Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 17
Polynucleotides HPT for Asian Skin Regeneration and Rejuvenation
Authors Lim TS , Liew S, Tee XJ, Chong I , Lo FJ, Ho MJ, Ong K, Cavallini M
Received 31 August 2023
Accepted for publication 30 January 2024
Published 13 February 2024 Volume 2024:17 Pages 417—431
DOI https://doi.org/10.2147/CCID.S437942
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Rungsima Wanitphakdeedecha
Video abstract presented by Lim.
Views: 105
Ting Song Lim,1 ShaoRong Liew,1 Xiang Jie Tee,1 Ian Chong,1 Fui Jun Lo,1 Meng Jun Ho,1 KuokTjun Ong,1 Maurizio Cavallini2
1Clique Clinic, Petaling Jaya, Selangor, Malaysia; 2Dermatology and Dermatosurgery Department, CDI Centro Diagnostico Italiano, Milan, Italy
Correspondence: Ting Song Lim, Clique Clinic, 4, Jalan 19/36, Seksyen 19, Petaling Jaya, Selangor, 46300, Malaysia, Email [email protected]
Introduction: Even lightly compromised skin may impact self-esteem and social behaviour. After intradermal infiltration, natural-origin Polynucleotides High Purification Technology (PN HPT) promote new collagen and extracellular matrix production, translating into a physiological correction of the ageing skin. The study aimed to explore the benefits of intradermal PN HPT on the four perceptual skin quality categories “Skin Tone Evenness”, “Skin Surface Evenness”, “Skin Firmness”, and “Skin Glow” in a representative sample of 30 Asian subjects (mean age 40.2± 11.4 years old).
Methods: Study protocol: three intradermal injections of a PN HPT-based Class III CE-marked medical device at T0 (baseline assessment and first treatment session), T1 (four weeks after baseline), and T2 (eight weeks after baseline), with efficacy and safety evaluations at T1, T2, T3 (four months after baseline) and T4 (six months after baseline). Quantitative and qualitative assessments: 3D skin analysis system QuantifiCare and Global Aesthetic Improvement Scale (GAIS, Investigator and Patient subscales).
Results: PN HPT treatment led to a meaningful and statistically significant improvement of the skin surface, firmness, pigmentation, and radiance, with no early- or late-onset adverse events and benefits persisting up to the sixth-month visit in all subjects. At T4, 33% and 43% of treated subjects felt “Much Improved” and “Very Much Improved” (optimal result); 56% and 44% of treated subjects felt “Satisfied” or “Very Satisfied”. At T4, the mean Investigator GAIS scores were 3.33 out of 5.0 for the “Skin Tone Evenness” skin quality perceptual category, 3.46 for the “Skin Surface Evenness” category, 3.61 for “Skin Firmness”, and 3.45 per for the radiance determinant of the “Skin Glow” category.
Conclusion: Intradermal treatment with the PN HPT-based medical device led to a meaningful improvement of the skin surface, firmness, pigmentation, and radiance with complete safety. The aesthetic benefits persisted up to the sixth-month visit in all subjects.
Keywords: emergent perceptual skin quality categories, EPCs, GAIS, global aesthetic improvement scale, polynucleotides HPT, PN HPT, QuantifiCare, skin quality
Introduction
Facial beauty and attractiveness are far-reaching cross-cultural concepts and contribute to dictating how individuals are socially judged and treated, with a young and beautiful appearance positively influencing social behaviour and acceptance.1–3
However, the ageing of organs begins with birth, and the skin is no exception. As the most voluminous organ of the body, the skin inevitably shows signs of ageing as life progresses.
