Back to Journals » International Journal of Nanomedicine » Volume 12
Polyethylenimine-based micro/nanoparticles as vaccine adjuvants
Authors Shen C , Li J, Zhang Y, Li Y , Shen G , Zhu J, Tao J
Received 25 March 2017
Accepted for publication 12 June 2017
Published 31 July 2017 Volume 2017:12 Pages 5443—5460
DOI https://doi.org/10.2147/IJN.S137980
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Lei Yang
Chen Shen,1 Jun Li,1 Yi Zhang,1 Yuce Li,2 Guanxin Shen,3 Jintao Zhu,2 Juan Tao1
1Department of Dermatology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China; 2School of Chemistry and Chemical Engineering, Huazhong University of Science and Technology, Wuhan, China; 3Department of Immunology, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
Abstract: Vaccines have shown great success in treating and preventing tumors and infections, while adjuvants are always demanded to ensure potent immune responses. Polyethylenimine (PEI), as one of the well-studied cationic polymers, has been used as a transfection reagent for decades. However, increasing evidence has shown that PEI-based particles are also capable of acting as adjuvants. In this paper, we briefly review the physicochemical properties and the broad applications of PEI in different fields, and elaborate on the intracellular processes of PEI-based vaccines. In addition, we sum up the proof of their in vivo and clinical applications. We also highlight some mechanisms proposed for the intrinsic immunoactivation function of PEI, followed by the challenges and future perspectives of the applications of PEI in the vaccines, as well as some strategies to elicit the desirable immune responses.
Keywords: cationic polymers, APCs, immunoactivation, danger signals, anti-infection, anticancer
Corrigendum for this paper has been published
Introduction
Due to their weak immunogenicity, conventional vaccines, especially the subunit vaccines, are always combined with adjuvants to ensure potent immune responses. Polyethylenimine (PEI), as a kind of cationic polymer, has been extensively applied as a nucleotide delivery reagent for decades. In recent years, the robust adjuvanticity of PEI has been continuously documented. Increasing evidence has shown that PEI-based particles are capable of improving the efficiency of conventional vaccines against infections and tumors. These efficiencies are characterized by direct indicators, such as enhanced maturation rates of antigen-presenting cells (APCs) and increased proliferation of effector cells, as well as amplified production of antigen-specific antibodies and various cytokines and chemokines.
In this review, we first introduce the physicochemical properties of PEI and its general applications in distinct areas. Then, we focus on the effects of neat PEI itself and PEI-based nanoparticles/microparticles (NPs/MPs) in antigen uptake and presentation, which is the foundation for understanding the interplay between PEI and APCs. We then extend the focus on the effects of PEI-relevant adjuvant potency from the cell level to preclinical studies or clinical trials. Subsequently, we are fascinated to figure out some possible underlying mechanisms of its intrinsic immunoactivation functions. Finally, we discuss the challenges and future perspectives of the applications of PEI in vaccines, and explore the promising approaches to optimize it’s immune responses while manipulating the toxicity properly.
