Back to Journals » OncoTargets and Therapy » Volume 10
Polypeptide N-acetylgalactosaminyltransferase-6 expression in gastric cancer
Authors Guo Y, Shi JJ, Zhang J, Li HX, Liu B, Guo H
Received 1 April 2017
Accepted for publication 14 June 2017
Published 7 July 2017 Volume 2017:10 Pages 3337—3344
DOI https://doi.org/10.2147/OTT.S138590
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Carlos E Vigil
Yan Guo,1–3 Jingjing Shi,1–3 Jun Zhang,4 Haixin Li,1–3 Ben Liu,2,3,5 Hua Guo2,3,6
1Department of Cancer Biobank, Tianjin Medical University Cancer Institute and Hospital, National Clinical Research Center for Cancer, 2Key Laboratory of Cancer Prevention and Therapy of Tianjin, 3Tianjin’s Clinical Research Center for Cancer, 4Department of Thoracic Surgery, The Second Hospital of Tianjin Medical University, 5Department of Epidemiology and Biostatistics, 6Department of Tumor Cell Biology, Tianjin Medical University Cancer Institute and Hospital, National Clinical Research Center for Cancer, Tianjin, People’s Republic of China
Abstract: Gastric cancer (GC) is one of the leading causes of cancer-related deaths, with limited improvement in its clinical outcome worldwide. Aberrant mucin-type O-glycosylation is a critical event widespread in the development of GC. Polypeptide N-acetylgalactosaminyltransferases (GALNTs) regulate the initial step and determine the sites of mucin-type O-glycoprotein biosynthesis. GALNT6 has considerable potential as a biomarker in various cancers. The roles of GALNT6 in GC were analyzed, and the results showed that GALNT6 expression markedly increased in GC tissues compared with those in adjacent gastric tissues. High intratumoral GALNT6 density was associated with the clinicopathological parameters of TNM stage and distant metastasis. GALNT6 was identified as an independent prognosticator for the poor prognosis of GC patients. Moreover, the high expression level of GALNT6 was significantly associated with the low expression levels of E-cadherin and β-catenin and the high expression levels of MMP9. These findings indicated that GALNT6 could provide new insights into the characterization of GC as well as contribute to the development of an efficient prognostic indicator and novel therapeutic modalities for GC.
Keywords: gastric cancer, O-glycosylation, GALNT6, prognosis
Introduction
Gastric cancer (GC) is characterized by high recurrence rates and a dismal prognosis, given the lack of early detection and effective treatment methods for this cancer type.1 The development of novel biological markers and molecular-targeting drugs that could predict GC and improve clinical management is essential. Carcinoembryonic antigen (CEA), cancer antigen 19-9 (CA19-9), and cancer antigen 72-4 (CA 72-4) are the most commonly used GC biomarkers.2–4 In this study, we identify and characterize N-acetylgalactosaminyltransferase-6 (GALNT6), which is infrequently reported in GC.
Mucin-type O-glycosylation is the most common and complex posttranslational modification of human proteins. This process involves several physiological events, such as the folding, stabilization, and targeting of various glycoproteins. Aberrant mucin-type O-glycosylation exists in a variety of epithelial cancers and has various effects on cell adhesion, invasion, and metastasis.5–9 Mucin-type O-glycosylation is initiated by a large family of polypeptide GALNTs.10 GALNTs transfer N-acetylgalactosamine (GalNAc) to serine or threonine residues during protein biosynthesis and determine O-glycosylation sites on proteins.11,12 Given their involvement in several features of tumor cell biology and behavior, the abnormal expression of GALNTs is a possible molecular mechanism that promotes malignant transformation and tumor progression.13–15 Accumulating evidence suggests that several GALNT family members are involved in various tumors of the breast, pancreas, and colon and in neuroblastomas.16–19 GALNT6 expression has been mainly reported in breast carcinomas.16,20–24 Prior studies have revealed that GALNT6 expression is significantly higher in breast cancer tissues than in normal breast tissues. Furthermore, GALNT6 is exclusively upregulated in breast cancer cells but is not expressed in normal human vital organs. Therefore, GALNT6 may be an ideal therapeutic target for inhibitors in therapeutic modalities against various cancers, with minimal risks of adverse effects.16 GALNT6 expression in GC is rarely studied. Moreover, oncology studies on GALNT6 are confined to cytological studies. This study is a retrospective cohort study with a large number of samples and was designed to evaluate the relationship between GALNT6 protein expression and the clinicopathological characteristics of GC. Meanwhile, the molecular mechanism of GALNT6 was tentatively explored to understand its critical role in gastric carcinoma. The results of the present study could provide insight into the characterization of GC for the development of diagnostic and therapeutic strategies.