Both intrinsic and extrinsic factors induce cutaneous ageing. Intrinsic ageing is an inevitable age-related process that leads to thin, dry skin, fine wrinkles, and gradual dermal atrophy. Conversely, external environmental factors like pollution, smoking, poor nutrition, and sun exposure ignite extrinsic ageing, expressed by coarse wrinkles, loss of skin elasticity with skin laxity, and rough-textured appearance. Whatever the mechanism, small changes in the skin surface, firmness and radiance, and pigmentation patterns may impact facial attractiveness and self-esteem across all ethnicities and genders. Conceptually, the skin quality compromised by ageing can be described across all ethnicities, age groups, and genders by four perceptual categories (EPCs, Text Box 1).4
 |
Box 1 EPCs Descriptors4 |
The Polynucleotides High Purification Technology
Aesthetic procedures aim to remove or reduce the signs of ageing and increase the attractiveness of an otherwise healthy face. Among the treatments available to correct the signs of ageing and damaged skin, substitutes of soft tissue molecules injected intradermally to fill wrinkles and depressions are safe and effective alternatives to plastic surgery.5 Moreover, they are better tolerated than non-resorbable implants.5 Even more, extensive pieces of evidence support intradermal infiltrations as one of the most effective strategies to improve skin health, as testified by a new line of products and original formulations based on Polynucleotides High Purification Technology (PN HPT) and developed by Mastelli (Sanremo, Italy) for physiological skin bio-revitalisation.6
Natural-origin polynucleotides, ubiquitous in the body, uniquely establish a hydrated, protrophic, and protective microenvironment that promotes cell development and vitality for reparative purposes. When infiltrated intradermally, highly purified PN HPT ignite a physiological healing process by supporting fibroblast activity and vitality. Repair of tissue damage, new extracellular matrix production, and self-limited collagen deposition with no tendency to fibrosis are the outcomes.7
Many studies demonstrate that intradermal PN HPT immediately counteracts the signs of ageing and damaged skin through its filling and moisturising effects; at the same time, it helps to restore the dermis physiology – firmness, hydration, elasticity, and brightness – in the face, neck, and décolleté areas.8,9 After intradermal injection, the biocompatible PN HPT physiologically release water molecules and smaller-sized oligonucleotides, prolonging the PN HPT moisturising and viscoelastic properties over time, allowing a more natural and in-depth tissue regeneration and leading to a healthier skin look.8,9
Intradermal PN HPT injections are increasingly popular in aesthetic medicine because they are a rapid outpatient procedure, usually lasting no more than 20–30 minutes and suited to all levels of ageing skin. Moreover, the treatment allows for returning to daily activities without interruptions.6
The primary difference between Caucasian and Asian skin is in the melanocyte function: the differences in sun exposure habits and natural defence mechanisms against the effects of chronic sunlight exposure might explain the different clinical manifestations of photoaging in Caucasian and Asian skin.6 Although showing similar basal trans-epidermal water loss (TEWL) levels to Caucasian skin and similar ceramide levels upon mechanical challenge, in Asians, the skin barrier function appears weaker than in Caucasians with reduced subcutaneous natural moisturising factor levels.7
The objective of the current clinical study was to explore the benefits of PN HPT intradermal treatment for skin rejuvenation in Asian subjects. Trained assistants to the investigator pool photographed the skin surface of thirty patients before and after three and six months of intradermal PN HPT treatment with a tridimensional (3D) stereophotogrammetry system. Acting blindly and independently, the specialists of the investigator pool then scored the 3D images with the validated Investigator GAIS subscale.
Materials and Methods
Study Design
Five independent physicians, co-ordinated by Dr Ting Song Lim, medical director at Clique Clinic, Petaling Jaya, Selangor, Malaysia, carried out this investigator-initiated study, designed as an open-label, non-comparative cohort investigation. Ageing and dry skin, big pores, skin laxity, and acne scars were the main indications for treatment. Other treated conditions included rough texture, dyschromia, refractory melasma, and enlarged pores.
