Back to Journals » Clinical Ophthalmology » Volume 17
Polypoidal Choroidal Vasculopathy: An Update on Diagnosis and Treatment
Authors Sen P, Manayath G, Shroff D, Salloju V, Dhar P
Received 11 August 2022
Accepted for publication 5 December 2022
Published 5 January 2023 Volume 2023:17 Pages 53—70
DOI https://doi.org/10.2147/OPTH.S385827
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Scott Fraser
Parveen Sen,1 George Manayath,2 Daraius Shroff,3 Vineeth Salloju,4 Priyanka Dhar4
1Shri Bhagwan Mahavir Vitreoretinal Services, Sankara Nethralaya, Chennai, Tamil Nadu India; 2Department of Retina and Vitreous Services, Aravind Eye Hospital and Postgraduate Institute of Ophthalmology, Coimbatore, India; 3Vitreoretinal Services, Shroff Eye Centre, New Delhi, India; 4Medical Affairs, Novartis Healthcare Private Limited, Mumbai, India
Correspondence: George Manayath, Department of Retina and Vitreous Services, Aravind Eye Hospital and Postgraduate Institute of Ophthalmology, Coimbatore, Tamil Nadu, India, Email [email protected]
Abstract: Polypoidal choroidal vasculopathy (PCV) is a vascular disease of the choroid that leads to hemorrhagic and exudative macular degeneration. It may cause significant vision loss and thus affect the quality-of-life and psychological well-being. Non-invasive, non-ICGA-based OCT criteria have shown reliable results to plan adjunct photodynamic therapy (PDT) treatment, with the complete and consistent coverage of polypoidal lesions (PL) and branching neovascular network (BNN). The safety and efficacy of anti-vascular endothelial growth factor (anti-VEGF) monotherapy and its combination with verteporfin PDT have been established. However, treatment is still challenging due to frequent follow-ups, non-availability of PDT, and need for multiple anti-VEGF injection visits that increase the treatment burden and lead to patients being lost to follow-up. Effective treatments that prolong intervals between injections while maintaining vision and anatomical gains remain a critical unmet need. Longer acting molecules, like brolucizumab, have shown non-inferiority in BCVA gains and superior anatomical outcomes compared to other anti-VEGF agents. Newer therapies in the pipeline to enhance the efficacy and longevity of treatment include Faricimab and a port delivery system (PDS). This review summarizes the most recent diagnostic and treatment approaches in PCV to offer better treatment avenues.
Keywords: polypoidal lesions, branching neovascular network, photodynamic therapy, best-corrected visual acuity, anti-vascular endothelial growth factor (anti-VEGF) agents, brolucizumab
Introduction
Polypoidal choroidal vasculopathy (PCV) is one of the “Pachychoroid spectra of diseases” of the retina. As per initial reports, PCV was common among black females by as much as 57.1% to 80%.1 Though predominantly reported from the Afro-Caribbean and Asian countries in the past, recent reports from the Caucasian population have also emerged,2,3 giving this disease more global recognition and attention. Recent hospital/clinic-based epidemiological studies show PCV prevalence as high as 22–62% among presumed neovascular age-related macular degeneration (nAMD) cases in the Asian population4,5 and about 10–20% in the Caucasian population. It is estimated that, by 2050, the number of people aged 65 or above will be 1.5 billion or more worldwide, placing this population at risk of developing nAMD and PCV.6
Yannuzzi et al7 initially described PCV in 1982. Clinically PCV may have a predominantly hemorrhagic or exudative presentation.4,5 PCV is characterized by recurrent serosanguineous maculopathy, neurosensory layer detachment, branching neovascular network, and abnormal polypoidal/aneurysmal neovascular lesions referred to as “polyps”.7–9 Unlike typical nAMD, eyes with PCV are less prone to intraretinal edema; however, they are more prone to massive subretinal hemorrhage, serous pigment epithelial detachment (PED), and break-through vitreous hemorrhage.8–11 Differentiating PCV from nAMD is vital because of its suboptimal response to anti-vascular endothelial growth factor (VEGF) agents and the need for additional photodynamic therapy (PDT) and/or laser. Diagnosis of PCV is based on a combination of fundus findings, dye-based angiography, and optical coherence tomography (OCT).
A landmark article published by Anantharaman et al12 in 2018 discussed the consensus recommendation of diagnosis and management of PCV based on existing literature up to November 2015. Most physicians used this as a gold standard, especially in the Indian setting. However, recent advances in imaging techniques and limited availability of PDT has led to a paradigm shift from Indocyanine green angiography (ICGA)-based diagnosis and treatment to a more non-invasive OCT-based treatment. Also, newer wide-field imaging technologies have added new information and improved our understanding of PCV. This review article is an update on our understanding of pathophysiology, diagnosis, and treatment strategies in PCV considering the knowledge provided by newer imaging modalities and the availability of newer treatment molecules and regimes.
Pathophysiology of PCV as Understood by Newer Imaging Modalities
PCV comes under the pachychoroid spectrum of diseases (PSD) that also includes pachychoroid epitheliopathy, pachychoroid neovasculopathy (PNV), central serous chorioretinopathy (CSC), and peripapillary pachychoroid syndrome (PPS). The most salient feature of PSDs is the dilation of Haller’s layer and thinning of choriocapillaris and Sattler’s layer.13 These dilated “pachyvessels”, often clustered at the macula, do not taper toward the posterior pole, and may show choroidal hyperpermeability on ICGA.
Ultra-widefield ICGA allows visualization of the vortex veins system. Dilated choroidal veins are believed to be branches of the vortex veins, and eyes with pachyvessels were seen to have dilated vortex vein ampulla with blood flow stasis.14 This stasis may occur secondary to the thickened sclera, as seen in nanophthalmos, or increased blood flow, as seen in increased sympathetic activity secondary to stress, a known risk factor for CSC.15 In recent years, it has been postulated that choroidal vessel dilatation seen in PCV is secondary to vortex vein congestion.15,16
In normal eyes, the choroidal outflow is divided into four quadrants separated by vertical and horizontal watershed zones. The vortex vein stasis or congestion seen in PSD results in anastomosis between the superior and inferior vortex vein and obliteration of the watershed zone. Macular vortex vein anastomosis may be a common morphologic feature of PSD,16 reported in about 90% of eyes with PSD.17 Anastomosis between the superior and inferior vortex vein results in obliteration of the horizontal watershed as seen in CSC or PCV. Obliteration of the vertical watershed temporal to papilla may result in PPS.15
Anastomosis between the superior and inferior vortex veins was observed in 90% of eyes with CSC, 95% of eyes with PNV, and 98% of eyes with PCV.17 These anastomotic channels may provide a new drainage route to ameliorate choroidal congestion.16 Takahashi18 suggested the pachyvessels to be the anastomotic channels lined by a single layer of thin endothelial cells. The stasis results in dilatation of the Haller’s layer in the outer choroid and thinning of the Sattler’s layer and the choriocapillaris. The thinning of choriocapillaris results in ischemia, causing the formation of neovascular complexes as seen in PNV and PCV. This choriocapillaris occlusion may be seen as a delay in capillary filling on ICGA or as choriocapillaris flow deficits (CCFD) on OCT angiography (OCTA). Increased CCFD has been reported not only in eyes with PCV but also in fellow unaffected eyes of PCV.19
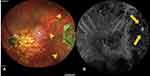
Unlike the retina, the choroid does not have a capillary complex. It is directly exposed to pulsatile blood flow that may result in dilatation of CNV tips leading to polyp formation.15 Thickened choroids are not seen in all PCV eyes. PCV has been classified into pachychoroid and non-pachychoroid PCV based on choroidal thickness (normal SFCT of about 267.5 μm).20 On imaging with OCT, nearly half of the PCV eyes observed normal choroidal thickness.14 Different pathological mechanisms and the clinical course are reported in eyes with pachychoroid and without pachychoroid.21 Patients with pachychoroid are younger, with fewer AMD-like features, more CSC-like features, more choroidal vascular hyperpermeability (CVH) (Figure 1), and resistant to anti-VEGF treatment.20 Recently peripheral exudative hemorrhagic chorioretinopathy (PEHCR), which showed manifestations of peripheral PCV, was added to the spectrum of pachychoroid disease entities.22
Genetics in PCV Pathophysiology
Numerous imaging studies have provided sufficient proof of concept that PCV, a part of the PDS, is a different disease entity from nAMD with some overlap perhaps seen in the form of the drusen-driven PCV or non-pachychoroid PCV.