Materials and methods
Clinical specimens
Paraffin-embedded samples and corresponding clinical information were obtained from a retrospective cohort of patients (n=275) who were randomly selected and had a confirmed diagnosis of GC between 2001 and 2009 at the Tianjin Medical University Cancer Institute and Hospital. A total of 15 frozen GC and matched adjacent tissues were evaluated using quantitative real-time polymerase chain reaction (qPCR) and Western blot analysis. Written informed consent was provided by the patients whose specimens were used. This research was approved by the ethics committees of Tianjin Medical University.
qPCR and Western blot analysis
Total RNA was extracted from frozen tissue specimens using Trizol reagent in accordance with the manufacturer’s protocol. Complementary DNA was synthesized by using a reverse transcription kit (TaKaRa Biotechnology Ltd, Dalian, People’s Republic of China). The PCR primer sequences for GALNT6 were 5′-CCAGACATCGTCACCATCG-3′ and 5′-AGGTCAGGCTCCAGTCAAAG-3′. The relative expression levels of GALNT6 were analyzed using the 2−ΔΔCt method. GAPDH was applied as the calibration control in all specimens. Total proteins were extracted by using lysis buffer containing a protease inhibitor cocktail. Then, equivalent protein samples were separated by 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred onto polyvinylidene fluoride (PVDF) membranes. GAPDH was used as a loading control.
Tissue microarray (TMA) construction
All candidate specimens were formalin-fixed paraffin-embedded (FFPE) tissue blocks and sections. FFPE sections were subjected to hematoxylin and eosin staining. TMAs were constructed using donor blocks taken from patients with GC. Representative tumor regions on slides were identified and marked by pathologists. The core selection of these donor blocks was guided by their corresponding marked slides. Tissue cores with a diameter of 2 mm were punched from the marked donor blocks. Then, recipient paraffin blocks were prepared. Finally, Quick-Ray was used to construct 15 TMA blocks representing 275 GC cases and their adjacent tissues.
Immunohistochemistry (IHC) analysis
TMA IHC staining was performed by using the EnVision two-step method in accordance with the manufacturer’s instructions. The tissue sections were immunostained with GALNT6, E-cadherin, β-catenin, and MMP9 antibodies. A semiquantitative approach was used to score the immunostaining frequency of each sample as follows: 0 for negative sample or <5% stained tumor tissue, 1 for samples stained between 6% and 25%, 2 for samples stained between 26% and 50%, 3 for samples stained between 56% and 75%, and 4 for samples stained >75%. Staining intensity was scored as 1 (weak), 2 (moderate), and 3 (strong). The total immunostaining score of each sample was the product of both parameters as mentioned earlier.
Statistical analysis
All data were analyzed by SPSS software (version 17.0; SPSS Inc., Chicago, IL, USA). The chi-square test or Fisher’s exact test was used for categorical variables. In addition, the analysis of continuous data was performed using the paired t-test or Wilcoxon matched-pairs test. Overall survival was defined as the time from diagnosis to the date of death or last follow-up. Cumulative survival time was calculated using the Kaplan–Meier method and performed with the use of log-rank test.
Results
Expression status of GALNT6 in GC
We utilized qPCR to assess GALNT6 mRNA levels in cancerous tissues from 15 matched patients with GC. qPCR results revealed that GALNT6 mRNA levels in GC tissues were at least twofold higher than those in corresponding adjacent gastric tissues (Figure 1A). The expression of GALNT6 mRNA in GC samples was significantly higher than those in other groups (P<0.001). We also performed Western blotting using the same samples (Figure 1B). Western blot results generally conformed to qPCR results and showed that GALNT6 protein expression was dramatically higher in GC tissues compared with those in adjacent tissues (P<0.001).
