginate for producing alginate nanofibers with exceptional antimicrobial properties of oregano essential oil (OEO) as a natural antimicrobial agent.71 The cross-linking of the alginate nanofibers with calcium chloride improves its mechanical properties, particularly the tensile strength. Antibacterial tests showed that OEO-loaded alginate nanofibers successfully inhibited the growth of gram-positive and gram-negative bacteria. These alginate nanofibers with good mechanical properties and bacteriostatic effects have great application prospects as wound dressings.
Hyaluronic Acid
Hyaluronic acid (HA) is an anionic aminoglycan produced naturally by the human body and widely distributed throughout the body in connective tissue, the eyes and the skin.72 Hyaluronic acid is a major component of the extracellular matrix, which plays a critical role in tissue regeneration, the inflammatory response, angiogenesis, skin wound repair, etc. Hyaluronic acid plays an important physiological role at multiple stages of wound healing, including promoting the proliferation and migration of fibroblasts and keratinocytes, regulating inflammatory responses, enhancing angiogenesis and collagen deposition at the wound site, and reducing scar formation.73 Hyaluronic acid nanofibers are novel bioactive wound dressings with unique properties such as similarity to the extracellular matrix and accelerated wound healing, which play an important role in wound management.74 However, the poor mechanical properties of hyaluronic acid nanofibers may limit their biological application.75 Hussein et al improved the mechanical properties of nanofibers by incorporating cellulose nanocrystals in hyaluronic acid nanofibers, and loaded L-arginine as a wound-healing promoter to enhance the wound-healing ability of nanofibers. The nanofibers exhibited good biological activities such as cell proliferation, cell adhesion, antibacterial activity and wound healing.76
Preparation Strategy of Polysaccharide Electrospun Nanofibers
With the continuous development of electrospinning technology, an increasing number of different preparation strategies for polysaccharide electrospun nanofibers as wound dressings are being developed to meet the different needs of wound treatment or to speed up wound healing. Several preparation strategies for electrospun polysaccharide nanofibers and their advantages in wound healing will be presented in detail.
Blend Electrospinning
Single-layer nanofiber membranes prepared by single nozzle electrospinning are the earliest and most studied wound dressings made by electrospinning.77 These membranes are typically prepared by blending polymer materials with antibacterial agents or substances that promote wound healing.78 The core requirement of blend electrospinning is that multiple components can be dissolved in the same solvent. The spinning process is simple, the spinning difficulty is low, and the solvent selection is flexible. Kharat et al prepared Calendula officinalis extract (CO)-loaded chitosan nanofibers by blending electrospinning to dissolve CO and chitosan in 50% aqueous acetic acid.79 The prepared nanofiber scaffolds had suitable properties including high biocompatibility, appropriate mechanical properties, proper biodegradability and excellent wettability. In addition, the antibacterial activity of chitosan nanofibers against Escherichia coli and Staphylococcus aureus was improved by adding CO into the nanofibers. The nanofibers exhibited excellent wound healing ability by improving collagen synthesis, tissue re-epithelialization and tissue remodeling.
Coaxial Electrospinning
The preparation of nanofibers by blending drugs with materials is simple and reproducible. However, the drugs are dispersed on nanofibers with a high specific surface area, which can easily lead to the explosive release of drugs on the surface of the nanofibers and thus cannot be sustained for an extended period of time. In addition, some fragile drugs (such as proteins) are not stable in organic solvents, which also limits their application in the field of wound treatment. Therefore, researchers developed coaxial electrospinning technology to prepare core-shell structure nanofibers to address the above problems.80 Coaxial electrospinning uses a concentric circular needle structure to spray two different components of the spinning solution at the same time to prevent the interference of blending between components.81 The drug or active substance is encapsulated in shell-core nanofibers by coaxial electrospinning. The drug in the core gradually dissolves through the shell, or the drug is released through hydrophilic holes in the shell, and the release rate is slower than that of the shell.82 Guo et al successfully prepared chitosan-PEO/PCL nanofibers using coaxial electrospinning technology with chitosan-PEO as the shell and PCL as the core, realizing the co-loading and sequential delivery of the two drugs (Figure 4).83 Lidocaine hydrochloride for pain relief was added to the shell, and the anti-inflammatory agent curcumin was added to the core. Sodium bicarbonate was also added to the core to provide microenvironment sensitivity at the wound site. The rapid release of lidocaine hydrochloride in the shell and the sustained release of curcumin in the core can provide immediate analgesic effects and long-term antibacterial activity during wound healing. In vitro experiments showed that the prepared nanofibers have sustained antibacterial properties against Escherichia coli and Staphylococcus aureus and have good coagulation, blood compatibility and cytocompatibility to L929 cells, making them ideal dressings for wound care in the future.
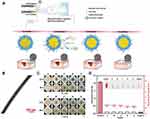 |
Figure 4 Sequential release of drugs forms a dual-delivery system based on pH-responsive nanofibrous mats towards wound care. (A) Illustration indicating wound healing with the help of microenvironment-responsive dual-drug-loaded wound dressings. (B) TEM images of core–shell nanofibers. (C) The inhibition zone of different samples against E. coli and S. aureus at 24 h and 48 h. (D) The results of hemolytic tests. Notes: Used with permission of Royal Society of Chemistry, from: Guo H, Tan S, Gao J, Wang L. Sequential release of drugs form a dual-delivery system based on pH-responsive nanofibrous mats towards wound care. J Mater Chem B. 2020;8(8):1759–1770. doi:10.1039/c9tb02522g.83 Copyright 2020; permission conveyed through Copyright Clearance Center, Inc. |
Multilayer Electrospun Nanofiber Membranes
The ideal wound dressings must have multiple functions such as acting as a protective barrier to prevent the invasion of microorganisms.84,85 In addition, these dressings should also have hemostatic and antibacterial properties, the ability to absorb wound exudate, suitable gas–liquid exchange capacity, and the ability to promote cell proliferation and migration, angiogenesis, tissue remodeling, etc. However, the single-layer nanofiber membranes prepared by blending electrospinning or coaxial electrospinning have relatively simple functions. Most single-layer nanofibers release antibacterial agents or substances that promote wound healing to the wound site, and cannot achieve more complex functions to meet the needs of wound healing. Therefore, electrospun polysaccharide nanofiber membranes with multilayer spatial structures have been further studied for wound dressings to meet the complex environment of wound healing.86–88 Miguel et al prepared two interconnected electrospun asymmetric hyaluronic acid films to mimic the epidermis and dermis of the skin.89 Hyaluronic acid provides high hydration and water absorption and retention and also allows cell attachment, migration and proliferation. Thus, the porous bottom layer composed of hyaluronic acid and silk fibroin forms a dermis-like structure, which can absorb wound exudate and promote cell adhesion and proliferation. Simultaneously, the addition of herbal thymol to the bottom layer of the membrane enhances its antibacterial and antioxidant properties. The top layer is prepared from silk fibroin and PCL to reproduce the compactness and water repellency of the epidermis. This bilayer membrane has multiple functions such barrier formation, waterproofing, absorbing wound exudate, and promoting cell proliferation, bacteriostasis and antioxidation, which enhance the application of this membrane as a wound dressing.
In addition, many researchers have developed three-layer nanofiber membranes to achieve more complex functions to accelerate wound healing. Chen et al prepared a three-layer chitosan wound dressing with a spatial structure by sequential electrospinning.90 This three-layer nanofiber membrane composed of chitosan, PVA and nanobioglass (nBG) provided the functions of hemostatic and antibacterial in the sub-layer (chitosan), moisture retention and exudate absorption in the mid-layer (chitosan-PVA), and promoting tissue generation in the top-layer (PVA-nBG). This layered multilayer structure is expected to organize the function of membrane components corresponding to the healing stage and to speed up the healing process. The results of in vitro and in vivo studies confirmed that the three-layer chitosan nanofiber membrane has great potential in acute and chronic wound healing (Figure 5).