Synthesis and broad applications of PEI
PEI is a kind of synthesized cationic polymer with topologies of linear or branched forms, and its molecular weight ranges from 1 kDa to 1,000 kDa.1 The most common and essential characteristic of PEI is its hydrophilic cationic polymeric structure. The strong positive-charged PEI condenses negative particles (such as the DNA, negative antigens) or plasma membranes in vivo. Moreover, the PEI backbone contains one nitrogen atom in every three atoms,2 forming amorphous net structures to work powerfully in lysosomes, as a “proton sponge”.3,4 These active amino groups, especially the primary and secondary amines, provide numerous possibilities for structural modifications, which enable them to target the agents and attenuate the potential toxicity.5
Synthesis and physiochemical properties of PEI
In general, branched PEI, which contains primary, secondary and tertiary amine groups, can be synthesized through the cationic ring-opening polymerization of aziridine (Figure 1A–C). Linear PEI (LPEI), however, includes secondary amines only, commonly derived from acidic hydrolysis of polyoxazoline (Figure 1D and E). Generally, the synthetic processes of gaining LPEI with narrow distribution of the molecular weight could be rather challenging.5 In PEI, amino groups with different sorts possess different properties. It is evidenced that primary and secondary amines have strong abilities to bind to nucleic acids, as well as targeting agents, drugs and other functional moieties. Yet, although with less binding capacity, tertiary amines efficiently buffer the pH decline in acidic conditions.6,7 In general, branched PEI is in the liquid state and water-soluble, whereas LPEI is solid at room temperature and less soluble in cold water, phenol, ethyl, ether, acetone and other solvants, and it turns more soluble in hot water, acidic aqueous solution and organic solutions (such as the methanol, ethanol or chloroform). Though PEIs of these two topologies are quite different, they both possess active amino groups, and the over-positive-charged nitrogens are always linked to the toxicity of PEI.5
  | Figure 1 (A) Molecular structure of branched PEI. (B) Polymerization of aziridine to branched PEI. (C) Mechanism of the cationic ring-opening polymerization of aziridine. (D) Molecular structure of linear PEI. (E) Synthesis of linear PEI from substituted 2-oxazolines. Reproduced from Jäger M, Schubert S, Ochrimenko S, Fischer D, Schubert US. Branched and linear poly(ethylene imine)-based conjugates: syn thetic modification, characterization, and application. Chem Soc Rev. 2012;41(13):4755–4767. With permission of The Royal Society of Chemistry.5 |
Broad applications of PEI
On the basis of the physicochemical properties, PEI is widely used in broad fields, including effluent treatments, carbon dioxide absorption, separation and purification of proteins, antibacterial operations and other procedures.8–12 In 1995, Boussif et al prepared a groundbreaking type of PEI/DNA NPs and successfully transferred DNA into nerve stem cells.2 Subsequently, PEI has drawn more attention in biomedical field, especially as drug carriers,13–15 biological labels16,17 and vaccine adjuvants (Table 1).18
Among all the delivered drugs, the most common ones are nucleic acids. PEI is the second acceptable nonviral nucleic acid transfer agent, besides poly-L-lysine (PLL).2 Nowadays, PEI transfection reagents are commercially available, which include ExGen500®, jetPEI® and PEIpro™.19 Besides nucleic acids, proteins or peptides are also included in the category.20,21 PEI also works as a biological label when conjugated with imaging agents (such as the Fe3O4 and fluorescent NPs), and reflects the specific cell or sub-cell information, such as migrations and other behaviors, under certain conditions.16,22,23 The adjuvant effect of PEI is an emerging area. In a series of recent studies, vaccines composed of PEI as the immunostimulants are quite competent in treating infections or tumors.24,25
Intracellular process of PEI/modified PEI as vaccine adjuvants
To better manipulate its adjuvanticity, strategies to construct the PEI-based vaccines are highly desirable. PEI could be incorporated into vaccine structures through different ways: directly binding with antigens, coating on antigen-loaded NPs/MPs, coating existing particles with antigens absorbed on the surface or co-encapsulated NPs/MPs with antigens and other constructed forms (Figure 2). During these processes, plenty of cytokines and ligands could also be added in the vaccines based on the desirable applications.
Cell uptake of PEI-based vaccines
The first step for a vaccine to take its effect is the endocytosis process by APCs. Similar to traditional vaccines, the factors influencing the cell uptake of PEI-based vaccines include size,33 shape,34 charge35 and surface chemistry of the particles.36,37
General factors influencing PEI-based polyplex uptake
With broad range of molecular weights, different topologies and plentiful modification possibilities, PEI-based polyplexes are rather versatile in size, shape and properties.5 Therefore, PEI-based vaccines demonstrate broad possibilities in applications.
Also, the strong positive charge in PEI is beneficial for cellular internalization. PEI can bind to the negative proteoglycan on cell membranes and mediate the uptake process through the electrostatic interaction.33,35 Generally, phagocytosis by immune cells of foreign particles is an actin-dependent process.34 The loosely organized PEI in neat state will disturb the actin remodeling when initiating the internalization. Yet, PEI compresses negative substances to condense small particles, or PEI-coating strategy will result in a smooth surface.25,38 These spherical particles are symmetrical and easily uptaken from any point of attachment.34
Size is one of the useful factors in the toolbox affecting the uptake of PEI-based particles, as well as other polymeric particulate carriers. Champion et al demonstrated that particle size played a role in phagocytosis only if the volume was larger than the cell size.34,39 It is relatively easy to achieve small-sized PEI-based polymers. For the PEI-coated large MPs, the uptake process is restricted; however, the electrostatic forces attach the huge particles to the cell membranes, and perform as a continuous depot of the antigens.40,41
Effect of surface properties of PEI-based polyplex on cellular uptake
In addition to the general parameters, significant attention has been paid to the chemical modifications of the surface chemistry.34 Some modifications would largely influence the cellular uptake of the PEI-based polyplexes.