Almost 34 loci have been identified to have an association with AMD with major susceptibility genes being complement factor H gene (CFH) and age-related maculopathy susceptibility 2 gene (ARMS2), and high-temperature requirement factor A1 (HTRA1). CFH was the first susceptibility gene identified for AMD and is the critical regulator of the complement system known to play a part in the pathogenesis of drusen-driven AMD. ARMS2/HTRA1 have also been found to play a significant role in drusen formation. Associations of these two genes have been confirmed with PCV, though the association of ARMS2/HTRAI may be stronger for Retinal Angiomatous Proliferation (RAP) than for PCV. Recent reports have suggested a higher association of ARMS2/HTRAI risk allele, particularly the A69S variant with PCV, especially drusen-driven PCV with thinner choroids.23,24 Also, the presence of CFH risk allele in patients with CSC makes them more susceptible to the development of PNV.25 Single nucleotide polymorphism (SNPs) in the angiopoietin 2 (ANGPT2) gene is also reported to be associated with AMD and PCV.26 Exome sequencing has identified a missense mutation in the FGD6 gene associated with an increased risk of PCV development via oxidized phospholipids (lipid metabolism). Multiple PCV-associated SNPs have been reported in PCV clinical expression, and it was reported that the HTRA1 rs2293870 variant is more likely involved in unilateral PCV development.27 The mutation reported in the first exon of HTRA1 (rs2293870) resulted in altered mRNA, although it could not change protein sequence and its expression.28 The role of altered mRNA and secondary protein structures involved in PCV pathogenesis needs further exploration. Additional research is needed to explore more etiological variants for PCV pathogenesis.
Diagnosis Through Clinical Imaging
Optimal treatment depends on the accurate diagnosis of PCV. Imaging techniques, especially the non-invasive modalities, have evolved radically to give better resolution and have greatly revolutionized our diagnostic skills and treatment algorithms.
Indocyanine Green Angiography
ICGA has been considered the gold standard for the definitive diagnosis of PCV.9,29,30 It enables the visualization of choroidal veins under RPE and choroidal blood circulation. In addition, the long operating wavelength of ICGA allows better visualization through subretinal or intraretinal fluid, RPE, lipid, and hemorrhage than fluorescein dye.31 But its lack of universal availability, contraindications, potential adverse reactions, and invasive nature limit its use.32
Fluorescein angiography, though of limited value in visualizing sub-RPE structures, including polypoidal lesions and BNN,9,33 is useful to determine the signs of activity12 and predicting the disease outcomes.34 Tan et al34 describe three subtypes of PCV: Type A with interconnecting channels, Type B with BNN with no leakage, and type C with BNN with leakage on FFA. The worst prognosis has been reported in Type C.
Multicolor Imaging
Multicolor imaging (MC) is a novel imaging technique using three different wavelengths to obtain a final reflectance image. Not many studies have reported the utility of multicolor imaging in PCV. In a report, Tan et al35 demonstrated that polyps are best seen on infrared reflectance images. Though BVN is not very well delineated, polyps are well highlighted as dark green oval lesions on the multicolor composite images with higher contrast on MC compared to the nodular orange appearance on CFP. Tan et al,36 in a comparison study, found multicolor images as useful as Color Fundus Photograph (CFP) for detecting PCV lesions.
Optical Coherence Tomography (OCT)
Optical coherence tomography (OCT) is a non-invasive technique that is widely available and provides high-resolution cross-sectional images of the retina and choroidal vasculature. With the advancement in technology, spectral-domain (SD) and swept source (SS) OCT with faster scanning speeds has allowed better resolution of retinal layers, as well as better imaging of choroidal vascular changes in PCV patients.37,38 In addition, recent studies suggested that OCT alone or with color fundus photography can differentiate PCV from nAMD with accuracy, high sensitivity, and specificity even in the absence of ICGA.39,40 Additionally, Enhanced Depth Imaging (EDI) is an innovative imaging technique using commercially available OCT which upgraded the evaluation of choroidal thickness and recognition of AMD and pachychoroid spectrum diseases.41,42
Recognizing the lack of widespread availability of ICGA amongst retina specialists, a panel of retinal specialists as part of the Asia-Pacific Ocular Imaging Society (APOIS) PCV workshop was established in 2019. It proposed a consensus revised nomenclature for PCV component lesions and established “major” and “minor” non-ICGA-based criteria for differentiating PCV from nAMD in treatment-naïve eyes.43 OCT, which has been widely used as a monitoring tool, was especially evaluated along with fundus photograph as a diagnostic tool. They recommended the term “polypoidal lesion” and “branching neovascular network” instead of “polyp” and “branching vascular network”, respectively. Various OCT reports and histological findings demonstrated that these “polyps” are not fleshy solid lesions, and the larger “aneurysmal lesions” may have some internal structures seen within them, as seen on OCTA.38 Therefore, the panel recommended the term “polyp” be replaced by “polypoidal lesion” or PL. The panel also suggested that PCV is a subgroup of type 1 neovascularization of nAMD; thus, “branching neovascular network” is more suitable than “branching vascular network”.43 Also, Type 1 CNV associated with thick choroid (or pachychoroid) may be a disease continuum, often referred to as pachychoroid neovasculopathy (PNV).
In addition to a revised uniform nomenclature, they developed a set of diagnostic features based on a combination of nine OCT signs (six based on cross-sectional OCT, two based on color fundus photograph or fundus examination, and one based on enface OCT) to differentiate PCV from typical nAMD without relying on ICGA. Further, the APOIS PCV panel evaluated the performance of non-ICGA-based imaging features for their sensitivity, specificity, predictive values, and accuracy for every distinct feature.44,45 The highest performance was reported by three individual features which had an AUC of >0.75 and were labelled the “major criteria”:
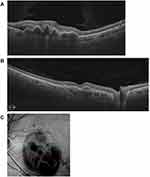
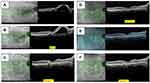
- Sharp-peaked pigment epithelial detachment (PED); also known as “thumb-like-protrusion”, narrow peaked with inverted “V” like arrangement, sharp vertical slope with more than 70° on at least one side, and more than one ratio of height versus thickness at the base (Figure 2A).
- Sub-retinal pigment epithelium (RPE) ring-like lesion within the PED; circular structure under PED may be associated with the hypo-reflective center, which may differ in intensity, surrounded by hyper-reflective outline (Figure 2B).
- Enface OCT Complex RPE elevation – Irregular elevation of RPE with a neovascular network (BNN) connecting multiple PEDs on enface OCT (Figure 2C).
These three features combined met the predictive accuracy (pre-specified area under the curve [AUC]) of 0.85 in workshop report-2 and 0.90 (AUC) in workshop report-1. Furthermore, validation of these OCT markers in another subset of patients achieved an accuracy of 82% (APOIS-1) to differentiate PCV from nAMD. Therefore, these three OCT markers are developed as “Major criteria” to differentiate PCV from nAMD. Authors also reported that in case of unavailability of en face OCT, the other two major criteria could achieve an AUC of 0.82.
Four other features matched with pre-specified AUC requirement for minor criteria (AUC<0.75 but >0.60) and were categorized as “Minor criteria”.44 These include:
- Orange nodule on CFP.
- Thick choroid (>300 microns) with dilated vessels in Haller’s layer.
- Complex/multilobular or notched PED.
- Double-layer sign or slight elevation of RPE due to underlying BNN.
Massive hemorrhage and fluid predominantly in the subretinal compartment were not included in the final diagnostic criteria as they did not meet the requisite requirements. However, based on the presence of these OCT features alone, diagnosis of PCV could be established with a sensitivity and specificity of 85–90% in various studies.45,46
While the above criteria are for treatment-naïve eyes, APOIS PCV Workgroup Report-2 also recommended criteria to detect PCV in presumed nAMD eyes that were seen to have a suboptimal response to three loading doses of anti-VEGF therapy.44
The three major criteria that could differentiate PCV from typical nAMD in these cases of “suboptimal responders” were:
- Sharp-peaked PED (AUC=0.76),
- Hyporeflective sub-RPE ring (AUC=0.73), and
- Orange nodule on fundus photography (AUC=0.67).
These may indicate needs for adjunct or rescue PDT treatment. A combination of these three criteria had a specificity of 0.82 and sensitivity of 0.65 (AUC of 0.85) in detecting PCV. En face RPE elevation considered one of the major criteria for treatment-naïve eyes, was not significant when evaluating the “suboptimal response eyes.” This could be due to the resolution of some subRPE fluid following treatment with anti-VEGF agents.