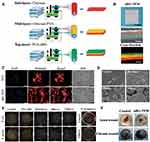 |
Figure 5 Electrospun chitosan/PVA/bioglass Nanofibrous membrane with spatially designed structure for accelerating chronic wound healing. (A) Schematic of nBG-TFM fabricated by sequential electrospinning. (B) Photographs, SEM images and fluorescent images of electrospining membranes. (C) Cell morphology of HDFs cells cultured on trilayer membranes with nBG (40%) (nBG-TFM) and without nBG (TFM) was observed by fluorescent staining and SEM. (D) The effect of membranes on the aggregation of red blood cells was observed by SEM. (E) Surface antibacterial activity of membranes for E. coli, S. aureus and P. aeruginosa. (F) Evaluation of nBG-TFM on acute and chronic wound healing in rats. Notes: Reprinted from: Chen Q, Wu J, Liu Y, et al. Electrospun chitosan/PVA/bioglass Nanofibrous membrane with spatially designed structure for accelerating chronic wound healing. Mater Sci Eng C Mater Biol Appl. 2019;105:110083. doi:10.1016/j.msec.2019.110083.90 © 2019 Elsevier B.V. All rights reserved. With permission from Elsevier. |
Electrospun Nanofiber Coating Polysaccharide
A high viscosity caused by inherently high molecular weights and electrical charges also produces poor electrospinnability of some polysaccharides. In addition, polysaccharide electrospun nanofibers have poorer mechanical properties and water resistance. Synthetic polymers have good spinnability and superior mechanical properties. The synthetic polymer nanofibers can be prepared by electrospinning, and then the polysaccharide is coated on the surface of the nanofibers to obtain the polysaccharide-coated nanofibers.51 These nanofibers not only ensure the excellent mechanical properties of synthetic polymers but also have the functions of polysaccharides such as moisture absorption, good biocompatibility and promotion of wound healing. Croisier et al prepared charged nanofibers by electrospinning poly(ε-caprolactone) (PCL) with a block-copolymer bearing carboxylic acid functional groups. After deprotonation of the acid groups, the layer-by-layer deposition of polyelectrolyte polysaccharides, notably chitosan and hyaluronic acid, was used to coat the electrospun fibers. A multilayered structure was achieved by alternating the deposition of positively charged chitosan with the deposition of a negatively charged polyelectrolyte. The polysaccharide-coated nanofibers retained the excellent biological properties of polysaccharides while avoiding the limitation of direct electrospinning of polysaccharides.91
Polysaccharide Electrospun Nanofibers with Different Functions for Use as Wound Dressings
Wounds can be classified into acute wounds and chronic wounds according to the length of the healing time.92 Acute wounds such as mechanical wounds, surgical wounds, burns and chemical injuries heal in a short period of time following an orderly wound healing process. Chronic wounds cannot be repaired through an orderly healing process and take longer to heal. The most common chronic wounds are diabetic foot ulcers, bedsores, venous leg ulcers, and burns.93 The main causes of delayed wound healing are chronic inflammation, growth factor secretion disorder, microbial infection and destruction of angiogenesis.94,95 In addition, reduced proliferation, migration, and relocation of fibroblasts severely impede the wound healing process. Therefore, the development of wound dressings with specific functions such as antibacterial, anti-inflammatory, promotion of cell proliferation and migration, and promotion of angiogenesis can correct the disrupted stages of wound healing and guide the wound to follow an orderly healing procedure.96,97
Antibacterial Activity
Natural polysaccharides have been widely used in wound dressings. However, most natural polysaccharides have no antibacterial properties except for a few polysaccharides, such as chitosan, that have limited antibacterial properties. Therefore, the application of natural polysaccharides in bacteria-infected wounds will be limited. Harmful bacteria will adhere to polysaccharide dressings, proliferate and secrete more harmful substances on the dressings, which will not only fail to promote wound healing, it will actually hinder wound healing.98 Therefore, the preparation of polysaccharide wound dressings with antibacterial properties can greatly improve the application of natural polysaccharides in wound dressings by loading suitable antibacterial agents. Researchers are currently preparing electrospun polysaccharide nanofibers functionalized with antimicrobial agents such as antibiotics, metal nanoparticles, plant extracts, and antimicrobial peptides (Table 2).99,100
 |
Table 2 Details of Recent Works on Antibacterial Polysaccharide Nanofibers Fabricated by Electrospinning |
Antibiotics have the advantages of quick efficacy and strong bactericidal properties, and are widely used in the clinical treatment of wound infections.123 Therefore, adding antibiotics into electrospun polysaccharide nanofibers can achieve a good antibacterial effect.124,125 Chronic wounds are caused by multiple factors, such as nosocomial infections, dermal bacteria, and surgical site infections. Therefore, Kalalinia et al prepared vancomycin-loaded chitosan/PEO nanofibers by blending electrospinning to control infection.104 Nanofibers exhibit good mechanical properties, biocompatibility, and antibacterial properties. Furthermore, in vivo experiments showed that chitosan/PEO nanofibers loaded with 2.5% vancomycin had a faster healing time compared with other treatment groups.
Antibiotics have a good antibacterial effect, but the widespread use of antibiotics leads to bacterial resistance.126,127 Unlike traditional antibiotics, metal nanoparticles do not bind to specific receptors on bacterial cells, which makes it difficult for bacteria to develop drug resistance and also expands the broad spectrum of antibacterial activity.128 Therefore, polysaccharide electrospun fibers loaded with metal nanoparticles were used to prevent bacterial infection at the wound site.129,130 Dodero et al prepared alginate nanofibers loaded with ZnO nanoparticles by electrospinning and crosslinked them with Sr2+ ions. These nanofibers can effectively inhibit the growth of Escherichia coli.111
Plants are a natural treasure trove of bioactive compounds, and there are many types of active components with antibacterial activity, such as volatile oils, alkaloids, flavonoids, phenolic alcohols, quinones, saponins, and glycosides.131,132 These ingredients come from a wide range of sources and have less biotoxicity and superior wound healing properties. Therefore, many researchers have loaded plant extracts with antibacterial activity into electrospun polysaccharide nanofibers to promote wound healing.133,134 Yousefi et al added henna leaf extract with antibacterial activity to chitosan/PEO nanofibers, which showed significant inhibitory effect on both gram-negative and gram-positive bacteria, enhancing the antibacterial performance of chitosan/PEO nanofibers alone. In vivo results confirmed that chitosan/PEO nanofibers loaded with henna extract significantly accelerated the wound healing process.117 Henna extract has a variety of pharmacological components for burn healing, so the wound dressing loaded with henna extract is promising for the treatment of severe burns.
Antioxidant or Anti-Inflammatory Activity
Chronic wounds exhibit chronic inflammation as they heal.93,135 Chronic inflammation is mainly caused by high levels of reactive oxygen species (ROS), proinflammatory chemokines and bacterial infections.136 The use of dressing materials to modulate the wound inflammatory microenvironment, including ROS removal, adsorption of inflammatory factors, and regulation of the phenotype and number of immune cells, can reduce the inflammatory response of the wound and accelerate the healing of chronic wounds.137,138 Therefore, polysaccharide electrospun nanofibers with the ability to regulate the wound inflammatory microenvironment have great potential in the treatment of chronic wounds.139,140
Honey is an ancient natural wound-healing agent with antibacterial, antioxidant and anti-inflammatory properties that has been reintroduced into modern clinical wound care. Tang et al added honey to alginate/PVA electrospun nanofibers to develop a wound dressing with antioxidant properties.141 With the increase in honey content, the nanofibers showed enhanced antioxidant activity, indicating that it could control the excessive production of reactive oxygen species. The addition of honey to nanofibers can also effectively inhibit the growth of gram-positive bacteria and gram-negative bacteria. The MTT test demonstrated that the nanofibers have good biocompatibility. Therefore, honey-loaded alginate/PVA nanofibers are expected to be an effective wound dressing.
High molecular weight hyaluronic acid (HHA) can promote the transformation of macrophages from the proinflammatory M1 phenotype to the M2 phenotype. M2 phenotype macrophages can greatly reduce inflammation and promote cell proliferation by releasing anti-inflammatory cytokines and growth factors.142 Liu et al developed a nanofiber-absorbable hyaluronic acid hydrogel for the synergistic regulation of the inflammatory microenvironment of diabetic wounds (Figure 6).149 The electrospun thioether grafted hyaluronic acid nanofibers (FHHA-S/Fe) can form hydrogels in situ on the wound bed. The hydrogels gradually degraded and were absorbed within three days. The grafted thioether on the surface of HHA can rapidly remove reactive oxygen species and reduce the inflammatory response in the early stage of inflammation. In addition, HHA can promote the transformation of aggregated M1 macrophages into the M2 phenotype, thus co-accelerating the transition from inflammation to proliferation and remodeling in the wound healing phase. In vitro and in vivo results demonstrated that the nanofiber hyaluronic acid hydrogel can significantly accelerate wound healing in chronic diabetes, particularly in the early stage of wound healing. Therefore, this simple dressing strategy with intrinsic dual regulatory mechanisms of the wound inflammatory microenvironment can be used as an effective and safe therapeutic strategy for diabetic wound management.