In APCs, phagocytosis is frequently mediated by receptors (via mannose receptor-, complement receptor-, Fcγ receptor- and scavenger receptor-mediated pathways or other pathways).34 Targeted modifications can obviously improve the cellular uptake efficiency and the subsequent biological effects of the polyplexes.36,37,42 For MPs or less positively charged particles, the tethering of targeting moieties on the particles’ surface is even more important for endocytosis.43,44 Hu et al prepared mannose-modified PEI-cell-penetrating peptide (CPP)/DNA particles and found an improved DC2.4-consuming efficiency.45 It is worth noting that Toll-like receptors (TLRs) are not included in the phagocytosis-related receptors, but they initiate the phagosome-mediated APCs maturation and inflammatory secretion,46 which is discussed in this review. Cho et al reported that the maltosylated PEI-mediated vaccines for cervical cancers were more effective than neat human papillomavirus antigens. Presumably, receptor-mediated endocytosis of PEI-based particles by maltose subsequently enhanced the transfection efficiency.18 For PEI-coated NPs/MPs, similar patterns of receptor-mediated gene delivery have also been demonstrated. Mesoporous silica NPs coupled with PEI also demonstrated high transfection efficiency due to the more effective targeting into APCs.47
Besides the specific receptor-mediated targeting strategy, another nonspecific targeting method is the hydrophobic modification of PEI. Given the lipophilic nature of cell membranes, the lipid-like structure facilitates the interactions between the hydrophobic species and plasma membranes (eg, lysosomal membranes and nuclear membranes).7 It is demonstrated that 76% and 96% of acetylated branched PEI (BPEI) enhanced the cellular uptake ability by four-fold, corresponding to the hydrophobic interaction theory.48,49 When the lipid components were incorporated, though possessing a relatively decreased positive charge, the electrostatic interaction could still be maintained and aid in binding.48,49 Recently, Parhiz et al reported that hexanoated-PEI vector was also more effective than the corresponding non-hydrophobic PEI in enhancing transfection rates.19 Besides the transfection benefits, Wang et al proved that hydrophobic modification of alkyl chains to PEI vaccines optimized the cross-presentation of antigens and upregulated IL-2 secretion. Yet, correlation between the properties of the synthesized hydrophobic-modified PEI and the vaccine potency still needs further investigation.50
CPPs are another valuable species that can optimize the interactions between cell membranes and the PEI-based polyplex. CPPs, characterized by the polycationic amino acid residues (ie, arginine and lysine), have been known for their specialized ability to penetrate the cellular membranes. Until now, most studies demonstrated that CPPs take effect by electrostatic binding to negatively charged cell membranes and induction of vesicle rupture by osmotic change and/or membrane lysis.19 Truncated Tat peptide and penetratin are the most popular CPPs, and both are arginine-rich peptides.19 Recently, Morris and Sharma constructed arginine-modified oligo-(alkylaminosiloxanes)-grafted PEI and found that the modified PEI/pDNA exhibited 98% cell viability and 150% more gene transfection efficiency than neat BPEI in human nasopharyngeal epidermoid carcinoma.51 Inhibitor studies, by use of various cellular uptake inhibitors such as wortmannin and genistein, indicated the role of arginine moiety in promoting internalization of the polyplex, and the process possibly contained a combination of multiple pathways.51
More modification strategies will be developed to magnify the advantages of PEI-based vaccines in the initiation process. Yet, after certain modifications, the particle size, charge and other parameters of the polymers may vary correspondingly. Though each of these areas can be studied separately, the interplay among these parameters must be considered as a whole to identify the best performance in boosting the uptake.34,52
Antigen presentation of PEI-based vaccines