Optical Coherence Tomography (OCT) Angiography
OCT angiography (OCTA) allows in-depth analysis of the microvascular circulation in the choriocapillaris and choroid vasculature, these are not assessed on routine FA or ICGA.47,48
Diagnosis of PCV on OCTA depends on detection and evaluation of the two components of PL and BNN. Various recent reports have suggested that OCTA is maybe comparable or even superior to ICGA in detecting BNN, but not for identifying polypoidal lesions.49–51 Also, OCTA allows a depth-resolved analysis which is not possible with ICGA. Whereas BNN was picked up in almost 100% of cases of PCV, the detection rate of PLs on OCTA is reported to be about 79%.44 BNN are seen as high flow lesions on OCTA, but polyps are difficult to visualize due to slow and turbulent blood flow within them. Very slow filling PLs on ICGA are particularly difficult to pick up on OCTA.51 Zhan et al52 reported some other features that may be associated with the non-visualization of polyps on OCTA including increased height of polyps, pulsatile polyps, and the presence of overlying subretinal hemorrhage as well as branches of normal retinal vessels. They hypothesized that increased fibrin and exudation in higher polyps might make visualization on OCTA difficult. Similarly, subretinal hemorrhage of more than 350 μm and overlying retinal blood vessels may create artifacts that may not allow visualization of underlying polyps.52
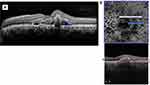
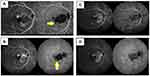
Previous studies have described polyps as aneurysmal dilatations. However, a recent study of PCV on swept-source OCTA (SS-OCTA) by Bo et al38 described polypoidal lesions as tangled vascular structures at the border of BNNs instead of a dilated pouch. SS-OCTA uses a longer wavelength (1,060 nm) that allows deeper and faster scans which can be more useful in imaging the PLs in PCV. Compared to SD-OCT it has a lower sensitivity roll-off under the RPE, providing better structural and angiographic images.53 The presence of tiny neovascular tufts in these PLs could explain the decrease in size and complexity of PLs in response to anti-VEGF therapy. With SS-OCTA, the internal structures were reported for the first time, and their precise location between the Bruch’s membrane and RPE was established (Figure 3). In keeping with this enhanced imaging of PCV lesions, SS-OCTA may score over the gold standard ICGA. But still not many studies have validated the concept of PLs being a neovascular structure.
Cheung et al54 used a combination of OCT and OCTA to differentiate PCV from AMD with a sensitivity and specificity of 82.6% and 100%, respectively. In addition, 10% of the BNN not adequately marked while determining the OCT-based greatest linear diameter (GLD) can also be well visualized on OCTA, making the combined OCT and OCTA a more comprehensive noninvasive imaging modality for PCV,44 though the technique is continuously evolving.
Imaging as a Guide for Treatment
OCT alone has been considered to plan adjunct rescue PDT treatment without requiring ICGA. Teo et al44 described the marking of Near Infrared (NIR) reflectance images on a 6*6 mm B scan on OCT to cover all PLs and BNN in a single circular spot which covered 100% of the PL area and 90% of the BNN area as compared to the area covered on ICGA. Since PDT largely targets the closure of polypoidal lesions, this circular spot or GLD as determined by OCT may give an acceptable treatment outcome. Another clue to detect the total extent of the BNN is to consider the complete extent of the “double layer sign” on OCT B scans. However, the efficacy of OCT-guided PDT in PCV remains to be ascertained in many PCV eyes.
Imaging to Determine Response to Therapy
OCT is largely used to evaluate response to therapy in PCV. The parameters assessed include: subretinal fluid (SRF), intraretinal cysts (IRC), PED height, and PED volume. These signs are similar to the ones used for nAMD. Features of activity and regression specific to PCV have recently been described.49
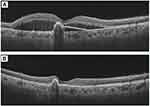
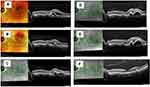
Perfused polyps on ICGA were defined in the EVEREST study presenting as nodular hyperfluorescence on stereoscopic examination with a late hypofluorescent halo around the nodule. Polyp closure was defined as the absence of the early nodular hyperfluorescence on ICGA.55 On OCT, polyp regression signs include a decrease in the height of polyp, sub-PL space becoming densely hyperreflective, absence of the subRPE hyperreflective ring, and absence of associated SRF since thrombosed or closed polyps will not leak56 (Figure 4).
Other signs of polyp closure on OCTA include the presence of indistinct RPE above PED, absent PED, absence of IRF, presence of fibrovascular PED vs serous PED, decrease in the height of PED, and increasing hyperreflectivity of PED contents.56 The authors concluded that polyp closure is an important landmark and suggested continued treatment even in the absence of SRF if perfused polyps as seen on OCT.56
BNN on OCTA has been described as a loose, dead-tree shape (more likely to be inactive), coral-bush type, anastomosis, or pseudopod-like extrusion (more likely to be active); especially the pseudopod-like may be more prone to recurrences.57
Mean choroidal volume, sub-foveal choroidal thickness, and CVI are some other parameters that show modulation under long-term anti-VEGF therapy. After anti-VEGF therapy, the blood flow through the anastomotic channels in PCV decreases resulting in decreased transudation into the choroidal stroma, and reduced exudation from the BNN resulted in thinner choroids and increased CVI.58
Imaging for Disease Prognostication
Huang et at59 described three different BNN on OCTA: Type 1(trunk form, larger), type 2 (glomeruli form), and type 3 (stick form, smallest). Ma et al60 described the effect of these patterns of BNN as seen on OCTA on treatment response and outcome in PCV. Type 2 and 3 were associated with thicker choroids and increased recurrences, while type 1 was associated with the thin choroid, drusen, and AMD-like features. Ma et al60 found that, for most patients, type 1, 2, and 3 BNN corresponded to Type C, B, and A BNN, respectively, as described by Tan et al based on FFA and ICGA. IRC was most seen with type 1, which also had the worse BCVA both at baseline and after treatment. Type 3 was associated with most favorable outcome following combination therapy with PDT and anti-VEGF.
With a high detection rate of BNN, near-accurate estimation of GLD for treatment, and the ability to gauge the treatment response and long-term prognosis, OCT and OCTA together can be a powerful tool for the management of PCV. The limitation of difficulty in acquisition, interpretation, and segmentation must always be kept in mind. Of course, there is no substitute for clinical acumen while meaningfully applying these strong imaging tools for better treatment and management of our patients.
Artificial Intelligence in Diagnosis
Before the treatment plans, PCV patients should be distinguished from typical AMD cases. Diagnosis is difficult for inexperienced ophthalmologists, and ICGA imaging is not always possible. The volumetric nature of the OCT also makes it tedious for clinicians to go through each B-scan and identify distinctive imaging features locally. The AI platform permits ophthalmologists to develop their own models, which will be helpful to provide insight in clinical applications. In a recent study automatic differential diagnosis between polypoidal choroidal vasculopathy (PCV) and wet age-related macular degeneration (AMD) from volumetric optical coherence tomography (OCT) images was done to identify clinically relevant pathological features, using an explainable deep-learning-based framework. Authors found that the application of non-invasive differential diagnosis using AI-driven OCT-based analysis, with minimal requirement of labeling efforts achieved through automatically detected disease-related pathologies.61,62
Treatment Updates in PCV Management
Various treatment options recommended for PCV include thermal laser photocoagulation, PDT monotherapy with verteporfin infusion, anti-VEGF monotherapy, and a combination therapy. Thermal laser photocoagulation has long been used to ablate extrafoveal polyps identified through ICGA.63 However, an individualized approach with adjunctive laser therapy and anti-VEGF therapy may be necessary in selected cases for optimum treatment.63
The anti-VEGF agents are known to reduce exudation from abnormal polyps and choroidal vessels, thereby reducing macular edema and thus preserving vision.64 Anti-VEGF therapy is the primary treatment for PCV when significant hemorrhage or exudation precludes the visualization of polyps in ICGA, and PDT cannot be undertaken. In the recent past clinical trials and real-world studies have demonstrated the efficacy and safety of anti-VEGF therapy in PCV management globally.64
Ranibizumab is a monoclonal antibody fragment that neutralizes VEGF-A and thus inhibits angiogenesis.65 The PEARL and PEARL2 studies demonstrated that monthly intravitreal ranibizumab monotherapy at 0.5 mg and 2.0 mg, respectively, preserved the vision by reducing subretinal hemorrhage and macular edema with polyp closure rates of 38% with 0.5 mg and 79% with 2 mg ranibizumab.66,67 Primarily the ICGA-guided Everest I study assessed the effect of Verteporfin PDT (vPDT) and ranibizumab (0.5 mg) in Asian patients with symptomatic macular PCV. The study reported that PDT, either alone or in combination, was significantly more effective compared to ranibizumab monotherapy in achieving complete regression of polyps (77.8% and 71.4% vs 28.6%) and BCVA (letters) 7.5±10.6 (vPDT), 10.9±10.9 (verteporfin PDT + ranibizumab), and 9.2±12.4 (ranibizumab).40 The EVEREST II study reported a 34.7% polyp regression rate with 0.5 mg ranibizumab. Among the 322 participants, combination therapy achieved superior BCVA gain (Monotherapy group, 5.5 letters; combination therapy group, 9.6 letters) at 2 years. There were increased odds of polyp regression, and fewer treatment episodes in combination therapy than with ranibizumab monotherapy.68 Fujisan’s study reported similar visual and anatomical improvements with initial or deferred PDT combined with ranibizumab at 12 months. The initial PDT regimen showed lesser additional treatment requirements.69
Aflibercept is an inhibitor of VEGF-A, VEGF-B, and placental growth factor (PLGF). Compared to ranibizumab, aflibercept may be more effective in causing polyp regression,55 reduction in BNN, and significant best-corrected visual acuity (BCVA) improvement.70,71 Aflibercept was evaluated in the PLANET study to compare the efficacy, safety, and tolerability of aflibercept monotherapy with combination therapy of aflibercept and PDT, in the treatment of PCV patients. Eighty-five percent of PCV patients treated with aflibercept monotherapy showed improvement in visual outcomes with no signs of leakage from polyps (Polyp Inactivation).72 Additionally, the efficacy of aflibercept monotherapy was comparable to aflibercept/PDT in best-corrected visual acuity (+10.7 vs +10.8 letters, respectively), with lesser than 15% of patients requiring rescue therapy with PDT.72 Other studies have reported polyp regression in 55–77% of eyes and BNN regression in 4.8–13.4% of eyes with aflibercept.73 However, frequent injection visits and increased treatment cost leading to patient follow-up loss is a major concern.