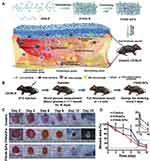 |
Figure 6 Absorbable thioether grafted hyaluronic acid nanofibrous hydrogel for synergistic modulation of inflammation microenvironment to accelerate chronic diabetic wound healing. (A) Illustration of the preparation procedure of FHHA-S/Fe, dressing of FHHA-S/Fe on full-thickness wound model in diabetic C57BL/6 mouse, and the mechanism of FHHA-S/Fe for enhanced chronic wound healing effect. (B) Schematic of the establishment and treatment of a chronic diabetic wound model. (C) Representative photographs of wounds at indicated days with nanofibrous hydrogel treatment. (D) Quantitative analysis of wound area at the indicated days in comparison with the original wound. Notes: Reprinted with permission from: Liu S, Zhang Q, Yu J, et al. Absorbable Thioether Grafted Hyaluronic Acid Nanofibrous Hydrogel for Synergistic Modulation ofInflammation Microenvironment to Accelerate Chronic Diabetic Wound Healing. Adv Healthc Mater. 2020;9(11):e2000198. doi:10.1002/adhm.202000198.149 © 2020 WILEY -VCH Verlag GmbH & Co. KGaA, Weinheim. |
Promoting the Proliferation and Migration of Fibroblasts
In the process of wound healing, normal fibroblasts at the edge of the wound continue to proliferate and migrate to the wound surface. Fibroblasts produce fibrous tissue and matrix, thereby promoting skin tissue remodeling to complete wound healing. Therefore, the proliferation and migration of wound tissue cells are of great significance for wound healing.143 Ghalei et al prepared an alginate coated electrospun fibroin capable of delivering amniotic fluid (AF) to the wound site.144 An alginate coating on the surface of nanofibers can enhance the cell-matrix interaction and promote the proliferation and migration of fibroblasts. In addition, AF contains a variety of growth factors, such as FGF, EGF and TGF-β, which can promote cellular response and wound healing. Cultured fibroblasts on manufactured dressings showed that the nanofibers could promote the proliferation and migration of fibroblasts and increase collagen secretion (Figure 7).
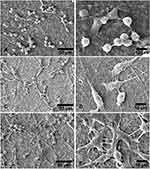 |
Figure 7 SEM images of L929 cells cultured on alginate hydrogel-electrospun silk fibroin fibers. (A and B) SF-ALG, (C and D) SF-ALG-AF1, and (E and F) SF-ALG-AF2 after 24 h. Notes: Reprinted from: Ghalei S, Nourmohammadi J, Solouk A, Mirzadeh H. Enhanced cellular response elicited by addition of amniotic fluid to alginate hydrogel-electrospun silk fibroin fibers for potential wound dressing application. Colloids Surf B Biointerfaces. 2018;172:82–89. doi:10.1016/j.colsurfb.2018.08.028.144 Copyright 2018, with permission from Elsevier. |
Enhanced Angiogenesis
Wound angiogenesis refers to the process of angiogenesis on the original microvessels after skin tissue injury under the stimulation of various factors. Neovascularization plays an important role in wound healing by providing oxygen, nutrition and bioactive substances to the wound site. People with diabetes develop hyperglycemia, which can cause the walls of blood vessels to harden, lowering blood flow and depriving the wound site of much-needed oxygen and nutrients, thereby reducing angiogenesis and hindering the healing of diabetic wounds.145 Promoting angiogenesis is a promising function of wound dressings.146,147 Mulholland et al investigated the delivery of siFKBPL nanocomplexes with RALA peptides as a novel gene therapy to reduce endogenous levels of FKBPL, thereby promoting angiogenesis and wound healing.148 The RALA/siFKBPL complex was added to electrospun alginate/chitosan/PVA nanofibers to enhance the drug delivery effect. In vivo wound healing studies in mice showed a significant increase in angiogenesis when RALA/siFKBPL nanoparticles were delivered from nanofibers, with a 326% increase in vascular density observed compared with untreated wounds.
Conclusions and Future Perspectives
The superior biological characteristics of natural polysaccharides, such as good biocompatibility and biodegradability, can maintain the moist microenvironment for wound healing, and the chemical structure of natural polysaccharides is similar to that of the natural cytoplasmic matrix, which has the function of promoting wound healing. Therefore, natural polysaccharides have been widely used as wound dressing materials. Electrospun nanofibers have a large specific surface, high porosity, good mechanical properties, and excellent biocompatibility, which are beneficial to wound moisturizing, cell growth and respiration, and skin regeneration. Electrospun polysaccharide nanofibers combine the characteristics of polysaccharide and electrospun nanofibers. They have the functions of hemostasis, absorption, breathability, bacteriostasis, anti-inflammation, and promotion of cell proliferation and tissue remodeling when used as a wound dressing. Therefore, electrospun polysaccharide nanofibers are ideal wound dressings. This article reviewed the preparation of polysaccharide nanofibers by electrospinning and their application in the field of wound healing. A variety of polysaccharide nanofibers, including chitosan, starch, hyaluronic acid, and alginate, were introduced.
Electrospun polysaccharide nanofibers have significant advantages as wound dressings. However, there are only a few types of polysaccharides that can be prepared by electrospinning technology, and the range of properties that can be obtained for wound dressings and the types of wounds that can be treated are limited. For example, electrospun alginate nanofibers have a strong liquid absorption capacity, and are suitable for wounds with a large amount exudate. Once applied to the wound with a little exudate, they will make the wound too dry, uncomfortable and scar. Most importantly, alginate nanofibers have no bacteriostatic properties. The electrospun chitosan nanofiber membrane can inhibit bacteria, but it has poor absorbability and moisture retention, which cannot ensure the moist environment required for wound healing. Therefore, the applicability of polysaccharide nanofibers in wound treatment can be improved by combining a variety of polysaccharide materials.
There have been many studies on polysaccharide electrospinning, but the preparation of polysaccharide nanofibers by electrospinning is still a challenge. This is mainly because polysaccharides are prone to form strong hydrogen bonds, which leads to extremely high viscosity or gelation of electrospinning solutions, resulting in unsatisfactory morphology and performance of nanofibers. This makes it difficult to transfer the production stage of polysaccharide nanofibers from the laboratory to commercial and industrial scales. The effective measures to solve this problem are to constantly try to adjust the solvent, material and structural design of polysaccharide electrospun nanofibers so as to improve the spinnability of polysaccharide wound dressings. Experiments with different solvent combinations can change the swelling and entanglement states of polysaccharide molecules and thus affect the nanofiber morphology. Blending polysaccharides with other electrospinning-friendly polymers, such as polyvinyl alcohol (PVA), polylactic acid (PLA), and polyethylene oxide (PEO), can also improve the spinning performance of polysaccharide nanofibers, which is a common method for preparing electrospun polysaccharide nanofibers. More importantly, the structural design of wound dressings with polysaccharide nanofibers is becoming increasingly complex, which leads to its increased difficulty in preparation technology and poor reproducibility, thus making it difficult to carry out industrial production. Polysaccharide nanofiber dressings with relatively simple structures, low production technology requirements, and excellent wound healing ability are attractive and feasible for commercial development. The addition of electrospun jet devices can also effectively increase the jet flow of the nozzle, which will improve the formation of fibers in the spinning process; thus, increasing the yield of polysaccharide nanofiber wound dressings is of great significance for their commercialization.
Disclosure
The authors declare no conflicts of interest in relation to this work.