After being uptaken, antigens are processed and transported through different cell compartments and are finally presented within the peptide-binding groove of a major histocompatibility complex (MHC) molecule (MHC class I molecules or class II molecules). T cell receptors can only recognize antigens presented in this pattern. Mostly, MHC class I molecules present endogenous peptides (such as transformed or infected cell components) and elicit the cell-mediated immunity (CMI), while class II molecules acquire exogenous peptides (extracellular pathogens or vaccine peptides) and induce abundance of antibodies.53 PEI-based vaccines with antigen peptides mainly follow the exogenous/class II pathway and stimulate the humoral immunity.54 PEI-based DNA vaccines provide the opportunities to synthesize multiple antigens in APCs and stimulate effective cellular immunity and long-lasting memory immunity. However, most DNA is actually delivered to bystander cells (such as myocytes and fibroblasts). In this case, newly synthesized antigen peptides are presented on or secreted out of bystander cells. They are shuttled to APCs, acting as the exogenous antigens.55,56
The “proton sponge effect” of PEI makes cross-presentation possible
As mentioned above, MHC class I molecules present endogenous peptides synthesized in the cells, while class II molecules present the exogenous internalized antigens. Occasionally, MHC class I molecules also participate in presenting exogenous antigens, known as the cross-presentation, which is very meaningful in biological evolution. In human beings, since the virus invasion does not commonly occur in professional APCs and the CMI is not naturally generated,57 virus antigens released from the bystander cells turn into exogenous antigens for APCs. The cross-presentation mechanism in APCs largely increases the efficiency of the immune system to generate CMI and eliminate all the infected cells. Yet, in application of vaccines for treating cancers and infections, which needs the CMI instead of mere humoral immunity, the cross-presentation is also of great importance.57 In the processes, phagocytosed or endocytosed antigens escape from the vacuole and gain entry to the cytosol (known as the “lysosomal escape”). Then, they become qualified clients for ubiquitination and subsequent degradation by the proteasome, followed by the transporter associated with antigen processing (TAP)-mediated transfer into the endoplasmic reticulum, and presentation by MHC class I molecules.57–59
There are two representative mechanisms of the “lysosomal escape”, that are, the variation of osmotic pressure and membrane lytic activity.19 Some pH-sensitive biomaterials possess the membrane-destabilizing property; thus, they are good candidates.60,61 PEI has the unique “proton sponge effect”, which buffers under acidic conditions.2,62 When involved in acidic conditions, the ATP-mediated pH-dependent proton pumps open, followed by passive influx of chloride ions and water molecules resulting in hyperosmolar state instantaneously, causing the vesicles to burst.2,62 The “lysosomal escape” process of PEI-based polyplex was well recorded by Merdan et al (Figure 3).6,7 PEI/ribozyme and PLL/ribozyme migrations in the living cells were identified under confocal laser scanning microscope, and they were found to first gather in acidic vesicles, most probably lysosomes. Unlike the PLL-based complex, the vesicles containing the PEI-based complex met with a sudden burst while letting out the contents throughout the cytoplasm.6,7 To verify the function of the pH change in the “lysosomal escape” process of PEI, they prepared the PEI/ribozyme group with bafilomycin A, a selective inhibitor of endosomal/lysosomal acidification. It showed no lysosomal rupture, suggesting the major role of acidification in the procedure.6,7 The escaped antigens (peptides or DNA) in the PEI-based vaccines in the cytoplasm are then prepared to undergo a cross-presentation process. The process has recently been verified again by Song et al. They found that PEI-coated poly(lactide-co-glycolide) (PLGA) (OVA) NPs induced efficient cross-presentation of antigens on MHC class I molecules through the endosome escape and lysosomal processing.63
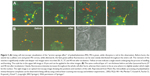  | Figure 3 (A) Living cell microscopic visualization of the “proton sponge effect” of polyethylenimine (PEI). PEI is green, while ribozyme is red in this observation. Before burst, the vesicle has a yellow core and green PEI corona, while afterwards, the faint green-yellow fluorescence can be seen evenly distributed throughout the entire cell. The remnant of the vesicles is significantly smaller and deeper red. Images were recorded 28, 31, 37, and 40 min after incubation. Yellow arrows indicate a single vesicle undergoing the process of swelling and bursting. The scale bar in the upper-left image is 10 μm and can be applied in the