After the US and EU, Brolucizumab, approved in India for the treatment of nAMD, is a high-affinity antibody fragment for VEGF. Being small allows more drug delivery per injection than other anti-VEGF agents and penetrates the tissue more effectively.74 HAWK and HARRIER Phase III trials reported non-inferior improvement in BCVA and superior anatomical outcomes with brolucizumab in nAMD cases compared to aflibercept.75,76 The HAWK PCV subpopulation analysis described robust BCVA gains with the mean change of +10.4 for brolucizumab (6 mg) and +11.6 for aflibercept (2 mg) at week 48. The achieved visual gains were sustained to week 96 with a change of +11.4 for brolucizumab (6 mg) and +11.1 for aflibercept (2 mg). After the loading dose, the probability of exclusively maintaining on q12w dosing interval through week 48 was 76% and through week 96 was 68% for the eyes treated with brolucizumab.77
The HAWK analysis revealed the presence of IRF and/or SRF in fewer brolucizumab (6 mg) treated eyes compared to aflibercept treatment (48.7% vs 70%), at week 4 after the first injection and the difference was observed through week 96. Similarly, fewer PCV eyes had sub-RPE fluid after brolucizumab (6 mg) treatment compared with aflibercept from week 4 (38.5% vs 60.0%) to week 96 (7.7% vs 13.3%).77 This fluid resolution could manifest the inactivation of polys.
Further, the study also reported a notable reduction in central subfield thickness (CST) in both brolucizumab and aflibercept treatment arms as 149 μm and 182 μm, respectively, at week 48 (baseline: 393 μm), and these comparable improvements were maintained up to week 96.77
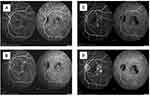
In addition, higher polypoidal regression rates of about 78.9% have been reported with 3-loading doses of brolucizumab 6 mg,39 compared to approximately 30% and 50% regression achieved by ranibizumab or aflibercept monotherapy, respectively.69,78–82 Compared to other anti-VEGF agents, brolucizumab provides advantages in morphological and angiographic outcomes (Figures 5 and 6). Reports of increased intraocular inflammation (IOI) have also been reported with brolucizumab. In early 2020, an external Safety Review Committee (SRC) reviewed cases of occlusive vasculitis and IOI, that sometimes resulted in vision loss, following injections and confirmed their association with brolucizumab.77 While there were no cases of moderate or severe vision loss related to the IOI events seen in the HAWK study occurred in the Japanese participants with PCV.77 Despite comparable or better efficacy and longevity of brolucizumab as compared to aflibercept, brolucizumab should be initiated after appropriate evaluation of benefit-risk analysis. There is another US-FDA-approved anti-VEGF agent Faricimab with an additional angiopoietin-2 inhibitory effect to treat wet AMD and Diabetic macular edema (DME).83 The inhibition of angiopoietin-2 may promote vascular stability and thus may be a promising therapy for PCV treatment with less frequent injections than other anti-VEGF therapies. However, its efficacy and safety are not established in PCV management yet.83
The port delivery system (PDS) of ranibizumab developed for long-term nAMD treatment is a surgically implanted small refillable reservoir placed at the pars plana. It can be periodically refilled via direct injection through the conjunctiva to reduce the treatment burden. PDS loaded with 100 mg/mL ranibizumab had a median reload time of 15 months and comparable efficacy to monthly ranibizumab injections in the Phase 2 LADDER trial.84 Though it shows promise for patients needing long-term anti-VEGF therapy like PCV and avoiding frequent injections, PDS has not been studied in eyes with PCV to-date.
Combination Therapy
Due to suboptimal effect of anti VEGF monotherapy on polyp regression, various clinical trials have demonstrated a reduction in disease activity and favorable visual gain with combination therapy compared to monotherapy in PCV treatment.40,85–87 Although, in the PLANET study anti-VEGF monotherapy demonstrated substantial VA gains and anatomical benefits with reported decreased in ICGA leakage or presence of PL. The addition of PDT to anti-VEGF monotherapy did not show the additional benefits in visual outcomes. The findings of the trial did not favor the superiority of combination therapy over anti-VEGF monotherapy.72
The combination therapy uses the thrombotic property of PDT to enhance polyp regression and the anti-permeability effect of anti-VEGF agents to decrease the exudation.5 The less intensive PDT strategies may reduce complications, further confirmed by a case-control review which compared the efficacy of reduced and standard-fluence PDT in combination therapy with anti-VEGF. The reduced-fluence PDT was comparable to standard-fluence PDT in terms of clinical, anatomical OCT outcomes, and visual gains88 (Figures 7 and 8). Therefore, the reduced fluence PDT combination therapy with OCT-based outcomes can be considered an optimal treatment paradigm for PCV management.
Treatment Regimens Followed in PCV Management
Three main approaches employed for the administration of anti-VEGF injections are:
- Fixed dosing regimens, which require monthly or bi-monthly dosing for at least one year,
- “Reactive” pro re nata (PRN) dosing regimens, which require monthly visits, with treatment reinstated on disease recurrence, and
- “Proactive” treat-and-extend (T&E) dosing regimens, which started with ≥3 consecutive monthly dosing until disease inactivity is seen and followed by gradual extension of 2–4 weeks to a maximum of 12–16 weeks treatment interval.
Among three major approaches, fixed dosing, pro re nata (PRN), and treat-and-extend (T&E) regimens, clinicians globally prefer T&E as it allows the extension of treatment intervals and therefore reduces the burden of clinic visits.89,90 Based on recent observational studies91–93 and the LAPTOP study reported better VA after initiation of ranibizumab as compared to PDT monotherapy at 2 years and through 5-year follow-up.89 ALTAIR94 trials, consensus recommendations for the T&E regimen allow adjustment of dosing intervals by 2–4 weeks, extension up to 16 weeks with aflibercept, and maximum extension of 12 weeks with ranibizumab or bevacizumab.91–94 In the PLANET trial, 41.2% of PCV patients achieved ≥12 weeks treatment intervals with VA gains of >10 letters up to Year 2.37,95 In the ALTAIR trial, around 37% of patients achieved VA gains of up to +4.9 letters by week 96.94 Multiple real-world studies also show the efficacy of aflibercept in the T&E regimen, with VA gains of up to +9.0 letters at Year 2.96 Almost 50% of patients experienced complete polyp regression after the loading dose.80,96,97 There is presently limited evidence on the T&E regimen for conbercept or brolucizumab in PCV.