The interventional protocol included three PN HPT injections at T0 (baseline assessment and first treatment visit), T1 (four weeks after baseline), and T2 (eight weeks after baseline) with efficacy evaluations at T1, T2, T3 (four months after baseline) and T4 (six months after baseline) and safety assessment throughout the study. Brand of the Class-III, CE-marked PN HPT-based device for intradermal injection used in the study: PLINEST (20 mg/mL PN HPT gel in 2-mL prefilled sterile syringes; Mastelli S.r.l., Sanremo, Italy).
Objective Efficacy Assessment — QuantifiCare
QuantifiCare (www.quantificare.com/clinical-trials-photography) identifies a proprietary technique used in more than 300 clinical trials for 3D skin analysis and simulation, allowing a unique visualisation of facial shapes from any angle. Tridimensional representations augmented with anatomical reference points are the only way to obtain standardised pictures over repeated visits to compare and quantify the skin changes arising from treatment vs baseline.
QuantifiCare measurements were performed on the 30-patient study cohort at T0 before treatment, T1, T2, T3, and T4. The five investigators independently assessed the QuantifiCare photographs at each assessment visit, scoring them with the Global Aesthetic Improvement Scale (GAIS, investigator’s subscale). The mean Investigator GAIS value at each assessment time was the average of the five independent GAIS evaluations by the five investigators. At the same time, the treated subjects, independently from each other and the evaluators, expressed their subjective judgement with the GAIS Patient subscale.
Visit Schedule
- T0 – Baseline session, clinical evaluation before treatment, and first injection.
- T1 – Second injection session four weeks after T0.
- T2 – Third injection session eight weeks after T0.
- T3 – First follow-up session four months after T0.
- T4 – Second follow-up session six months after T0.
Primary Endpoint
Using the Investigator GAIS and Patient GAIS evaluation tools, assessment of the device efficacy on the four emergent perceptual skin quality categories “Skin Tone Evenness”, “Skin Surface Evenness”, “Skin Firmness”, and “Skin Glow” (see Text Box 1, Introduction section).
Global Aesthetic Improvement Scale
The Global Aesthetic Improvement Scale (GAIS) is a validated five-point scale widely used to rate the global aesthetic improvement in facial appearance compared to baseline, as judged by the investigator and the patient.10 It was developed in 2003 to evaluate injectable biomaterials for correcting nasolabial folds and then extensively used with other cosmetic treatments or surgeries for facial rejuvenation, skin tightening, and scar treatment. The rating categories are “worse”, “no change”, “improved”, “much improved”, and “very much improved” (Table 1).
 |
Table 1 Descriptors of the Global Aesthetic Improvement Scale |
Five-point scoring scales like GAIS have the statistical advantage of distributing according to unimodal and relatively symmetric frequency distributions in contrast to the highly skewed J- and U-shaped distributions originating from seemingly more discriminating ten-point scales.11 Moreover, the five-point scale data have significantly lower item means, floor, and ceiling effects. At the same time, regression analysis shows that this type of scale explains a significant fraction of variation in floor and ceiling effects.11
Safety Assessment
Based on spontaneous reporting of adverse events by cohort subjects and investigators, with evaluation for severity and persistence.
Inclusion and Exclusion Criteria
The study enrolled Asian subjects at least 18 years old, without gender preference.
As reported in the device use instructions, known hypersensitivity or previous allergic reactions from fish-derived products and ingredients of the study device excluded participation. Exclusion criteria also included pregnancy, diabetes mellitus, history of connective tissue diseases, known susceptibility to keloid formation, hypertrophic scarring, or clinically significant skin pigmentation disorders; moreover, the investigators could exclude all subjects considered unsuitable for whatever reason. Previous treatments in the same areas with permanent, semi‐permanent, or resorbable fillers within one year also prevented inclusion in the study cohort. The same was true for any other aesthetic procedure such as laser, ultrasound, surgery, or chemical peels for the previous six months.