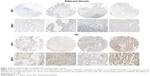
IHC analysis of GALNT6 expression in patients with GC
IHC staining of GALNT6 was evaluated in tissues from randomly chosen patients. As shown in Figure 2, GALNT6 expression was negative or very weakly detectable in the glandular epithelium of adjacent gastric mucosa. Only 64 of 275 samples (23%) showed GALNT6 expression, whereas the positive rate of GALNT6 expression in GC was observed in 218 of 275 samples (Table 1). In these samples, GALNT6 expression was predominantly localized in the cytoplasm of tumor cells. The immunostaining pattern of GALNT6 was either diffused or perinuclear in GC cells. As illustrated in Table 1, GALNT6 expression in GC cases was significantly stronger than that in adjacent tissues, and staining intensity usually varied. Therefore, GALNT6 might be a candidate regulator of GC initiation and progression.
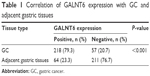  | Table 1 Correlation of GALNT6 expression with GC and adjacent gastric tissues |
Association of GALNT6 expression with the clinicopathological features of patients with GC
To identify associations between GALNT6 expression and clinicopathological characteristics, the valuable variables of GC are listed in Table 2. GALNT6 expression was divided into two groups of low and high expression levels based on the criteria for immunostaining. The relationships between the clinicopathological parameters of GC patients and GALNT6 expression levels are also summarized in Table 2. Statistical analysis was performed to determine the correlations between clinicopathological parameters and GALNT6 expression. GALNT6 expression showed a significant correlation with clinical stage and distant metastasis (P<0.05). GALNT6 tended to be highly expressed with more advanced clinical stages. In addition, GC metastasis was more frequent among patients in the strong GALNT6 expression group (42/73), compared with those in other groups (31/73). Our results showed that GALNT6 expression in GC was not correlated with the age, gender, tumor size, and histologic grade of patients. Thus, the expression level of GALNT6 in patients has a crucial impact on clinical outcome.
  | Table 2 Relationship between GALNT6 expression and clinical characteristics |
Influence of GALNT6 expression level on the survival of patients with GC
Kaplan–Meier survival analysis was employed to evaluate the correlation of GALNT6 expression with patient prognosis. The survival curves demonstrated that patients with high expression levels of GALNT6 had significantly shorter overall survival time (Figure 3). Therefore, GC patients with high positive staining for GALNT6 had a poorer prognosis than those with a low positive rate. Furthermore, the high expression level of GALNT6 was associated with poorer prognosis and shorter disease survival in GC patients. GALNT6 expression may be a useful marker to predict the overall survival of patients with GC.
Relationship between GALNT6 expression and MMP9, E-cadherin, and β-catenin protein
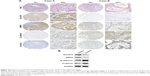
To understand the role of GALNT6 in GC, we assessed the correlation of GALNT6 expression with several molecules detected by IHC (Table 3). Our data showed that the staining intensity of GALNT6 was associated with increased MMP9 expression. The expression levels of GALNT6 and MMP9 were statistically and significantly associated. In addition, the relative expression of GALNT6 was negatively correlated with the expression of E-cadherin and β-catenin. Cases A and B were representative cases for the illustration of the correlation among GALNT6 and related protein molecules based on relative expression (Figure 4A). Meanwhile, the representative case A and case B were subjected to Western blotting (Figure 4B), which revealed correlations between cases A and B.