References
1. Zhang Z, Dai Q, Zhang Y, et al. Design of a multifunctional biomaterial inspired by ancient Chinese medicine for hair regeneration in burned skin. ACS Appl Mater Interfaces. 2020;12(11):12489–12499. doi:10.1021/acsami.9b22769
2. Su Y, Mainardi VL, Wang H, et al. Dissolvable microneedles coupled with nanofiber dressings eradicate biofilms via effectively delivering a database-designed antimicrobial peptide. ACS Nano. 2020;14(9):11775–11786. doi:10.1021/acsnano.0c04527
3. Guo X, Liu Y, Bera H, et al. Alpha-lactalbumin-based nanofiber dressings improve burn wound healing and reduce scarring. ACS Appl Mater Interfaces. 2020;12(41):45702–45713. doi:10.1021/acsami.0c05175
4. Khalid A, Bai D, Abraham AN, et al. Electrospun nanodiamond-silk fibroin membranes: a multifunctional platform for biosensing and wound-healing applications. ACS Appl Mater Interfaces. 2020;12(43):48408–48419. doi:10.1021/acsami.0c15612
5. Chen S, Li R, Li X, Xie J. Electrospinning: an enabling nanotechnology platform for drug delivery and regenerative medicine. Adv Drug Deliv Rev. 2018;132:188–213. doi:10.1016/j.addr.2018.05.001
6. Lv F, Wang J, Xu P, et al. A conducive bioceramic/polymer composite biomaterial for diabetic wound healing. Acta Biomater. 2017;60:128–143. doi:10.1016/j.actbio.2017.07.020
7. Ghosal K, Kovacova M, Humpolicek P, Vajdak J, Bodik M, Spitalsky Z. Antibacterial photodynamic activity of hydrophobic carbon quantum dots and polycaprolactone based nanocomposite processed via both electrospinning and solvent casting method. Photodiagnosis Photodyn Ther. 2021;35:102455. doi:10.1016/j.pdpdt.2021.102455
8. Dong R, Li Y, Chen M, et al. In situ electrospinning of aggregation-induced emission nanofibrous dressing for wound healing. Small Methods. 2022;6(5):e2101247. doi:10.1002/smtd.202101247
9. Bhardwaj N, Kundu SC. Electrospinning: a fascinating fiber fabrication technique. Biotechnol Adv. 2010;28(3):325–347. doi:10.1016/j.biotechadv.2010.01.004
10. Norouzi M, Boroujeni SM, Omidvarkordshouli N, Soleimani M. Advances in skin regeneration: application of electrospun scaffolds. Adv Healthc Mater. 2015;4(8):1114–1133. doi:10.1002/adhm.201500001
11. Xu T, Yang R, Ma X, et al. Bionic poly(gamma-glutamic acid) electrospun fibrous scaffolds for preventing hypertrophic scars. Adv Healthc Mater. 2019;8(13):e1900123. doi:10.1002/adhm.201900123
12. Ahmed MK, Zayed MA, El-Dek SI, Hady MA, El Sherbiny DH, Uskokovic V. Nanofibrous epsilon-polycaprolactone scaffolds containing Ag-doped magnetite nanoparticles: physicochemical characterization and biological testing for wound dressing applications in vitro and in vivo. Bioact Mater. 2021;6(7):2070–2088. doi:10.1016/j.bioactmat.2020.12.026
13. Augustine R, Dan P, Schlachet I, Rouxel D, Menu P, Sosnik A. Chitosan ascorbate hydrogel improves water uptake capacity and cell adhesion of electrospun poly(epsilon-caprolactone) membranes. Int J Pharm. 2019;559:420–426. doi:10.1016/j.ijpharm.2019.01.063
14. Zahedi E, Esmaeili A, Eslahi N, Shokrgozar MA, Simchi A. Fabrication and characterization of core-shell electrospun fibrous mats containing medicinal herbs for wound healing and skin tissue engineering. Mar Drugs. 2019;17(1):27. doi:10.3390/md17010027
15. Khazaeli P, Alaei M, Khaksarihadad M, Ranjbar M. Preparation of PLA/chitosan nanoscaffolds containing cod liver oil and experimental diabetic wound healing in male rats study. J Nanobiotechnology. 2020;18(1):176. doi:10.1186/s12951-020-00737-9
16. Hu H, Xu FJ. Rational design and latest advances of polysaccharide-based hydrogels for wound healing. Biomater Sci. 2020;8(8):2084–2101. doi:10.1039/d0bm00055h
17. Wang Z, Wang Y, Yan J, et al. Pharmaceutical electrospinning and 3D printing scaffold design for bone regeneration. Adv Drug Deliv Rev. 2021;174:504–534. doi:10.1016/j.addr.2021.05.007
18. Lee KY, Jeong L, Kang YO, Lee SJ, Park WH. Electrospinning of polysaccharides for regenerative medicine. Adv Drug Deliv Rev. 2009;61(12):1020–1032. doi:10.1016/j.addr.2009.07.006
19. GU P, BS U, MG A, et al. Electrospun polysaccharide scaffolds: wound healing and stem cell differentiation. J Biomater Sci Polym Ed. 2022;33(7):858–877. doi:10.1080/09205063.2021.2024053
20. Rahimi M, Noruzi EB, Sheykhsaran E, et al. Carbohydrate polymer-based silver nanocomposites: recent progress in the antimicrobial wound dressings. Carbohydr Polym. 2020;231:115696. doi:10.1016/j.carbpol.2019.115696
21. Luo CJ, Stoyanov SD, Stride E, Pelan E, Edirisinghe M. Electrospinning versus fibre production methods: from specifics to technological convergence. Chem Soc Rev. 2012;41(13):4708–4735. doi:10.1039/c2cs35083a
22. Fu Y, Li X, Ren Z, Mao C, Han G. Multifunctional electrospun nanofibers for enhancing localized cancer treatment. Small. 2018;e1801183. doi:10.1002/smll.201801183
23. Chen Y, Shafiq M, Liu M, Morsi Y, Mo X. Advanced fabrication for electrospun three-dimensional nanofiber aerogels and scaffolds. Bioact Mater. 2020;5(4):963–979. doi:10.1016/j.bioactmat.2020.06.023
24. Inagaki M, Yang Y, Kang F. Carbon nanofibers prepared via electrospinning. Adv Mater. 2012;24(19):2547–2566. doi:10.1002/adma.201104940
25. Park SM, Kim DS. Electrolyte-assisted electrospinning for a self-assembled, free-standing nanofiber membrane on a curved surface. Adv Mater. 2015;27(10):1682–1687. doi:10.1002/adma.201404741
26. Feng X, Li J, Zhang X, Liu T, Ding J, Chen X. Electrospun polymer micro/nanofibers as pharmaceutical repositories for healthcare. J Control Release. 2019;302:19–41. doi:10.1016/j.jconrel.2019.03.020
27. Graca MFP, de Melo-Diogo D, Correia IJ, Moreira AF. Electrospun asymmetric membranes as promising wound dressings: a review. Pharmaceutics. 2021;13(2). doi:10.3390/pharmaceutics13020183
28. Moheman A, Alam MS, Mohammad A. Recent trends in electrospinning of polymer nanofibers and their applications in ultra thin layer chromatography. Adv Colloid Interface Sci. 2016;229:1–24. doi:10.1016/j.cis.2015.12.003
29. Ahmed J, Gultekinoglu M, Edirisinghe M. Bacterial cellulose micro-nano fibres for wound healing applications. Biotechnol Adv. 2020;41:107549. doi:10.1016/j.biotechadv.2020.107549
30. Mehta P, Rasekh M, Patel M, et al. Recent applications of electrical, centrifugal, and pressurised emerging technologies for fibrous structure engineering in drug delivery, regenerative medicine and theranostics. Adv Drug Deliv Rev. 2021;175:113823. doi:10.1016/j.addr.2021.05.033
31. Kopp A, Smeets R, Gosau M, et al. Effect of process parameters on additive-free electrospinning of regenerated silk fibroin nonwovens. Bioact Mater. 2020;5(2):241–252. doi:10.1016/j.bioactmat.2020.01.010
32. Cui C, Sun S, Wu S, Chen S, Ma J, Zhou F. Electrospun chitosan nanofibers for wound healing application. Eng Regener. 2021;2:82–90. doi:10.1016/j.engreg.2021.08.001
33. Cui C, Sun S, Li X, et al. Optimizing the chitosan-PCL based membranes with random/aligned fiber structure for controlled ciprofloxacin delivery and wound healing. Int J Biol Macromol. 2022;205:500–510. doi:10.1016/j.ijbiomac.2022.02.118