Newer Monotherapy Strategies and Polyp Regression
The current disease activity indicators used for guiding subsequent retreatment include the presence of fluid or hemorrhage on OCT and visual acuity status. However, the persistence of PL is responsible for much of the exudation and recurrences in PCV. Hence, Polyp regression should be an important consideration in anti-VEGF monotherapy strategies in PCV. An important limitation of anti-VEGF monotherapy in PCV is the limited PL closure rate compared with PDT strategies.68,72 Recently, Chaikitmongkol et al98 showed a 50% complete polyp closure rate within 2 months of aflibercept monotherapy initiation and remained closed in 100% on ICGA. No regression or incomplete polypoidal regression may be associated with higher risk of long-term massive submacular hemorrhage.98 In contrast, complete polypoidal regression has been reported to be associated with less recurrence, longer intervals between treatments, and fewer numbers of treatments for PCV, though in a study with smaller sample size.99 Other indicators of disease activity such as fluid status and retinal volume fluctuation have not been evaluated in PCV eyes through prospective trials.
In a recent randomized controlled trial, Teo et al100 studied a novel personalized T&E regimen with aflibercept for PCV. They showed that eyes with persistent PL on ICGA after three initial monthly loading injections were treated with an additional 3-monthly injection and achieved comparable visual gain and higher PL closure rate at month 6 and month 12, than fixed 8-weekly dosing.100 Based on this novel protocol, a repeat ICGA should be performed after the third monthly treatment. If PL is absent, a T&E regimen can be commenced. If PL is present, regardless of other signs of disease activity, an additional three injections are administered monthly apart as a prolonged induction phase may be considered an alternative approach to deferred PDT for physicians who value PL closure as an important treatment outcome. This strategy may be particularly relevant in settings where PDT is unavailable.100
Recurrence of Polypoidal Lesions
PCV eyes have a period of lesion reactivation that occurs around 2–3 years after the initial presentation that requires re-initiation of an aggressive treatment regimen.101 Kim et al102 showed that clusters of multiple PL and larger lesion sizes are associated with PCV reactivation at 1 year, though it is unclear what features dictate reactivation in the long-term. In addition, Cho et al103 reported submacular hemorrhage to occur in 10% of eyes at 5 years and 30% at 10 years in PCV, which is significantly higher than that of typical nAMD, which is reported to occur in only 4% in 5 years and 10% in 10 years. Though most of the current anti-VEGF monotherapy protocols consider polyp inactivation as the treatment end point, the recurrences and late reactivation of PL may suggest considering PL closure status also as a treatment endpoint in the management of PCV, especially in the presence of multiple clusters of PL at baseline.
Surgical Management
Surgery is not frequently indicated in PCV. Indications for surgery include breakthrough vitreous hemorrhage and large submacular hemorrhage. In a series of cases from India, Narayanan et al104 reported that vitrectomy led to a significant visual improvement in patients with vitreous hemorrhage due to PCV. The authors found that the incidence of retinal breaks during vitrectomy was high in PCV and advised that caution should be exercised while inducing PVD in these cases.
Various approaches have been used for large submacular hemorrhages. Some authors have used intravitreal recombinant tissue plasminogen activator (rt-PA) injection without vitrectomy (“non-vitrectomizing technique”) (Figure 9). Other studies have described using submacular rt-PA injection with vitrectomy (“vitrectomizing techniques”). Gas injection and/or anti-VEGF agents are generally added in both the techniques. The non-vitrectomizing and vitrectomizing techniques do not differ in the postoperative response rate of complete submacular hemorrhage displacement and the complication rates of recurrent submacular hemorrhage and vitreous hemorrhage.105 Ranibizumab is recommended to be used concomitantly with rt-PA because this agent is not cleaved or functionally compromised by rt-PA or plasmin, whereas Aflibercept is cleaved, if co-administered with plasmin, reducing its efficacy.106
 |
Figure 9 Optos color photo. Notes: (A) OD having a submacular hemorrhage due to PCV (blue arrow). (B) Post-treatment with tPA, gas, and anti-VEGF, the hemorrhage has displaced inferiorly (blue arrow). |
Thus, current practices for the management of submacular hemorrhage include observation, intravitreal anti-VEGF, intravitreal gas therapy, intravitreal rtPA or subretinal rtPA with vitrectomy, and different other combinations. To the best of our understanding, no large-scale clinical study was conducted/published to compare the surgical management options. A Phase 3, pan-European surgical trial – TIGER – is underway to optimize the treatment for large submacular hemorrhage.97
Conclusion
In this review, we highlight how in recent years multimodal imaging studies have revolutionized our understanding of PCV and have resulted in newer treatment strategies. PCV is more prevalent in Asian populations and in India most cases remain undiagnosed due to the unavailability of ICGA facilities in many places. Advanced multimodal imaging, including OCT and OCTA, have characterized the biomarkers that increase the sensitivity and specificity of PCV diagnosis and allow treatment without ICGA. Thus, the diagnostic paradigm for PCV has shifted from invasive to non-invasive techniques.
Randomized therapeutic trials described the risks and benefits of the existing treatment protocols, which have substantially improved the visual outcomes in PCV over the past decade. With the absence of universal availability of PDT, monotherapy with anti VEGF therapy has become the mainstay of treatment. But, high injection burden, poor regression of PL and high recurrence rates remain a challenge for PCV management. Variable treatment response is also responsible for significant heterogenicity in PCV treatment approaches among physicians. There is a need for more comprehensive follow-up studies to assess the disease’s long-term visual outcomes and recurrence rates. Additionally, there is an unmet need to titrate the imaging biomarkers for evaluation of treatment response. Further strategies are required to overcome the challenges associated with PCV management in the form of newer therapies, increased treatment durability, and reduced treatment burden.
Acknowledgments
The authors would like to thank Dr Shweta Varshney, IQVIA, for her writing and editing support.
Disclosure
Vineeth Salloju and Priyanka Dhar are employees of Novartis Healthcare Private Limited, India. The authors report no other conflicts of interest in this work.
References
1. Kabedi NN, Kayembe DL, Elongo GM, Mwanza JC. Polypoidal choroidal vasculopathy in congolese patients. J Ophthalmol. 2020;2020:4103871.
2. Castro-Navarro V, Behar-Cohen F, Chang W, et al. Pachychoroid: current concepts on clinical features and pathogenesis. Graefes Arch Clin Exp Ophthalmol. 2021;259:1385–1400. doi:10.1007/s00417-020-04940-0
3. Bhoomibunchoo C, Yospaiboon Y, Thoongsuwan S, et al. Idiopathic polypoidal choroidal vasculopathy in Thai patients with clinical and angiographic choroidal neovascularization. Clin Ophthalmol. 2017;11:317. doi:10.2147/OPTH.S126226
4. Chan WM, Lam DS, Lai TY, et al. Photodynamic therapy with verteporfin for symptomatic polypoidal choroidal vasculopathy: one-year results of a prospective case series. Ophthalmol. 2004;11:1576–1584. doi:10.1016/j.ophtha.2003.12.056
5. Koh AH, Chen LJ, Chen SJ, et al. Polypoidal choroidal vasculopathy: evidence-based guidelines for clinical diagnosis and treatment. Retina. 2013;33:686–716. doi:10.1097/IAE.0b013e3182852446
6. Chaikitmongkol V, Sagong M, Lai TY, et al. Treat-and-extend regimens for the management of neovascular age-related macular degeneration and polypoidal choroidal vasculopathy: consensus and recommendations from the Asia-Pacific Vitreo-Retina Society. Asia-Pac J Ophthal. 2021;10:507. doi:10.1097/APO.0000000000000445
7. Yannuzzi LA, Sorenson J, Spaide RF, et al. Idiopathic polypoidal choroidal vasculopathy (IPCV). Retina. 1990;10:1–8. doi:10.1097/00006982-199010010-00001
8. Kleiner RC, Brucker AJ, Johnston RL. The posterior uveal bleeding syndrome. Retina. 1990;10:9–17. doi:10.1097/00006982-199010010-00002
9. Kumar A, Kumawat D, Sundar MD, et al. Polypoidal choroidal vasculopathy: a comprehensive clinical update. Ther Adv Ophthalmol. 2019;11:2515841419831152. doi:10.1177/2515841419831152
10. Ozawa S, Ishikawa K, Ito Y, et al. Differences in macular morphology between polypoidal choroidal vasculopathy and exudative age-related macular degeneration detected by optical coherence tomography. Retina. 2009;29:793–802. doi:10.1097/IAE.0b013e3181a3b7d9
11. Hirami Y, Tsujikawa A, Otani A, et al. Hemorrhagic complications after photodynamic therapy for polypoidal choroidal vasculopathy. Retina. 2007;27:335–341. doi:10.1097/01.iae.0000233647.78726.46
12. Anantharaman G, Sheth J, Bhende M, et al. Polypoidal choroidal vasculopathy: pearls in diagnosis and management. Indian J Ophthalmol. 2018;66(7):896. doi:10.4103/ijo.IJO_1136_17
13. Lee WK, Baek J, Dansingani KK, et al. Choroidal morphology in eyes with polypoidal choroidal vasculopathy and normal or subnormal choroidal thickness. Retina. 2016;36(suppl 1):S73–S82. doi:10.1097/IAE.0000000000001346
14. Qiu B, Zhang X, Li Z, et al. Characterization of choroidal morphology and vasculature in the phenotype of pachychoroid diseases by swept-source OCT and OCTA. J Clin Med. 2022;11(11):3243. doi:10.3390/jcm11113243
15. Kishi S, Matsumoto H. A new insight into pachychoroid diseases: remodeling of choroidal vasculature. Graefes Arch Clin Exp Ophthalmol. 2022;16:1–3.