Statistics
Descriptive statistics: median values and 95% confidence intervals. Inferential statistics: comparison of the mean GAIS at T1 and T4 vs baseline with the paired-sample t-test after preliminary control for normal distribution (null hypothesis: zero mean difference between each paired set of observations).12 All statistical tests were two-sided with a 5% significance level. Moreover, the differences in the per cent distribution of patients in each rating category (Investigator and Patient GAIS) were assessed with the χ2 test for proportions. The quantitative and qualitative analysis of reported side effects helped to estimate the incidence of known and previously unknown side effects.12
Sample Size Calculation
Based on polynucleotide efficacy in published literature and the effect size “dz” set at a conservative 0.7 (70% mean improvement in GAIS scores), 30 subjects were the estimated sample size. Statistical program: GPower version 3.1; Table 2 shows the estimate’s parameters.13
 |
Table 2 Preliminary Sample Size and Power Estimate for the Two-Tailed Paired-Sample t-test |
Results
Baseline Demographics and Other Cohort Characteristics
Table 3 illustrates the demographic data of the 30-patient treatment cohort.
 |
Table 3 Study Cohort Demographics |
The PN HPT Performance on the Skin Quality Perceptual Categories
According to Investigators
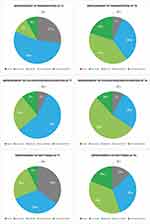
The following figures illustrate the Patient GAIS outcomes of intradermal PN HPT on the four emergent skin quality perceptual categories or EPCs at T1 and T4 vs baseline T0. Figure 1 shows the outcomes for the determinants of Skin Tone Evenness (Pigmentation and Skin Colour).
Figure 2 shows the outcomes for the determinants of Skin Surface Evenness (Pores, Wrinkles, Scars), while Figure 3 shows the determinants of Skin Firmness (Hydration and Elasticity) and Skin Glow (Radiance).
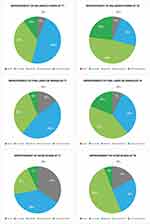 |
Figure 2 Subjective assessment by PN HPT-treated patients on some of the determinants (pore size, wrinkles and lines, acne scars) of the skin quality perceptual category “Skin Surface Evenness”. |
Figure 4 illustrates the overall short-time (T1) and longer-time (T4) judgements by treated subjects about the impact of the intradermal PN HPT-based device on their overall skin quality. At the end of the six-month follow-up period after baseline and the first treatment session, 43% of patients judged their appearance as “Very much improved”—for them, the optimal outcome. No patient experienced a worsening condition.
 |
Figure 4 Subjective short-term (T1) and longer-term (T4) assessments of the impact of the intradermal PN HPT-based treatment on skin quality by treated patients vs baseline (T0). |
Most subjects rated the intradermal infiltration as “comfortable” and “very comfortable” at T1 (26% and 42%, respectively) and T4 (42% and 32%, respectively). Comparatively, only a decreasing fraction of patients rated the method as “uncomfortable” or “very uncomfortable” (22% at T1 and 15% at T4). The cannula intradermal administration received more positive ratings than the needle injections with 30 or 32-gauge needle and microdroplet or linear retrograde technique as early as the first T1 visit—“comfortable” and “very comfortable”, 48% and 35%, respectively, with only 10% evaluations of “uncomfortable” and none of “very uncomfortable”. Nearly all subjects rated themselves as “satisfied” or “very satisfied” as early as the first visit (Figure 5).
 |
Figure 5 Subjective assessment by PN HPT-treated patients about satisfaction with treatment. |
According to Surveyed Subjects
Table 4 illustrates how the five investigators independently rated the four emergent perceptual skin quality categories, with a consistently and highly significant (p < 0.001 vs baseline) upward trend of the Investigator GAIS score from visit T1 to T4 for each of the four emerging perceptual skin quality categories. Figure 6 graphically summarises the progression from short-term assessment T1 to longer-term T4, with the mean Investigator GAIS score differences between T1 and T4 still highly significant (p < 0.01). In only one subject out of thirty, one investigator reported a slight worsening in Skin Surface Evenness and Skin Firmness at T4, possibly due to the persisting effects of a previous injection trauma or occasional intolerance to an unknown ingredient of the intradermal formulation.