34. Dodero A, Scarfi S, Mirata S, et al. Effect of crosslinking type on the physical-chemical properties and biocompatibility of chitosan-based electrospun membranes. Polymers. 2021;13(5). doi:10.3390/polym13050831
35. Jirofti N, Golandi M, Movaffagh J, Ahmadi FS, Kalalinia F. Improvement of the wound-healing process by curcumin-loaded chitosan/collagen blend electrospun nanofibers: in vitro and in vivo studies. ACS Biomater Sci Eng. 2021;7(8):3886–3897. doi:10.1021/acsbiomaterials.1c00131
36. Hajilou H, Farahpour MR, Hamishehkar H. Polycaprolactone nanofiber coated with chitosan and Gamma oryzanol functionalized as a novel wound dressing for healing infected wounds. Int J Biol Macromol. 2020;164:2358–2369. doi:10.1016/j.ijbiomac.2020.08.079
37. Naeimi A, Payandeh M, Ghara AR, Ghadi FE. In vivo evaluation of the wound healing properties of bio-nanofiber chitosan/ polyvinyl alcohol incorporating honey and Nepeta dschuparensis. Carbohydr Polym. 2020;240:116315. doi:10.1016/j.carbpol.2020.116315
38. Mahmoudi N, Simchi A. On the biological performance of graphene oxide-modified chitosan/polyvinyl pyrrolidone nanocomposite membranes: in vitro and in vivo effects of graphene oxide. Mater Sci Eng C Mater Biol Appl. 2017;70(Pt 1):121–131. doi:10.1016/j.msec.2016.08.063
39. Poornima B, Korrapati PS. Fabrication of chitosan-polycaprolactone composite nanofibrous scaffold for simultaneous delivery of ferulic acid and resveratrol. Carbohydr Polym. 2017;157:1741–1749. doi:10.1016/j.carbpol.2016.11.056
40. Milanesi G, Vigani B, Rossi S, Sandri G, Mele E. Chitosan-coated Poly(lactic acid) nanofibres loaded with essential oils for wound healing. Polymers. 2021;13(16). doi:10.3390/polym13162582
41. Ho TT, Doan VK, Tran NM, et al. Fabrication of chitosan oligomer-coated electrospun polycaprolactone membrane for wound dressing application. Mater Sci Eng C Mater Biol Appl. 2021;120:111724. doi:10.1016/j.msec.2020.111724
42. Mistry P, Chhabra R, Muke S, et al. Fabrication and characterization of starch-TPU based nanofibers for wound healing applications. Mater Sci Eng C Mater Biol Appl. 2021;119:111316. doi:10.1016/j.msec.2020.111316
43. Waghmare VS, Wadke PR, Dyawanapelly S, Deshpande A, Jain R, Dandekar P. Starch based nanofibrous scaffolds for wound healing applications. Bioact Mater. 2018;3(3):255–266. doi:10.1016/j.bioactmat.2017.11.006
44. Li S, Kong L, Ziegler GR. Electrospinning of octenylsuccinylated starch-pullulan nanofibers from aqueous dispersions. Carbohydr Polym. 2021;258:116933. doi:10.1016/j.carbpol.2020.116933
45. Movahedi M, Asefnejad A, Rafienia M, Khorasani MT. Potential of novel electrospun core-shell structured polyurethane/starch (hyaluronic acid) nanofibers for skin tissue engineering: in vitro and in vivo evaluation. Int J Biol Macromol. 2020;146:627–637. doi:10.1016/j.ijbiomac.2019.11.233
46. Dodero A, Alloisio M, Castellano M, Vicini S. Multilayer alginate-polycaprolactone electrospun membranes as skin wound patches with drug delivery abilities. ACS Appl Mater Interfaces. 2020;12(28):31162–31171. doi:10.1021/acsami.0c07352
47. Nie J, Zhang S, Wu P, Liu Y, Su Y. Electrospinning with lyophilized platelet-rich fibrin has the potential to enhance the proliferation and osteogenesis of MC3T3-E1 cells. Front Bioeng Biotechnol. 2020;8:595579. doi:10.3389/fbioe.2020.595579
48. Sobhanian P, Khorram M, Hashemi SS, Mohammadi A. Development of nanofibrous collagen-grafted poly (vinyl alcohol)/gelatin/alginate scaffolds as potential skin substitute. Int J Biol Macromol. 2019;130:977–987. doi:10.1016/j.ijbiomac.2019.03.045
49. Shiny PJ, Vimala Devi M, Felciya SJG, Ramanathan G, Fardim P, Sivagnanam UT. In vitro and in vivo evaluation of poly-3-hydroxybutyric acid-sodium alginate as a core-shell nanofibrous matrix with arginine and bacitracin-nanoclay complex for dermal reconstruction of excision wound. Int J Biol Macromol. 2021;168:46–58. doi:10.1016/j.ijbiomac.2020.12.025
50. Zhu L, Liu X, Du L, Jin Y. Preparation of asiaticoside-loaded coaxially electrospinning nanofibers and their effect on deep partial-thickness burn injury. Biomed Pharmacother. 2016;83:33–40. doi:10.1016/j.biopha.2016.06.016
51. Lima LL, Taketa TB, Beppu MM, Sousa IMO, Foglio MA, Moraes AM. Coated electrospun bioactive wound dressings: mechanical properties and ability to control lesion microenvironment. Mater Sci Eng C Mater Biol Appl. 2019;100:493–504. doi:10.1016/j.msec.2019.03.005
52. Sandri G, Rossi S, Bonferoni MC, et al. Chitosan/glycosaminoglycan scaffolds for skin reparation. Carbohydr Polym. 2019;220:219–227. doi:10.1016/j.carbpol.2019.05.069
53. Ji Y, Ghosh K, Li B, Sokolov JC, Clark RA, Rafailovich MH. Dual-syringe reactive electrospinning of cross-linked hyaluronic acid hydrogel nanofibers for tissue engineering applications. Macromol Biosci. 2006;6(10):811–817. doi:10.1002/mabi.200600132
54. Shin YC, Shin DM, Lee EJ, et al. Hyaluronic Acid/PLGA Core/Shell fiber matrices loaded with EGCG beneficial to diabetic wound healing. Adv Healthc Mater. 2016;5(23):3035–3045. doi:10.1002/adhm.201600658
55. Cheng L, Sun X, Li B, et al. Electrospun Ginsenoside Rg3/poly(lactic-co-glycolic acid) fibers coated with hyaluronic acid for repairing and inhibiting hypertrophic scars. J Mater Chem B. 2013;1(35):4428–4437. doi:10.1039/c3tb20441c
56. Maeda N, Miao J, Simmons TJ, Dordick JS, Linhardt RJ. Composite polysaccharide fibers prepared by electrospinning and coating. Carbohydr Polym. 2014;102:950–955. doi:10.1016/j.carbpol.2013.10.038
57. Saudi S, Bhattarai SR, Adhikari U, et al. Nanonet-nano fiber electrospun mesh of PCL-chitosan for controlled and extended release of diclofenac sodium. Nanoscale. 2020;12(46):23556–23569. doi:10.1039/d0nr05968d
58. Moutsatsou P, Coopman K, Georgiadou S. Biocompatibility assessment of conducting PANI/chitosan nanofibers for wound healing applications. Polymers. 2017;9(12):Dec. doi:10.3390/polym9120687
59. Deng A, Yang Y, Du S, Yang S. Electrospinning of in situ crosslinked recombinant human collagen peptide/chitosan nanofibers for wound healing. Biomater Sci. 2018;6(8):2197–2208. doi:10.1039/c8bm00492g
60. Augustine R, Rehman SRU, Ahmed R, et al. Electrospun chitosan membranes containing bioactive and therapeutic agents for enhanced wound healing. Int J Biol Macromol. 2020;156:153–170. doi:10.1016/j.ijbiomac.2020.03.207
61. Bayat S, Amiri N, Pishavar E, Kalalinia F, Movaffagh J, Hashemi M. Bromelain-loaded chitosan nanofibers prepared by electrospinning method for burn wound healing in animal models. Life Sci. 2019;229:57–66. doi:10.1016/j.lfs.2019.05.028
62. Adeli H, Khorasani MT, Parvazinia M. Wound dressing based on electrospun PVA/chitosan/starch nanofibrous mats: fabrication, antibacterial and cytocompatibility evaluation and in vitro healing assay. Int J Biol Macromol. 2019;122:238–254. doi:10.1016/j.ijbiomac.2018.10.115
63. Deineka V, Sulaieva O, Pernakov M, et al. Hemostatic and tissue regeneration performance of novel electrospun chitosan-based materials. Biomedicines. 2021;9(6). doi:10.3390/biomedicines9060588