16. Matsumoto H, Hoshino J, Mukai R, et al. Vortex vein anastomosis at the watershed in pachychoroid spectrum diseases. Ophthalmol Retina. 2020;4:938–945. doi:10.1016/j.oret.2020.03.024
17. Matsumoto H, Hoshino J, Arai Y, et al. Quantitative measures of vortex veins in the posterior pole in eyes with pachychoroid spectrum diseases. Sci Rep. 2020;10:19505. doi:10.1038/s41598-020-75789-w
18. Takahashi K. Bioimaging of choroidal neovascularization and pathological correlation. Nippon Ganka Gakkai Zasshi. 2019;123:284–311.
19. Luo M, Zhao X, Zhao N, et al. Comparison of choriocapillary flow density between fellow eyes of polypoidal choroidal vasculopathy and neovascular age-related macular degeneration. BMC Ophthalmol. 2020;20:162. doi:10.1186/s12886-020-01386-0
20. Chang YC, Cheng CK. Difference between pachychoroid and nonpachychoroid polypoidal choroidal vasculopathy and their response to anti-vascular endothelial growth factor therapy. Retina. 2020;40:1403–1411. doi:10.1097/IAE.0000000000002583
21. Kim H, Lee SC, Kwon KY, et al. Subfoveal choroidal thick- ness as a predictor of treatment response to anti-vascular endo- thelial growth factor therapy for polypoidal choroidal vasculopathy. Graefe’s Arch Clin Exp Ophthalmol. 2016;254:1497–1503. doi:10.1007/s00417-015-3221-x
22. Shroff D, Sharma M, Chhablani J, et al. Peripheral exudative hemorrhagic chorioretinopathy-A new addition to the spectrum of pachychoroid disease. Retina. 2021;41:1518–1525. doi:10.1097/IAE.0000000000003063
23. Sakurada Y, Kubota T, Imasawa M, et al. Angiographic lesion size associated with LOC387715 A69S genotype in subfoveal polypoidal choroidal vasculopathy. Retina. 2009;29:1522–1526. doi:10.1097/IAE.0b013e3181af0d72
24. Tanaka K, Nakayama T, Mori R, et al. Associations of complement factor H (CFH) and age-related maculopathy susceptibility 2 (ARMS2) genotypes with subtypes of polypoidal choroidal vasculopathy. Invest Ophthalmol Vis Sci. 2011;52:7441–7444. doi:10.1167/iovs.11-7546
25. Yamashiro K, Hosoda Y, Miyake M, et al. Characteristics of pachychoroid diseases and age-related macular degeneration: multimodal imaging and genetic backgrounds. J Clin Med. 2020;9:2034. doi:10.3390/jcm9072034
26. Chaikitmongkol V, Cheung CM, Koizumi H, et al. Latest developments in polypoidal choroidal vasculopathy: epidemiology, etiology, diagnosis, and treatment. Asia-Pac J Ophthalmol. 2020;9:260. doi:10.1097/01.APO.0000656992.00746.48
27. Luo M, Zhao X, Yang J, et al. The association of polypoidal choroidal vasculopathy clinical phenotypes with previously reported genetic markers. Graefe’s Arch Clin Exp Ophthalmol. 2020;258:1199–1203. doi:10.1007/s00417-020-04702-y
28. Wang G, Dubovy SR, Kovach JL, et al. Variants at chromosome 10q26 locus and the expression of HTRA1 in the retina. Exp Eye Res. 2013;112:102–105. doi:10.1016/j.exer.2013.04.019
29. Dansingani KK, Balaratnasingam C, Naysan J, et al. En face imaging of pachychoroid spectrum disorders with swept-source optical coherence tomography. Retina. 2016;36:499–516. doi:10.1097/IAE.0000000000000742
30. Kumar S, Nakashizuka H, Jones A, et al. Proteolytic degradation and inflammation play critical roles in polypoidal choroidal vasculopathy. Am J Pathol. 2017;187:2841–2857. doi:10.1016/j.ajpath.2017.08.025
31. Okubo A, Sameshima M, Uemura A, et al. Clinicopathological correlation of polypoidal choroidal vasculopathy revealed by ultrastructural study. Br J Ophthal. 2002;86:1093–1098. doi:10.1136/bjo.86.10.1093
32. Saito M, Iida T, Nagayama D. Cross-sectional and en face optical coherence tomographic features of polypoidal choroidal vasculopathy. Retina. 2008;28:459–464. doi:10.1097/IAE.0b013e318156db60
33. Pang CE, Shah VP, Sarraf D, et al. Ultra-widefield imaging with autofluorescence and indocyanine green angiography in central serous chorioretinopathy. Am J Ophthalmol. 2014;158:362–371. doi:10.1016/j.ajo.2014.04.021
34. Tan CS, Ngo WK, Lim LW, et al. A novel classification of the vascular patterns of polypoidal choroidal vasculopathy and its relation to clinical outcomes. Br J Ophthalmol. 2014;98:
35. Tan CS, Ting DS, Lim LW. Multicolor fundus imaging of polypoidal choroidal vasculopathy. Ophthalmol Retina. 2019;3:400–409. doi:10.1016/j.oret.2019.01.009
36. Tan ACS, Yanagi Y, Cheung GCM. Comparison of multicolor imaging and color fundus photography in the detection of pathological findings in eyes with polypoidal choroidal vasculopathy. Retina. 2020;40:1512–1519. doi:10.1097/IAE.0000000000002638
37. Ng DS, Bakthavatsalam M, Lai FH, et al. Classification of exudative age-related macular degeneration with pachyvessels on en face swept-source optical coherence tomography. Invest. Ophthalmol Vis Sci. 2017;58:1054–1062. doi:10.1167/iovs.16-20519
38. Bo Q, Yan Q, Shen M, et al. Appearance of polypoidal lesions in patients with polypoidal choroidal vasculopathy using swept source optical coherence tomographic angiography. JAMA Ophthalmol. 2019;137:642–650. doi:10.1001/jamaophthalmol.2019.0449
39. Matsumoto H, Hoshino J, Mukai R, et al. Short-term outcomes of intravitreal brolucizumab for treatment-naïve neovascular age-related macular degeneration with type 1 choroidal neovascularization including polypoidal choroidal vasculopathy. Sci Rep. 2021;11:1–8. doi:10.1038/s41598-021-86014-7
40. Koh A, Lee WK, Chen LJ, et al. EVEREST study: efficacy and safety of verteporfin photodynamic therapy in combination with ranibizumab or alone versus ranibizumab monotherapy in patients with symptomatic macular polypoidal choroidal vasculopathy. Retina. 2012;32:1453–1464. doi:10.1097/IAE.0b013e31824f91e8
41. Ikuno Y, Maruko I, Yasuno Y, et al. Reproducibility of retinal and choroidal thickness measurements in enhanced depth imaging and high-penetration optical coherence tomography. Invest Ophthalmol Vis Sci. 2011;52:5536–5540. doi:10.1167/iovs.10-6811
42. Lee JG, Rosen RB. Newest applications of enhanced-depth imaging and swept-source optical coherence tomography facilitation detailed imaging of the choroid. Retin Physician. 2017;14:41–43.