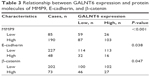  | Table 3 Relationship between GALNT6 expression and protein molecules of MMP9, E-cadherin, and β-catenin |
Discussion
Molecular biomarkers and targeted therapies have been widely developed and implemented for various tumors. CEA, CA 72-4, and CA19-9 are widely used as biomarkers for GC. The low sensitivity and high false-positive rate of these biomarkers, however, have greatly limited their clinical value. Combining these biomarkers could improve their sensitivity and detection rate.4,25 In this study, we identified a promising molecule that could be used as an efficient prognostic indicator for the development of novel GC treatment strategies. Previous studies have shown that GALNT6 is a potential biomarker in breast cancer, as confirmed by cytological experiments.16,20,21 Park et al16 considered that the knockdown of GALNT6 suppresses the growth of breast cancer cells. Subsequently, the knockdown of GALNT6 significantly decreases cell viability in pancreatic cancer cells. These results indicated that GALNT6 may be essential for the survival of cancer cells.26 In this study, we evaluated several hundreds of tissue samples from GC patients to investigate the role of GALNT6 in gastric carcinoma. qPCR, Western blot, and IHC results revealed that GALNT6 was upregulated in GC. In addition, the expression levels of GALNT6 and several clinicopathological parameters were significantly associated. As shown in Table 2, patients with GC and with high expression levels of GALNT6 exhibited advanced TNM stages and a higher possibility of distant metastases. Furthermore, intratumoral GALNT6 expression could influence the overall survival of patients with GC. Kaplan–Meier analysis showed that GC patients with high expression levels of GALNT6 had a poor survival rate. GALNT6 is a member of the GALNT family that regulates the initiation of O-linked protein glycosylation. The considerable increase in GALNT6 expression in GC leads to the production of abnormal O-glycans, which influence cell biological activities and contribute to the invasion and metastases of GC.27,28 We previously demonstrated that GALNT6 expression is associated with certain clinicopathological characteristics of patients with GC. In summary, the results presented in the previous study provided clinicopathological evidence that verifies the hypothesis that GALNT6 is associated with tumor invasion and metastases in GC.14,16,20,26
GALNT6 expression is a potential independent predictor for the prognosis of patients with GC. Thus, GC patients who present high expression levels of GALNT6 should receive particular attention from oncologists for more aggressive treatment. An increasing number of studies on the important roles of GALNT6 have been reported. However, the exact mechanism of action of GALNT6 in tumor progression remains unclear. A recent study showed that the knockdown of GALNT6 by small interfering RNA slows down breast cancer cell growth by increasing cell adhesion.14 More interestingly, another study demonstrated that the knockdown of GALNT6 accelerates the cell adhesion complex, including E-cadherin and β-catenin, thus attenuating the invasiveness of pancreatic cancer cells.26 To confirm previous results and further characterize the biological significance of GALNT6, we examined and analyzed the status of E-cadherin and β-catenin in the regulation of cell morphology and carcinogenesis. Results showed that E-cadherin expression was inversely associated with the expression level of GALNT6. In addition, another cell membrane molecule, β-catenin, which mediates cell adhesion along with E-cadherin, presented a consistent relationship with GALNT6 similar to that with E-cadherin. Moreover, the upregulated expression of GALNT6 enhanced the transformational potentials of epithelial cells and epithelial–mesenchymal transition (EMT)-like cellular alterations through O-glycosylation.29 Prior studies presented similar conclusions that the deregulation of GALNT6 and O-glycosylation affects cellular adhesion and invasion in different carcinomas.30,31 GALNT6 regulates the biochemical properties of cell surface proteins to maintain cellular polarity and adhesion. Our findings further revealed that the high expression of GALNT6 was accompanied by the strong staining of MM9. Furthermore, we selected representative cases to illustrate the relationship of GALNT6, E-cadherin, β-catenin, and MMP9 in GC tissues. These results are also consistent with those of a study on GALNT13 in cancer metastasis, where the high expression of the GALNT13 gene generated trimeric Tn antigen on Syndecan 1; this expression pattern could increase cell adhesion to fibronectin under the reduced expression of monosialotetrahexosyl ganglioside. This phenomenon enhances invasion and metastasis via the formation of a molecular complex that consists of integrinα5β1, Syndecan 1, and MMP9 in glycolipid-enriched microdomain/rafts.32 Thus, the abnormal expression of GALNT6 in GC may induce changes in the adhesive and invasive functions of cells. The present cohort study shows that GALNT6 is an independent and efficient negative biomarker of clinical management in GC. Moreover, our results contribute to the development of a target molecular inhibitor that could facilitate early diagnosis and treatment.
Conclusion
We demonstrated that GALNT6 expression was markedly increased in GC. High intratumoral GALNT6 density was associated with several clinicopathological parameters. Furthermore, it can be used as an independent prognosticator for poor prognosis of GC patients. These findings indicate that GALNT6 could provide new insights into GC characterization and contribute to the development of efficient prognostic indicator and novel therapeutic modalities.