 |
Table 4 Evolution of the Four Emergent Perceptual Skin Quality Categories at All Assessment Visits According to Investigators (Investigator GAIS Scoring, Mean ± Standard Deviation) |
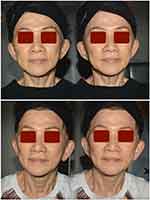
Figures 7 and 8 summarise how the investigators rated the evolution in the four emergent perceptual skin quality categories at the last (T4) follow-up assessment visit, six months after baseline and the first treatment session (per cent ratings out of 150 evaluations). “Improved” was the most frequent evaluation for the categories “Skin Tone Evenness” and “Skin Tone Firmness”; still, for 45% and 58% of the cohort subjects, the combined “Much Improved” and “Very Much Improved” categories qualified their “Skin Tone Evenness” and “Skin Tone Firmness”, respectively. Fifty per cent of subjects evaluated their “Skin Glow” as “Much Improved” or “Very Much Improved”. Figure 9 illustrates two representative examples of the skin quality benefits foreseeable after a PN HPT treatment in an anonymised middle-aged woman (photographs taken from two slightly different perspectives).
 |
Figure 7 Emergent perceptual skin quality categories Skin Tone Evenness and Skin Firmness, ratings at T4. |
 |
Figure 8 Emergent perceptual skin quality categories Skin Firmness and Skin Glow, ratings at T4. |
Discussion
The 30-subject blinded-evaluator cohort study in Asian subjects of all Fitzpatrick skin phototypes, predominantly in their late thirties and early forties, confirmed the benefits for the four perceptual categories defining skin quality suggested by literature for the PN HPT-based gel for intradermal injection.4 The Investigator GAIS subscale, administered at T1, T2, T3, and T4 by five independent clinicians and scored by blinded evaluators, and the Patient GAIS subscale guaranteed the validation of study outcomes.
Compared with baseline, almost all participants reported an enhancement in skin quality parameters during the sixth-month follow-up period, with noticeable and progressive GAIS improvements for each perceptual category, notably “Much Improved” and “Very Much Improved”, between visits T1 and T4. For only one subject, an investigator found a worsening in Skin Surface Evenness and Skin Firmness at the six-month follow-up, possibly due to previous injection trauma or occasional intolerance to some formulation ingredient. Most surveyed subjects rated the treatment comfort as “Comfortable” or “Very Comfortable” at the sixth-month follow-up for both the cannula and needle injections.
There were no side effects, and satisfaction with treatment was high as early as the first visit T1 and steadily elevated throughout the study period, with all subjects satisfied at the final T4 follow-up visit.
Regarding how PN HPT position themselves within the leading skin rejuvenation strategies, the authors believe PN HPT belong to a different class from fillers. Although PN HPT immediately enhance volumes (like hyaluronans, they are highly hydrophilic), over the longer term, they support and fine-tune fibroblast activity and improve the dermal environment by passively replenishing the dermal pools of critical precursors. Sharing similar dermal restructuring purposes, the energy-based laser and radiofrequency technologies and Platelet-Rich Plasma (PRP) might be the most congruous comparators to PN HPT.14–17 However, only PRP shares the intradermal administration technique with PN HPT. PRP is a cocktail of growth factors like platelet‑derived growth factor (PDGF), vascular endothelial growth factor (VEGF), transforming growth factor beta (TGFβ), and epithelial growth factor (EGF) that improve fibroblast proliferation and differentiation, contributing to skin rejuvenation and smoother and tighter skin.15–17 Unfortunately, exposure to pro- and anti-inflammatory cytokines like IL-4, IL-8, IL-13, and IL-17 may lead to untoward and unpredictable tissue effects. Moreover, good-quality randomised trials with consistent reporting of treatment parameters, adequate controls, and objective outcomes are lacking, and comparisons across studies are difficult because of different and unreliable classification systems of PRP preparations. The available PRP evidence documents the increased dermal elasticity, collagen production, and extracellular polymer synthesis. However, pain during injections requiring topical anaesthetic creams before, during, and after the procedure and even nerve block is rife; erythema, oedema, and bruising are also frequent.15–17 Comparatively, PN HPT intradermal injections are usually pain-free, and minor oedema and bruising are the only occasional side effects.6 Finally, the two strategies differ markedly regarding contraindications. Blood and liver disorders may be a serious problem for PRP and require preliminary baseline haemoglobin, platelet count, HIV, HbsAg, and HCV assessment, and drug interactions with NSAIDs, prednisolone more than 20 mg per day, and anticoagulants are a severe risk.17 There are no such problems with PN HPT.6