43. Cheung CM, Lai TY, Teo K, et al. Polypoidal choroidal vasculopathy: consensus nomenclature and non–indocyanine green angiograph diagnostic criteria from the Asia-pacific ocular imaging society PCV workgroup. Ophthalmol. 2021;128:443–452. doi:10.1016/j.ophtha.2020.08.006
44. Teo KY, Sadda SR, Cheung CM, et al. Non-ICGA treatment criteria for suboptimal anti-VEGF response for polypoidal choroidal vasculopathy: APOIS PCV Workgroup report 2. Ophthalmol Retina. 2021;5:945–953. doi:10.1016/j.oret.2021.04.002
45. De Salvo G, Vaz-Pereira S, Keane PA, et al. Sensitivity and specificity of spectral-domain optical coherence tomography in detecting idiopathic polypoidal choroidal vasculopathy. Am J Ophthalmol. 2014;158:1228–1238. doi:10.1016/j.ajo.2014.08.025
46. Chaikitmongkol V, Khunsongkiet P, Patikulsila D, et al. Color fundus photography, optical coherence tomography, and fluorescein angiography in diagnosing polypoidal choroidal vasculopathy. Am J Ophthalmol. 2018;192:77–83. doi:10.1016/j.ajo.2018.05.005
47. Cheung CMG, Yanagi Y, Mohla A, et al. Characterization and differentiation of polypoidal choroidal vasculopathy using swept source optical coherence tomography angiography. Retina. 2017;37:1464–1474. doi:10.1097/IAE.0000000000001391
48. Srour M, Querques G, Semoun O, et al. Optical coherence tomography angiography characteristics of polypoidal choroidal vasculopathy. Br J Ophthalmol. 2016;100:1489–1493. doi:10.1136/bjophthalmol-2015-307892
49. Tan CS. The Role of optical coherence tomography angiography in polypoidal choroidal vasculopathy. JAMA Ophthalmol. 2017;135:1316–1317. doi:10.1001/jamaophthalmol.2017.4453
50. Singh SR, Goyal P, Parameswarappa DC, et al. Angiographic features of polypoidal choroidal vasculopathy using indocyanine green angiography and optical coherence tomography angiography: a comparative study. Eur J Ophthalmol. 2019;22:1120672119850075.
51. Fukuyama H, Iwami H, Araki T, et al. Indocyanine green dye filling time for polypoidal lesions in polypoidal choroidal vasculopathy affects the visibility of the lesions on OCT angiography. Ophthalmol Retina. 2018;2:803–807. doi:10.1016/j.oret.2017.11.016
52. Zhan Z, Sun L, Jin C, et al. Comparison between non-visualized polyps and visualized polyps on optical coherence tomography angiography in polypoidal choroidal vasculopathy. Graefes Arch Clin Exp Ophthalmol. 2019;257:2349–2356. doi:10.1007/s00417-019-04445-5
53. Ting DS, Cheung GC, Lim LS, et al. Comparison of swept source optical coherence tomography and spectral domain optical coherence tomography in polypoidal choroidal vasculopathy. Clin Exp Ophthal. 2015;43:815–819. doi:10.1111/ceo.12580
54. Cheung CMG, Yanagi Y, Akiba M, et al. Improved detection and diagnosis of polypoidal choroidal vasculopathy using a combination of optical coherence tomography and optical coherence tomography angiography. Retina. 2019;39:1655–1663. doi:10.1097/IAE.0000000000002228
55. Koh A, Lai TYY, Takahashi K, et al. Efficacy and safety of ranibizumab with or without verteporfin photodynamic therapy for polypoidal choroidal vasculopathy: a randomized clinical trial. JAMA Ophthalmol. 2017;135:1206–1213. doi:10.1001/jamaophthalmol.2017.4030
56. Tan A, Jordan-Yu JM, Vyas CH, et al. Optical coherence tomography features of polypoidal lesion closure in polypoidal choroidal vasculopathy treated with aflibercept. Retina. 2022;42:114–122. doi:10.1097/IAE.0000000000003285
57. Azar G, Vasseur V, Lahoud C, et al. Polypoidal choroidal vasculopathy diagnosis and neovascular activity evaluation using optical coherence tomography angiography. BioMed Res Internat. 2021;2021. doi:10.1155/2021/1637377
58. Shen M, Zhou H, Kim K, et al. Choroidal changes in eyes with polypoidal choroidal vasculopathy after anti-VEGF therapy imaged with swept-source OCT angiography. J Invest Ophthalmol Vis Sci. 2021;62:5. doi:10.1167/iovs.62.15.5
59. Huang CH, Yeh P-T, Hsieh Y-T, Ho T-C, Yang C-M, Yang C-H. Characterizing branching vascular network morphology in polypoidal choroidal vasculopathy by optical coherence tomography angiography. Sci Rep. 2019;9:595. doi:10.1038/s41598-018-37384-y
60. Ma ST, Huang CH, Chang YC, et al. Clinical features and prognosis of polypoidal choroidal vasculopathy with different morphologies of branching vascular network on optical coherence tomography angiography. Sci Rep. 2021;11:17848. doi:10.1038/s41598-021-97340-1
61. Yang J, Zhang C, Wang E, et al. Utility of a public-available artificial intelligence in diagnosis of polypoidal choroidal vasculopathy. Graefes Arch Clin Exp Ophthalmol. 2020;258:17–21. doi:10.1007/s00417-019-04493-x
62. Ma D, Kumar M, Khetan V, et al. Clinical explainable differential diagnosis of polypoidal choroidal vasculopathy and age-related macular degeneration using deep learning. Comput Biol Med. 2022;143:105319. doi:10.1016/j.compbiomed.2022.105319
63. Kwok AK, Lai TY, Chan CW, et al. Polypoidal choroidal vasculopathy in Chinese patients. Br J Ophthalmol. 2002;86:892–897. doi:10.1136/bjo.86.8.892
64. Ho CPS, Lai TYY. Current management strategy of polypoidal choroidal vasculopathy. Ind J Ophthalmol. 2018;66:1727–1735. doi:10.4103/ijo.IJO_975_18
65. Ferrara N, Damico L, Shams N, et al. Development of ranibizumab, an anti-vascular endothelial growth factor antigen binding fragment, as therapy for neovascular age-related macular degeneration. Retina. 2006;26:859–870. doi:10.1097/01.iae.0000242842.14624.e7
66. Kokame GT, Yeung L, Lai JC. Continuous anti-VEGF treatment with ranibizumab for polypoidal choroidal vasculopathy: 6-month results. Br J Ophthalmol. 2010;94:297–301. doi:10.1136/bjo.2008.150029
67. Kokame GT. Prospective evaluation of subretinal vessel location in polypoidal choroidal vasculopathy (PCV) and response of hemorrhagic and exudative PCV to high-dose antiangiogenic therapy (an American Ophthalmological Society thesis). Trans Am Ophthalmol Soc. 2014;112:74–93.
68. Lim TH, Lai TY, Takahashi K, et al. Comparison of ranibizumab with or without verteporfin photodynamic therapy for polypoidal choroidal vasculopathy: the EVEREST II randomized clinical trial. JAMA Ophthalmol. 2020;138:935–942. doi:10.1001/jamaophthalmol.2020.2443
69. Gomi F, Oshima Y, Mori R, et al. Initial versus delayed photodynamic therapy in combination with ranibizumab for treatment of polypoidal choroidal vasculopathy: the Fujisan Study. Retina. 2015;35:1569–1576. doi:10.1097/IAE.0000000000000526
70. Cho HJ, Kim KM, Kim HS, et al. Intravitreal aflibercept and ranibizumab injections for polypoidal choroidal vasculopathy. Am J Ophthalmol. 2016;165:1–6. doi:10.1016/j.ajo.2016.02.019
71. Azuma K, Obata R, Nomura Y, et al. Angiographic findings of ranibizumab-resistant polypoidal choroidal vasculopathy after switching to a treat-and-extend regimen with intravitreal aflibercept. Retina. 2016;36:2158–2165. doi:10.1097/IAE.0000000000001047
72. Lee WK, Iida T, Ogura Y, et al. Efficacy and safety of intravitreal aflibercept for polypoidal choroidal vasculopathy in the PLANET study: a randomized clinical trial. JAMA Ophthalmol. 2018;136:786–793. doi:10.1001/jamaophthalmol.2018.1804
73. Wakabayashi T, Gomi F, Sawa M, et al. Intravitreal bevacizumab for exudative branching vascular networks in polypoidal choroidal vasculopathy. Br J Ophthalmol. 2012;96:394–399. doi:10.1136/bjo.2011.204123
74. Matsumoto H, Morimoto M, Mimura K, Ito A, Akiyama H. Treat-and-extend regimen with afibercept for neovascular age-related macular degeneration: efficacy and macular atrophy development. Ophthalmol Retina. 2018;2:462–468. doi:10.1016/j.oret.2017.09.002
75. Nguyen QD, Das A, Do DV, et al. Brolucizumab: evolution through preclinical and clinical studies and the implications for the management of neovascular age-related macular degeneration. Ophthalmol. 2020;127:963–976. doi:10.1016/j.ophtha.2019.12.031
76. Dugel PU, Koh A, Ogura Y, et al. Hawk and harrier: phase 3, multicenter, randomized, double-masked trials of Brolucizumab for neovascular age-related macular degeneration. Ophthalmol. 2020;127:72–84. doi:10.1016/j.ophtha.2019.04.017
77. Ogura Y, Jaffe GJ, Cheung CM, et al. Efficacy and safety of brolucizumab versus aflibercept in eyes with polypoidal choroidal vasculopathy in Japanese participants of HAWK. Br J Ophthalmol. 2021;2021:319090.