64. Liu G, Gu Z, Hong Y, Cheng L, Li C. Electrospun starch nanofibers: recent advances, challenges, and strategies for potential pharmaceutical applications. J Control Release. 2017;252:95–107. doi:10.1016/j.jconrel.2017.03.016
65. Hemamalini T, Giri Dev VR. Comprehensive review on electrospinning of starch polymer for biomedical applications. Int J Biol Macromol. 2018;106:712–718. doi:10.1016/j.ijbiomac.2017.08.079
66. Salehi H, Mehrasa M, Nasri-Nasrabadi B, et al. Effects of nanozeolite/starch thermoplastic hydrogels on wound healing. J Res Med Sci. 2017;22:110. doi:10.4103/jrms.JRMS_1037_16
67. Dodero A, Donati I, Scarfi S, et al. Effect of sodium alginate molecular structure on electrospun membrane cell adhesion. Mater Sci Eng C Mater Biol Appl. 2021;124:112067. doi:10.1016/j.msec.2021.112067
68. Rashtchian M, Hivechi A, Bahrami SH, Milan PB, Simorgh S. Fabricating alginate/poly(caprolactone) nanofibers with enhanced bio-mechanical properties via cellulose nanocrystal incorporation. Carbohydr Polym. 2020;233:115873. doi:10.1016/j.carbpol.2020.115873
69. Wang S, Ju J, Wu S, et al. Electrospinning of biocompatible alginate-based nanofiber membranes via tailoring chain flexibility. Carbohydr Polym. 2020;230:115665. doi:10.1016/j.carbpol.2019.115665
70. Ashraf SS, Parivar K, Hayati Roodbari N, Mashayekhan S, Amini N. Fabrication and characterization of biaxially electrospun collagen/alginate nanofibers, improved with Rhodotorula mucilaginosa sp. GUMS16 produced exopolysaccharides for wound healing applications. Int J Biol Macromol. 2022;196:194–203. doi:10.1016/j.ijbiomac.2021.11.132
71. Lu H, Butler JA, Britten NS, Venkatraman PD, Rahatekar SS. Natural antimicrobial nano composite fibres manufactured from a combination of alginate and oregano essential oil. Nanomaterials. 2021;11(8):Aug. doi:10.3390/nano11082062
72. Bazmandeh AZ, Mirzaei E, Fadaie M, Shirian S, Ghasemi Y. Dual spinneret electrospun nanofibrous/gel structure of chitosan-gelatin/chitosan-hyaluronic acid as a wound dressing: in-vitro and in-vivo studies. Int J Biol Macromol. 2020;162:359–373. doi:10.1016/j.ijbiomac.2020.06.181
73. Chen X, Lu B, Zhou D, Shao M, Xu W, Zhou Y. Photocrosslinking maleilated hyaluronate/methacrylated poly (vinyl alcohol) nanofibrous mats for hydrogel wound dressings. Int J Biol Macromol. 2020;155:903–910. doi:10.1016/j.ijbiomac.2019.11.048
74. Chanda A, Adhikari J, Ghosh A, et al. Electrospun chitosan/polycaprolactone-hyaluronic acid bilayered scaffold for potential wound healing applications. Int J Biol Macromol. 2018;116:774–785. doi:10.1016/j.ijbiomac.2018.05.099
75. Castro KC, Campos MGN, Mei LHI. Hyaluronic acid electrospinning: challenges, applications in wound dressings and new perspectives. Int J Biol Macromol. 2021;173:251–266. doi:10.1016/j.ijbiomac.2021.01.100
76. Hussein Y, El-Fakharany EM, Kamoun EA, et al. Electrospun PVA/hyaluronic acid/L-arginine nanofibers for wound healing applications: nanofibers optimization and in vitro bioevaluation. Int J Biol Macromol. 2020;164:667–676. doi:10.1016/j.ijbiomac.2020.07.126
77. Fathi A, Khanmohammadi M, Goodarzi A, et al. Fabrication of chitosan-polyvinyl alcohol and silk electrospun fiber seeded with differentiated keratinocyte for skin tissue regeneration in animal wound model. J Biol Eng. 2020;14(1):27. doi:10.1186/s13036-020-00249-y
78. Datta S, Rameshbabu AP, Bankoti K, et al. Oleoyl-chitosan-based nanofiber mats impregnated with amniotic membrane derived stem cells for accelerated full-thickness excisional wound healing. ACS Biomater Sci Eng. 2017;3(8):1738–1749. doi:10.1021/acsbiomaterials.7b00189
79. Kharat Z, Amiri Goushki M, Sarvian N, Asad S, Dehghan MM, Kabiri M. Chitosan/PEO nanofibers containing Calendula officinalis extract: preparation, characterization, in vitro and in vivo evaluation for wound healing applications. Int J Pharm. 2021;609:121132. doi:10.1016/j.ijpharm.2021.121132
80. Ramalingam R, Dhand C, Mayandi V, et al. Core-shell structured antimicrobial nanofiber dressings containing herbal extract and antibiotics combination for the prevention of biofilms and promotion of cutaneous wound healing. ACS Appl Mater Interfaces. 2021;13(21):24356–24369. doi:10.1021/acsami.0c20642
81. Yoon J, Yang HS, Lee BS, Yu WR. Recent progress in coaxial electrospinning: new parameters, various structures, and wide applications. Adv Mater. 2018;30(42):e1704765. doi:10.1002/adma.201704765
82. Tort S, Acarturk F, Besikci A. Evaluation of three-layered doxycycline-collagen loaded nanofiber wound dressing. Int J Pharm. 2017;529(1–2):642–653. doi:10.1016/j.ijpharm.2017.07.027
83. Guo H, Tan S, Gao J, Wang L. Sequential release of drugs form a dual-delivery system based on pH-responsive nanofibrous mats towards wound care. J Mater Chem B. 2020;8(8):1759–1770. doi:10.1039/c9tb02522g
84. Li W, Yu Q, Yao H, et al. Superhydrophobic hierarchical fiber/bead composite membranes for efficient treatment of burns. Acta Biomater. 2019;92:60–70. doi:10.1016/j.actbio.2019.05.025
85. Zhang X, Lv R, Chen L, et al. A multifunctional janus electrospun nanofiber dressing with biofluid draining, monitoring, and antibacterial properties for wound healing. ACS Appl Mater Interfaces. 2022;14(11):12984–13000. doi:10.1021/acsami.1c22629
86. Yu B, He C, Wang W, et al. Asymmetric wettable composite wound dressing prepared by electrospinning with bioinspired micropatterning enhances diabetic wound healing. ACS Applied Bio Mater. 2020;3(8):5383–5394. doi:10.1021/acsabm.0c00695
87. Pal P, Dadhich P, Srivas PK, Das B, Maulik D, Dhara S. Bilayered nanofibrous 3D hierarchy as skin rudiment by emulsion electrospinning for burn wound management. Biomater Sci. 2017;5(9):1786–1799. doi:10.1039/c7bm00174f
88. Mirmajidi T, Chogan F, Rezayan AH, Sharifi AM. In vitro and in vivo evaluation of a nanofiber wound dressing loaded with melatonin. Int J Pharm. 2021;596:120213. doi:10.1016/j.ijpharm.2021.120213
89. Miguel SP, Simoes D, Moreira AF, Sequeira RS, Correia IJ. Production and characterization of electrospun silk fibroin based asymmetric membranes for wound dressing applications. Int J Biol Macromol. 2019;121:524–535. doi:10.1016/j.ijbiomac.2018.10.041
90. Chen Q, Wu J, Liu Y, et al. Electrospun chitosan/PVA/bioglass Nanofibrous membrane with spatially designed structure for accelerating chronic wound healing. Mater Sci Eng C Mater Biol Appl. 2019;105:110083. doi:10.1016/j.msec.2019.110083
91. Croisier F, Atanasova G, Poumay Y, Jerome C. Polysaccharide-coated PCL nanofibers for wound dressing applications. Adv Healthc Mater. 2014;3(12):2032–2039. doi:10.1002/adhm.201400380
92. Iacob AT, Dragan M, Ionescu OM, et al. An overview of biopolymeric electrospun nanofibers based on polysaccharides for wound healing management. Pharmaceutics. 2020;12(10). doi:10.3390/pharmaceutics12100983
93. Zhang H, Zhang M, Wang X, et al. Electrospun multifunctional nanofibrous mats loaded with bioactive anemoside B4 for accelerated wound healing in diabetic mice. Drug Deliv. 2022;29(1):174–185. doi:10.1080/10717544.2021.2021319