The study design has some strong points deserving mention, like an adequate six-month follow-up period allowing to discriminate between short-term and evanescent effects and longer-term in-depth skin rejuvenation, a technologically advanced system for tridimensional skin analysis and assessment (QuantifiCare) and simulation), and efficacy scoring with the validated GAIS subscales. Conversely, the non-randomised cohort design lacking a control group is a liability that must be acknowledged. The future powerfully designed confirmatory studies being planned will overcome this liability.
Conclusions
The treatment with a PN HPT-based intradermal device led to a meaningful and statistically significant improvement of the skin surface, firmness, pigmentation, and radiance with complete safety, including the lack of late-onset adverse events. Response persisted up to the sixth-month visit in all subjects, while objective and subjective GAIS improvement went hand in hand with consistently high patient satisfaction.
Data Sharing Statement
The datasets generated and analysed in the study are not publicly available. However, the authors currently archive all datasets with full details of participating subjects. After conversion in an anonymous form, all datasets are available from the Corresponding Author upon reasonable request.
Ethical Statement
The authors performed their activities in agreement with the Declaration of Helsinki and Good Clinical Practice principles. All authors actively contributed to the study; Dr Maurizio Cavallini did not contribute subjects to the study cohort but had a crucial role in devising the study design and materials. The participants had freely sought treatment to improve skin elasticity and quality and promote facial and periocular remodelling. After proper information by the investigators, they knew the benefits and risks they could reasonably expect from the proposed procedure and routine minimally invasive practice for remodelling moderate-to-severe facial and periocular wrinkles presented to them and agreed in writing.
The study protocol excluded any approach different from Polynucleotides HPT infiltrations, a long-established and extensively documented practice. All procedures were within the regulatorily accepted medical device’s indication stated in the Patient Information leaflet.
Regarding the requirement for an Ethical Committee (EC) to formally approve the study (a protocol developed in Italy and Malaysia), Article 62 to Article 82 of the European Union (EU) regulation MDR 2017/745 and Article 16 of the Legislative Decree 137/2022 allow waiving the EC requirement for studies not falling within the EU formal notion of “clinical investigation”, as is the case for studies narratively describing clinical evolutions or comparisons with one or more medical devices, general satisfaction inquiries or Health Technology Assessment studies.
Photo Consent Statement
All photographs (Figure 9) were taken by and belong to the principal author, who agrees to their submission. The photographed woman also agreed to publish her pictures in writing, provided she was unrecognisable.
Acknowledgments
The authors acknowledge the contribution of Mastelli S.r.l., Sanremo, Italy, holder of the Polynucleotides High Purification Technology and the intradermally injectable Polynucleotides HPT-based gel formulation tested in this study, for supporting the publication costs.
Funding
Support with the article processing charges required by the Clinical, Cosmetic and Investigational Dermatology journal will be the only funding the corporate sponsor will provide.
Disclosure
Dr Maurizio Cavallini received research grants as an R&D steering board member from Mastelli and Allergan; a tutor in continuous medical education activities. The authors state they have no other conflict of interest related to the study.