78. Morimoto M, Matsumoto H, Mimura K, et al. Two-year results of a treat-and-extend regimen with afibercept for polypoidal choroidal vasculopathy. Graefes Arch Clin Exp Ophthalmol. 2017;255:1891–1897. doi:10.1007/s00417-017-3718-6
79. Dugel PU, Singh RP, Koh A, et al. Hawk and harrier: ninety-six-week outcomes from the phase 3 trials of Brolucizumab for neovascular age-related macular degeneration. Ophthalmol. 2021;128:89–99. doi:10.1016/j.ophtha.2020.06.028
80. Ogura Y, Terasaki H, Gomi F, et al. Efficacy and safety of intravitreal aflibercept injection in wet age-related macular degeneration: outcomes in the Japanese subgroup of the VIEW 2 study. Br J Ophthalmol. 2015;99:92–97. doi:10.1136/bjophthalmol-2014-305076
81. Tsujikawa A, Ooto S, Yamashiro K, et al. Treatment of polypoidal choroidal vasculopathy by intravitreal injection of bevacizumab. Jpn J Ophthalmol. 2010;54:310–319. doi:10.1007/s10384-010-0813-1
82. Kikushima W, Sakurada Y, Sugiyama A, et al. Comparison of initial treatment between 3-monthly intravitreal afibercept monotherapy and combined photodynamic therapy with single intravitreal afibercept for polypoidal choroidal vasculopathy. Graefes Arch Clin Exp Ophthalmol. 2017;255:311–316. doi:10.1007/s00417-016-3467-y
83. FDA approves faricimab to treat wet AMD and DME. Available from: https://www.ajmc.com/view/fda-approves-fariximab-to-treat-wet-amd-and-dme.
84. Campochiaro PA, Marcus DM, Awh CC, et al. The port delivery system with ranibizumab for neovascular age-related macular degeneration: results from the randomized phase 2 ladder clinical trial. Ophthalmol. 2019;126:1141–1154. doi:10.1016/j.ophtha.2019.03.036
85. Gomi F, Sawa M, Wakabayashi T, et al. Efficacy of intravitreal bevacizumab combined with photodynamic therapy for polypoidal choroidal vasculopathy. Am J Ophthalmol. 2010;150:48–54. doi:10.1016/j.ajo.2010.02.008
86. Ruamviboonsuk P, Tadarati M, Vanichvaranont S, et al. Photodynamic therapy combined with ranibizumab for polypoidal choroidal vasculopathy: results of a 1-year preliminary study. Br J Ophthalmol. 2010;94:1045–1051. doi:10.1136/bjo.2009.173120
87. Saito M, Iida T, Kano M, et al. Two-year results of combined intravitreal ranibizumab and photodynamic therapy for polypoidal choroidal vasculopathy. Graefes Arch Clin Exp Ophthalmol. 2013;251:2099–2110. doi:10.1007/s00417-013-2323-6
88. Ngo WK, Chee WK, Tan CS, et al. Comparing efficacy of reduced-fluence and standard-fluence photodynamic therapy in the treatment of polypoidal choroidal vasculopathy. BMC Ophthalmol. 2020;20:1–7. doi:10.1186/s12886-020-01419-8
89. Miyamoto N, Mandai M, Oishi A, et al. Long-term results of photodynamic therapy or ranibizumab for polypoidal choroidal vasculopathy in LAPTOP study. Br J Ophthalmol. 2018;103:844–848. doi:10.1136/bjophthalmol-2018-312419
90. Rishi P, Rishi E, Sharma M, et al. Incidence, outcomes, and risk factors for hemorrhagic complications in eyes with polypoidal choroidal vasculopathy following photodynamic therapy in Indian subjects. Ind J Ophthalmol. 2017;65:712–718. doi:10.4103/ijo.IJO_174_17
91. Eleftheriadou M, Gemenetzi M, Lukic M, et al. Three-year outcomes of aflibercept treatment for neovascular age-related macular degeneration: evidence from a clinical setting. Ophthalmol Ther. 2018;7:361–368. doi:10.1007/s40123-018-0139-5
92. Traine PG, Pfister IB, Zandi S, et al. Long-term outcome of intravitreal aflibercept treatment for neovascular age-related macular degeneration using a “treat-and-extend” regimen. Ophthalmol Retina. 2019;3:393–399. doi:10.1016/j.oret.2019.01.018
93. Lukic M, Eleftheriadou M, Hamilton RD, et al. Four-year outcomes of aflibercept treatment for neovascular age-related macular degeneration: results from real-life setting. Eur J Ophthalmol. 2020;31:1940–1944. doi:10.1177/1120672120938565
94. Ohji M, Takahashi K, Okada AA, et al. Efficacy and safety of intravitreal aflibercept treat-and-extend regimens in exudative age-related macular degeneration: 52- and 96-week findings from ALTAIR: a randomized controlled trial. Adv Ther. 2020;37:1173–1187. doi:10.1007/s12325-020-01236-x
95. Wong TY, Ogura Y, Lee WK, et al. Efficacy and safety of intravitreal aflibercept for polypoidal choroidal vasculopathy: two-year results of the aflibercept in polypoidal choroidal vasculopathy study. Am J Ophthalmol. 2019;204:80–89. doi:10.1016/j.ajo.2019.02.027
96. Hosokawa M, Morizane Y, Hirano M, et al. One-year outcomes of a treat and-extend regimen of intravitreal aflibercept for polypoidal choroidal vasculopathy. Jpn J Ophthalmol. 2017;61:150–158. doi:10.1007/s10384-016-0492-7
97. Jackson TL, Bunce C, Desai R, et al. Vitrectomy, subretinal Tissue plasminogen activator and Intravitreal Gas for submacular haemorrhage secondary to Exudative Age-Related macular degeneration (TIGER): study protocol for a phase 3, pan-European, two-group, non-commercial, active-control, observer-masked, superiority, randomised controlled surgical trial. Trials. 2022;23:1–23.
98. Chaikitmongkol V, Upaphong P, Patikulsila D, et al. Timing of complete polypoidal regression following intravitreous aflibercept treatments in polypoidal choroidal vasculopathy. Ophthalmol Retina. 2022;6:21–28. doi:10.1016/j.oret.2021.03.012
99. Morizane-Hosokawa M, Morizane Y, Kimura S, et al. Impact of polyp regression on 2-year outcomes of intravitreal aflibercept injections: a treat and-extend regimen for polypoidal choroidal vasculopathy. Acta Med Okayama. 2018;72:379–385. doi:10.18926/AMO/56175
100. Teo KYC, Jordan-Yu JM, Tan AC, et al. Efficacy of a novel personalised aflibercept monotherapy regimen based on polypoidal lesion closure in participants with polypoidal choroidal vasculopathy. Br J Ophthalmol. 2021;10:987–993.
101. Wataru K, Sugiyama A, Yoneyama S, et al. Five-year outcomes of photodynamic therapy combined with intravitreal injection of ranibizumab or aflibercept for polypoidal choroidal vasculopathy. PLoS One. 2020;15:e0229231. doi:10.1371/journal.pone.0229231
102. Kim JH, Chang YS, Kim JW, et al. Submacular hemorrhage and grape-like polyp clusters: factors associated with reactivation of the lesion in polypoidal choroidal vasculopathy. Eye. 2017;31:1678–1684. doi:10.1038/eye.2017.126
103. Cho JH, Ryoo NK, Cho KH, et al. Incidence rate of massive submacular hemorrhage and its risk factors in polypoidal choroidal vasculopathy. Am J Ophthalmol. 2016;169:79–88. doi:10.1016/j.ajo.2016.06.014
104. Narayanan R, Mithal K, Jalali S, et al. Vitreous haemorrhage in massive hemorrhagic polypoidal choroidal vasculopathy: clinical characteristics and surgical outcomes. Int J Ret Vit. 2015;1:1–6.
105. Kitagawa Y, Shimada H, Mori R, et al. One-year outcome of intravitreal tissue plasminogen activator, ranibizumab, and gas injections for submacular hemorrhage in polypoidal choroidal vasculopathy. J Clin Med. 2022;11:2175. doi:10.3390/jcm11082175
106. Klettner A, Grotelüschen S, Treumer F, et al. Compatibility of recombinant tissue plasminogen activator (rtPA) and aflibercept or ranibizumab coapplied for neovascular age-related macular degeneration with submacular haemorrhage. Br J Ophthalmol. 2015;99:864–869. doi:10.1136/bjophthalmol-2014-306454
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.