Acknowledgment
This study was supported by the National Key Scientific Instrument and Equipment Development Project (2013YQ16055106), National Natural Science Foundation of China (81572445), Research Project of Tianjin Medical University Cancer Institute and Hospital (1202), and Natural Science Foundation of Tianjin (16JCYBJC24700).
Disclosure
The authors report no conflicts of interest in this work.
References
Van Cutsem E, Sagaert X, Topal B, Haustermans K, Prenen H. Gastric cancer. Lancet. 2016;388(10060):2654–2664. | ||
Tocchi A, Costa G, Lepre L, et al. The role of serum and gastric juice levels of carcinoembryonic antigen, CA19.9 and CA72.4 in patients with gastric cancer. J Cancer Res Clin Oncol. 1998;124(8):450–455. | ||
Lee JC, Lee SY, Kim CY, Yang DH. Clinical utility of tumor marker cutoff ratio and a combination scoring system of preoperative carcinoembryonic antigen, carbohydrate antigen 19-9, carbohydrate antigen 72-4 levels in gastric cancer. J Korean Surg Soc. 2013;85(6):283–289. | ||
Zheng TH, Zhao JL, Guleng B. Advances in molecular biomarkers for gastric cancer. Crit Rev Eukaryot Gene Expr. 2015;25(4):299–305. | ||
Springer GF. T and Tn, general carcinoma autoantigens. Science. 1984;224(4654):1198–1206. | ||
Chia J, Goh G, Bard F. Short O-GalNAc glycans: regulation and role in tumor development and clinical perspectives. Biochim Biophys Acta. 2016;1860(8):1623–1639. | ||
Stowell SR, Ju T, Cummings RD. Protein glycosylation in cancer. Annu Rev Pathol. 2015;10:473–510. | ||
Miles DW, Happerfield LC, Smith P, et al. Expression of sialyl-Tn predicts the effect of adjuvant chemotherapy in node-positive breast cancer. Br J Cancer. 1994;70(6):1272–1275. | ||
Pinho SS, Reis CA. Glycosylation in cancer: mechanisms and clinical implications. Nat Rev Cancer. 2015;15(9):540–555. | ||
Schjoldager KT, Clausen H. Site-specific protein O-glycosylation modulates proprotein processing – deciphering specific functions of the large polypeptide GalNAc-transferase gene family. Biochim Biophys Acta. 2012;1820(12):2079–2094. | ||
Kitada S, Yamada S, Kuma A, et al. Polypeptide N-acetylgalactosaminyl transferase 3 independently predicts high-grade tumours and poor prognosis in patients with renal cell carcinomas. Br J Cancer. 2013;109(2):472–481. | ||
He H, Shen Z, Zhang H, et al. Clinical significance of polypeptide N-acetylgalactosaminyl transferase-5 (GalNAc-T5) expression in patients with gastric cancer. Br J Cancer. 2014;110(8):2021–2029. | ||
Nomoto M, Izumi H, Ise T, et al. Structural basis for the regulation of UDP-N-acetyl-alpha-D-galactosamine: polypeptide N-acetylgalactosaminyl transferase-3 gene expression in adenocarcinoma cells. Cancer Res. 1999;59(24):6214–6222. | ||
Liesche F, Kolbl AC, Ilmer M, Hutter S, Jeschke U, Andergassen U. Role of N-acetylgalactosaminyltransferase 6 in early tumorigenesis and formation of metastasis. Mol Med Rep. 2016;13(5):4309–4314. | ||
Pinho SS, Carvalho S, Marcos-Pinto R, et al. Gastric cancer: adding glycosylation to the equation. Trends Mol Med. 2013;19(11):664–676. | ||
Park JH, Nishidate T, Kijima K, et al. Critical roles of mucin 1 glycosylation by transactivated polypeptide N-acetylgalactosaminyltransferase 6 in mammary carcinogenesis. Cancer Res. 2010;70(7):2759–2769. | ||
Taniuchi K, Cerny RL, Tanouchi A, et al. Overexpression of GalNAc-transferase GalNAc-T3 promotes pancreatic cancer cell growth. Oncogene. 2011;30(49):4843–4854. | ||