94. Rehman SRU, Augustine R, Zahid AA, Ahmed R, Tariq M, Hasan A. Reduced graphene oxide incorporated GelMA hydrogel promotes angiogenesis for wound healing applications. Int J Nanomedicine. 2019;14:9603–9617. doi:10.2147/IJN.S218120
95. Anand S, Rajinikanth PS, Arya DK, et al. Multifunctional biomimetic nanofibrous scaffold loaded with asiaticoside for rapid diabetic wound healing. Pharmaceutics. 2022;14(2):273. doi:10.3390/pharmaceutics14020273
96. Li A, Li L, Zhao B, et al. Antibacterial, antioxidant and anti-inflammatory PLCL/gelatin nanofiber membranes to promote wound healing. Int J Biol Macromol. 2022;194:914–923. doi:10.1016/j.ijbiomac.2021.11.146
97. Qian S, Wang J, Liu Z, et al.. Secretory fluid-aggregated janus electrospun short fiber scaffold for wound healing. Small. 2022:e2200799. doi:10.1002/smll.202200799
98. Currie S, Shariatzadeh FJ, Singh H, Logsetty S, Liu S. Highly sensitive bacteria-responsive membranes consisting of core-shell polyurethane polyvinylpyrrolidone electrospun nanofibers for in situ detection of bacterial infections. ACS Appl Mater Interfaces. 2020;12(41):45859–45872. doi:10.1021/acsami.0c14213
99. Weishaupt R, Zund JN, Heuberger L, et al. Antibacterial, cytocompatible, sustainably sourced: cellulose membranes with bifunctional peptides for advanced wound dressings. Adv Healthc Mater. 2020;9(7):e1901850. doi:10.1002/adhm.201901850
100. Mayandi V, Wen Choong AC, Dhand C, et al. Multifunctional antimicrobial nanofiber dressings containing epsilon-polylysine for the eradication of bacterial bioburden and promotion of wound healing in critically colonized wounds. ACS Appl Mater Interfaces. 2020;12(14):15989–16005. doi:10.1021/acsami.9b21683
101. Iqbal H, Khan BA, Khan ZU, et al. Fabrication, physical characterizations and in vitro antibacterial activity of cefadroxil-loaded chitosan/poly(vinyl alcohol) nanofibers against Staphylococcus aureus clinical isolates. Int J Biol Macromol. 2020;144:921–931. doi:10.1016/j.ijbiomac.2019.09.169
102. Fazli Y, Shariatinia Z. Controlled release of cefazolin sodium antibiotic drug from electrospun chitosan-polyethylene oxide nanofibrous Mats. Mater Sci Eng C Mater Biol Appl. 2017;71:641–652. doi:10.1016/j.msec.2016.10.048
103. Schulte-Werning LV, Murugaiah A, Singh B, et al. Multifunctional nanofibrous dressing with antimicrobial and anti-inflammatory properties prepared by needle-free electrospinning. Pharmaceutics. 2021;13(9):1527. doi:10.3390/pharmaceutics13091527
104. Kalalinia F, Taherzadeh Z, Jirofti N, et al. Evaluation of wound healing efficiency of vancomycin-loaded electrospun chitosan/poly ethylene oxide nanofibers in full thickness wound model of rat. Int J Biol Macromol. 2021;177:100–110. doi:10.1016/j.ijbiomac.2021.01.209
105. Hashemikia S, Farhangpazhouh F, Parsa M, Hasan M, Hassanzadeh A, Hamidi M. Fabrication of ciprofloxacin-loaded chitosan/polyethylene oxide/silica nanofibers for wound dressing application: in vitro and in vivo evaluations. Int J Pharm. 2021;597:120313. doi:10.1016/j.ijpharm.2021.120313
106. Liu X, Nielsen LH, Klodzinska SN, et al. Ciprofloxacin-loaded sodium alginate/poly (lactic-co-glycolic acid) electrospun fibrous mats for wound healing. Eur J Pharm Biopharm. 2018;123:42–49. doi:10.1016/j.ejpb.2017.11.004
107. Fu R, Li C, Yu C, et al. A novel electrospun membrane based on moxifloxacin hydrochloride/poly(vinyl alcohol)/sodium alginate for antibacterial wound dressings in practical application. Drug Deliv. 2016;23(3):828–839. doi:10.3109/10717544.2014.918676
108. Faccendini A, Ruggeri M, Miele D, et al. Norfloxacin-loaded electrospun scaffolds: montmorillonite nanocomposite vs. free drug. Pharmaceutics. 2020;12(4):325. doi:10.3390/pharmaceutics12040325
109. Hassiba AJ, El Zowalaty ME, Webster TJ, et al. Synthesis, characterization, and antimicrobial properties of novel double layer nanocomposite electrospun fibers for wound dressing applications. Int J Nanomedicine. 2017;12:2205–2213. doi:10.2147/IJN.S123417
110. El-Aassar MR, Ibrahim OM, Fouda MMG, El-Beheri NG, Agwa MM. Wound healing of nanofiber comprising Polygalacturonic/Hyaluronic acid embedded silver nanoparticles: in-vitro and in-vivo studies. Carbohydr Polym. 2020;238:116175. doi:10.1016/j.carbpol.2020.116175
111. Dodero A, Scarfi S, Pozzolini M, Vicini S, Alloisio M, Castellano M. Alginate-based electrospun membranes containing ZnO nanoparticles as potential wound healing patches: biological, mechanical, and physicochemical characterization. ACS Appl Mater Interfaces. 2020;12(3):3371–3381. doi:10.1021/acsami.9b17597
112. Sun L, Han J, Liu Z, Wei S, Su X, Zhang G. The facile fabrication of wound compatible anti-microbial nanoparticles encapsulated Collagenous Chitosan matrices for effective inhibition of poly-microbial infections and wound repairing in burn injury care: exhaustive in vivo evaluations. J Photochem Photobiol B. 2019;197:111539. doi:10.1016/j.jphotobiol.2019.111539
113. Ahmed R, Tariq M, Ali I, et al. Novel electrospun chitosan/polyvinyl alcohol/zinc oxide nanofibrous mats with antibacterial and antioxidant properties for diabetic wound healing. Int J Biol Macromol. 2018;120(Pt A):385–393. doi:10.1016/j.ijbiomac.2018.08.057
114. Hadisi Z, Farokhi M, Bakhsheshi-Rad HR, et al. Hyaluronic Acid (HA)-Based Silk Fibroin/Zinc Oxide core-shell electrospun dressing for burn wound management. Macromol Biosci. 2020;20(4):e1900328. doi:10.1002/mabi.201900328
115. Ghasemian Lemraski E, Jahangirian H, Dashti M, et al. Antimicrobial double-layer wound dressing based on chitosan/polyvinyl alcohol/copper: in vitro and in vivo assessment. Int J Nanomedicine. 2021;16:223–235. doi:10.2147/IJN.S266692
116. Wang S, Yan F, Ren P, et al. Incorporation of metal-organic frameworks into electrospun chitosan/poly (vinyl alcohol) nanofibrous membrane with enhanced antibacterial activity for wound dressing application. Int J Biol Macromol. 2020;158:9–17. doi:10.1016/j.ijbiomac.2020.04.116
117. Yousefi I, Pakravan M, Rahimi H, Bahador A, Farshadzadeh Z, Haririan I. An investigation of electrospun Henna leaves extract-loaded chitosan based nanofibrous mats for skin tissue engineering. Mater Sci Eng C Mater Biol Appl. 2017;75:433–444. doi:10.1016/j.msec.2017.02.076
118. Mouro C, Dunne CP, Gouveia IC. Designing new antibacterial wound dressings: development of a dual layer cotton material coated with Poly(Vinyl Alcohol)_Chitosan nanofibers incorporating Agrimonia eupatoria L. extract. Molecules. 2020;26(1):83. doi:10.3390/molecules26010083
119. Yin J, Xu L. Batch preparation of electrospun polycaprolactone/chitosan/aloe vera blended nanofiber membranes for novel wound dressing. Int J Biol Macromol. 2020;160:352–363. doi:10.1016/j.ijbiomac.2020.05.211
120. Khosravimelal S, Chizari M, Farhadihosseinabadi B, Moosazadeh Moghaddam M, Gholipourmalekabadi M. Fabrication and characterization of an antibacterial chitosan/silk fibroin electrospun nanofiber loaded with a cationic peptide for wound-dressing application. J Mater Sci Mater Med. 2021;32(9):114. doi:10.1007/s10856-021-06542-6