References
1. Samson N, Fink B, Matts P. Interaction of skin color distribution and skin surface topography cues in the perception of female facial age and health: perception of skin color and topography. J Cosmet Dermatol. 2011;10(1):78–84. doi:10.1111/j.1473-2165.2010.00538.x
2. Samson N, Fink B, Matts PJ. Visible skin condition and perception of human facial appearance. Int J Cosmet Sci. 2010;32(3):167–184. doi:10.1111/j.1468-2494.2009.00535.x
3. Fink B, Neave N. The biology of facial beauty. Int J Cosmet Sci. 2005;27(6):317–325. doi:10.1111/j.1467-2494.2005.00286.x
4. Goldie KM, Guillen Fabi S, Fabi SG, et al. Skin Quality – a holistic 360° view: consensus results. Clin Cosmet Invest Dermatol. 2021;Volume 14:643–654. doi:10.2147/CCID.S309374
5. Cassuto D, Bellia G, Schiraldi C. An overview of soft tissue fillers for cosmetic dermatology: from filling to regenerative medicine. Clin Cosmet Invest Dermatol. 2021;Volume 14:1857–1866. doi:10.2147/CCID.S276676
6. Cavallini M, Bartoletti E; on behalf of The Polynucleotides HPT Priming Board, Collegio Italiano delle Società Scientifiche di Medicina Estetica (Italian College of the Aesthetic Medicine Scientific Societies) — SIME, AGORÀ, SIES. Consensus report on the use of PN HPT (polynucleotides highly purified technology) in aesthetic medicine. J Cosmet Dermatol. 2021;20(3):922–928. doi:10.1111/jocd.13679
7. Colangelo MT, Govoni P, Belletti S, et al. Polynucleotide biogel enhances tissue repair, matrix deposition and organization. J Biol Regul Homeost Agents. 2021;35(1):355–362. doi:10.23812/20-320-L
8. Massirone A. Polynucleotides Highly Purified Technology and the face skin, a history of innovative skin priming. Aesthetic Med. 2021;7:35–40.
9. Cavallini M, Papagni M. Long chain polynucleotides gel and skin biorevitalization. Int J Plast Dermatol. 2007;3:27–32.
10. Narins RS, Brandt F, Leyden J, et al. A randomized, double-blind, multicenter comparison of the efficacy and tolerability of Restylane versus Zyplast for the correction of nasolabial folds. Dermatol Surg. 2003. doi:10.1046/j.1524-4725.2003.29150.x
11. Garratt AM, Helgeland J, Gulbrandsen P. Five-point scales outperform 10-point scales in a randomized comparison of item scaling for the Patient Experiences Questionnaire. J Clin Epidemiol. 2011;64(2):200–207. doi:10.1016/j.jclinepi.2010.02.016
12. Mishra P, Singh U, Pandey CM, et al. Application of Student’s t-test, analysis of variance, and covariance. Ann Card Anaesth. 2019;22(4):407. doi:10.4103/aca.ACA_94_19
13. Faul F, Erdfelder E, Buchner A, et al. Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behav Res Methods. 2009;41(4):1149–1160. doi:10.3758/BRM.41.4.1149
14. Shin J-W, Kwon S-H, Choi J-Y, et al. Molecular mechanisms of dermal aging and antiaging approaches. Int J Mol Sci. 2019. doi:10.3390/ijms20092126
15. Pensato R, La Padula S. The use of Platelet-Rich Plasma in aesthetic and regenerative medicine: a comprehensive review. Aesthetic Plast Surg. 2023. doi:10.1007/s00266-022-02781-2
16. Xiao H, Xu D, Mao R, et al. Platelet-Rich Plasma in facial rejuvenation: a systematic appraisal of the available clinical evidence. Clin Cosmet Invest Dermatol. 2021:1697–724. doi:10.2147/CCID.S340434
17. Nanda S, Chauhan K, Shetty V, et al. Platelet-Rich Plasma in aesthetics. Indian Dermatol Online J. 2021;12(7):41. doi:10.4103/idoj.idoj_290_21
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.