Guda K, Moinova H, He J, et al. Inactivating germ-line and somatic mutations in polypeptide N-acetylgalactosaminyltransferase 12 in human colon cancers. Proc Natl Acad Sci U S A. 2009;106(31):12921–12925. | ||
Berois N, Gattolliat CH, Barrios E, et al. GALNT9 gene expression is a prognostic marker in neuroblastoma patients. Clin Chem. 2013;59(1):225–233. | ||
Berois N, Mazal D, Ubillos L, et al. UDP-N-acetyl-D-galactosamine: polypeptide N-acetylgalactosaminyltransferase-6 as a new immunohistochemical breast cancer marker. J Histochem Cytochem. 2006;54(3):317–328. | ||
Freire T, Berois N, Sonora C, Varangot M, Barrios E, Osinaga E. UDP-N-acetyl-D-galactosamine: polypeptide N-acetylgalactosaminyltransferase 6 (ppGalNAc-T6) mRNA as a potential new marker for detection of bone marrow-disseminated breast cancer cells. Int J Cancer. 2006;119(6):1383–1388. | ||
Patani N, Jiang W, Mokbel K. Prognostic utility of glycosyltransferase expression in breast cancer. Cancer Genomics Proteomics. 2008;5(6):333–340. | ||
Milde-Langosch K, Schutze D, Oliveira-Ferrer L, et al. Relevance of betaGal-betaGalNAc-containing glycans and the enzymes involved in their synthesis for invasion and survival in breast cancer patients. Breast Cancer Res Treat. 2015;151(3):515–528. | ||
Kolbl AC, Hiller RA, Ilmer M, et al. Glycosyltransferases as marker genes for the quantitative polymerase chain reaction-based detection of circulating tumour cells from blood samples of patients with breast cancer undergoing adjuvant therapy. Mol Med Rep. 2015;12(2):2933–2938. | ||
Shimada H, Noie T, Ohashi M, Oba K, Takahashi Y. Clinical significance of serum tumor markers for gastric cancer: a systematic review of literature by the Task Force of the Japanese Gastric Cancer Association. Gastric Cancer. 2014;17(1):26–33. | ||
Tarhan YE, Kato T, Jang M, et al. Morphological changes, cadherin switching, and growth suppression in pancreatic cancer by GALNT6 knockdown. Neoplasia. 2016;18(5):265–272. | ||
Wagner KW, Punnoose EA, Januario T, et al. Death-receptor O-glycosylation controls tumor-cell sensitivity to the proapoptotic ligand Apo2L/TRAIL. Nat Med. 2007;13(9):1070–1077. | ||
Ichikawa S, Sorenson AH, Austin AM, et al. Ablation of the Galnt3 gene leads to low-circulating intact fibroblast growth factor 23 (Fgf23) concentrations and hyperphosphatemia despite increased Fgf23 expression. Endocrinology. 2009;150(6):2543–2550. | ||
Park JH, Katagiri T, Chung S, Kijima K, Nakamura Y. Polypeptide N-acetylgalactosaminyltransferase 6 disrupts mammary acinar morphogenesis through O-glycosylation of fibronectin. Neoplasia. 2011;13(4):320–326. | ||
Alisson-Silva F, Freire-de-Lima L, Donadio JL, et al. Increase of O-glycosylated oncofetal fibronectin in high glucose-induced epithelial-mesenchymal transition of cultured human epithelial cells. PLoS One. 2013;8(4):e60471. | ||
Freire-de-Lima L, Gelfenbeyn K, Ding Y, et al. Involvement of O-glycosylation defining oncofetal fibronectin in epithelial-mesenchymal transition process. Proc Natl Acad Sci U S A. 2011;108(43):17690–17695. | ||
Matsumoto Y, Zhang Q, Akita K, et al. Trimeric Tn antigen on syndecan 1 produced by ppGalNAc-T13 enhances cancer metastasis via a complex formation with integrin alpha5beta1 and matrix metalloproteinase 9. J Biol Chem. 2013;288(33):24264–24276. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.