121. Zou P, Lee WH, Gao Z, et al. Wound dressing from polyvinyl alcohol/chitosan electrospun fiber membrane loaded with OH-CATH30 nanoparticles. Carbohydr Polym. 2020;232:115786. doi:10.1016/j.carbpol.2019.115786
122. Yang Q, Xie Z, Hu J, Liu Y. Hyaluronic acid nanofiber mats loaded with antimicrobial peptide towards wound dressing applications. Mater Sci Eng C Mater Biol Appl. 2021;128:112319. doi:10.1016/j.msec.2021.112319
123. Gong M, Wan P, Ma D, et al. Flexible breathable nanomesh electronic devices for on‐demand therapy. Adv Funct Mater. 2019;29(26):1902127. doi:10.1002/adfm.201902127
124. Alavarse AC, de Oliveira Silva FW, Colque JT, et al. Tetracycline hydrochloride-loaded electrospun nanofibers mats based on PVA and chitosan for wound dressing. Mater Sci Eng C Mater Biol Appl. 2017;77:271–281. doi:10.1016/j.msec.2017.03.199
125. Saha K, Dutta K, Basu A, Adhikari A, Chattopadhyay D, Sarkar P. Controlled delivery of tetracycline hydrochloride intercalated into smectite clay using polyurethane nanofibrous membrane for wound healing application. Nano-Struct Nano-Obj. 2020;21:100418. doi:10.1016/j.nanoso.2019.100418
126. Wang L, Yang J, Yang X, et al. Mercaptophenylboronic acid-activated gold nanoparticles as nanoantibiotics against multidrug-resistant bacteria. ACS Appl Mater Interfaces. 2020;12(46):51148–51159. doi:10.1021/acsami.0c12597
127. Fras Zemljic L, Maver U, Krasevac Glaser T, et al. Electrospun composite nanofibrous materials based on (Poly)-Phenol-Polysaccharide formulations for potential wound treatment. Materials. 2020;13(11):2631. doi:10.3390/ma13112631
128. Ren X, Hu Y, Chang L, Xu S, Mei X, Chen Z. Electrospinning of antibacterial and anti-inflammatory Ag@hesperidin core-shell nanoparticles into nanofibers used for promoting infected wound healing. Regen Biomater. 2022;9:rbac012. doi:10.1093/rb/rbac012
129. Shi L, Liu X, Wang W, Jiang L, Wang S. A self-pumping dressing for draining excessive biofluid around wounds. Adv Mater. 2019;31(5):e1804187. doi:10.1002/adma.201804187
130. Zhou F, Cui C, Sun S, et al. Electrospun ZnO-loaded chitosan/PCL bilayer membranes with spatially designed structure for accelerated wound healing. Carbohydr Polym. 2022;282:119131. doi:10.1016/j.carbpol.2022.119131
131. Al-Musawi S, Albukhaty S, Al-Karagoly H, et al. Antibacterial activity of honey/chitosan nanofibers loaded with capsaicin and gold nanoparticles for wound dressing. Molecules. 2020;25(20):4770. doi:10.3390/molecules25204770
132. Shokrollahi M, Bahrami SH, Nazarpak MH, Solouk A. Multilayer nanofibrous patch comprising chamomile loaded carboxyethyl chitosan/poly(vinyl alcohol) and polycaprolactone as a potential wound dressing. Int J Biol Macromol. 2020;147:547–559. doi:10.1016/j.ijbiomac.2020.01.067
133. Garcia-Salinas S, Gamez E, Landa G, Arruebo M, Irusta S, Mendoza G. Antimicrobial wound dressings against fluorescent and methicillin-sensitive intracellular pathogenic bacteria. ACS Appl Mater Interfaces. 2020;12(46):51302–51313. doi:10.1021/acsami.0c17043
134. Asghari F, Rabiei Faradonbeh D, Malekshahi ZV, et al. Hybrid PCL/chitosan-PEO nanofibrous scaffolds incorporated with A. euchroma extract for skin tissue engineering application. Carbohydr Polym. 2022;278:118926. doi:10.1016/j.carbpol.2021.118926
135. Malgarim Cordenonsi L, Faccendini A, Rossi S, et al. Platelet lysate loaded electrospun scaffolds: effect of nanofiber types on wound healing. Eur J Pharm Biopharm. 2019;142:247–257. doi:10.1016/j.ejpb.2019.06.030
136. Sannasimuthu A, Ramani M, Paray BA, et al. Arthrospira platensis transglutaminase derived antioxidant peptide-packed electrospun chitosan/ poly (vinyl alcohol) nanofibrous mat accelerates wound healing, in vitro, via inducing mouse embryonic fibroblast proliferation. Colloids Surf B Biointerfaces. 2020;193:111124. doi:10.1016/j.colsurfb.2020.111124
137. Khan AUR, Huang K, Khalaji MS, et al. Multifunctional bioactive core-shell electrospun membrane capable to terminate inflammatory cycle and promote angiogenesis in diabetic wound. Bioact Mater. 2021;6(9):2783–2800. doi:10.1016/j.bioactmat.2021.01.040
138. Pillai MM, Dandia H, Checker R, Rokade S, Sharma D, Tayalia P. Novel combination of bioactive agents in bilayered dermal patches provides superior wound healing. Nanomedicine. 2021;40:102495. doi:10.1016/j.nano.2021.102495
139. Truskewycz A, Truong VK, Ball AS, et al. Fluorescent magnesium hydroxide nanosheet bandages with tailored properties for biocompatible antimicrobial wound dressings and pH monitoring. ACS Appl Mater Interfaces. 2021;13(24):27904–27919. doi:10.1021/acsami.1c05908
140. Hajiali H, Summa M, Russo D, et al. Alginate-lavender nanofibers with antibacterial and anti-inflammatory activity to effectively promote burn healing. J Mater Chem B. 2016;4(9):1686–1695. doi:10.1039/c5tb02174j
141. Tang Y, Lan X, Liang C, et al. Honey loaded alginate/PVA nanofibrous membrane as potential bioactive wound dressing. Carbohydr Polym. 2019;219:113–120. doi:10.1016/j.carbpol.2019.05.004
142. Las Heras K, Igartua M, Santos-Vizcaino E, Hernandez RM. Chronic wounds: current status, available strategies and emerging therapeutic solutions. J Control Release. 2020;328:532–550. doi:10.1016/j.jconrel.2020.09.039
143. Yuan TT, DiGeorge Foushee AM, Johnson MC, Jockheck-Clark AR, Stahl JM. Development of electrospun chitosan-polyethylene oxide/fibrinogen biocomposite for potential wound healing applications. Nanoscale Res Lett. 2018;13(1):88. doi:10.1186/s11671-018-2491-8
144. Ghalei S, Nourmohammadi J, Solouk A, Mirzadeh H. Enhanced cellular response elicited by addition of amniotic fluid to alginate hydrogel-electrospun silk fibroin fibers for potential wound dressing application. Colloids Surf B Biointerfaces. 2018;172:82–89. doi:10.1016/j.colsurfb.2018.08.028
145. Suliman Maashi M, Felemban SG, Almasmoum HA, Jarahian M. Nicaraven-loaded electrospun wound dressings promote diabetic wound healing via proangiogenic and immunomodulatory functions: a preclinical investigation. Drug Deliv Transl Res. 2022. doi:10.1007/s13346-022-01176-9
146. Zhu Z, Liu Y, Xue Y, et al. Tazarotene released from aligned electrospun membrane facilitates cutaneous wound healing by promoting angiogenesis. ACS Appl Mater Interfaces. 2019;11(39):36141–36153. doi:10.1021/acsami.9b13271
147. Ren X, Han Y, Wang J, et al. An aligned porous electrospun fibrous membrane with controlled drug delivery - An efficient strategy to accelerate diabetic wound healing with improved angiogenesis. Acta Biomater. 2018;70:140–153. doi:10.1016/j.actbio.2018.02.010
148. Mulholland EJ, Ali A, Robson T, Dunne NJ, McCarthy HO. Delivery of RALA/siFKBPL nanoparticles via electrospun bilayer nanofibres: an innovative angiogenic therapy for wound repair. J Control Release. 2019;316:53–65. doi:10.1016/j.jconrel.2019.10.050
149. Liu S, Zhang Q, Yu J, et al. Absorbable Thioether Grafted Hyaluronic Acid Nanofibrous Hydrogel for Synergistic Modulation of Inflammation Microenvironment to Accelerate Chronic Diabetic Wound Healing. Adv Healthc Mater. Jun 2020;9(11):e2000198. doi:10.1002/adhm.202000198
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