other images. (B) The same confocal layer of 1 nm thickness before and after lysosomal burst (37 and 40 min after incubation). Clearly, fluorescence intensity increases throughout the whole cell after burst, whereas there seems to be an area where it is slightly weaker which might be the nucleus. The right image is an optical microscopy image showing the positions and numbers of the cells. Reproduced from Pharm Res, Intracellular processing of poly(ethylene imine)/ribozyme complexes can be observed in living cells by using confocal laser scanning microscopy and inhibitor experiments., 2002;19(2):140–146, Merdan T, Kunath K, Fischer D, Kopecek J, Kissel T, (copyright 2002 Springer). With permission of Springer.6 |
Effect of surface properties of PEI-based polyplex on antigen presentation
There is a group of membrane-destabilizing peptides, which have shown the possibility of not only promoting cell uptake but also improving “lysosomal escape” and even nuclear translocation.64,65 Although PEI has the proton sponge effect to carry out the “lysosomal escape” itself, sometimes it is incompetent, and addition of membrane-destabilizing peptides (such as CPPs) in the vaccines can improve the cross-presentation effect. Ogris et al have explained the conditions in their study.52 The transfection efficiency was 10-fold (in B16F10 cells) to more than 100-fold (in Neuro2A cells, K562 cells) lower in small PEI/DNA particles compared with the large ones. Introduction of the lysosomotropic drug chloroquine or the pH-specific, membrane-active peptide (INF5) really promoted a substantial increase in antigen genes expression, which confirmed the hypothesis and importance of adding CPP moieties.52 Tan et al bounded two truncated peptides with penetrating properties to BPEI, resulting in higher transfection efficiency without causing cytotoxicity in CHO-K1, B16F10 and 293FT cell lines.66 These results can be ascribed to the enhanced endosomal disrupting activity of CPP-bound PEI carriers than to their parent carriers.
In PEI/DNA vaccines, PEI helps with the nucleic acids protection and endosomal escape. Yet, the transfection efficiency may still be limited in these vaccines.62 Particles of over 30 nm diameter or over 40 kDa molecular weight will require the active aid of the coupled nuclear localization signal (NLS) in nuclear translocation.60 NLS peptides are usually short conservative sequences, which bind to cytoplasmic importins and dock to the nuclear pore complex, thus aiding the nucleic acids transcription. The most commonly used NLS contains the PKKKRKV sequence.67 In addition, the well-known arginine-rich peptides, such as Tat and penetratin, also possess such nuclear transport activities.67 Besides, many other viral-origin peptides and even histone H1, protamine, ribonucleoprotein A1, high-motility-group proteins and others containing the above polycationic amino acids also act as effective NLS.60 Parhiz et al investigated the efficiency of PEI/DNA vaccines by attaching different arginine-rich sequences. It was found that the arginine-rich derivatives of PEI induced higher DNA and siRNA transfection efficiency than the groups without modifications. Moreover, PEI/DNA vaccines conjugated with arginine-rich peptides and hydrophobic derivatives demonstrated the highest DNA transfection efficiency, indicating that rational designs of two or more modifications are promising, to get a better transfection efficiency (antigen DNA expression) and lower cytotoxicity.68 All the efforts to elevate DNA transfection promise antigenic DNA translation and presentation. The advantages and modification strategies of PEI-based vaccines related to antigen uptake and presentation are illustrated in Figure 4.
In vivo and clinical applications of PEI and modifiers as vaccine adjuvants
To assess the performance of the PEI-based vaccines, we must consider the following three levels: in vitro, in vivo and clinical trials. Clearly, the clinical trials of large samples are the most convincing data for evaluating the efficiency of newly developed vaccines. Although we have got much more data from animal trials supporting the superior effects of PEI-based vaccines, only a few trials of PEI-based vaccines have been processed into clinical level. We summarize the application of PEI-based vaccines in animal experiments against infections and cancers, in Tables 2 and 3, respectively.
In vivo applications of vaccines
The in vivo applications of vaccines are confronted with bottlenecks sometimes, for two main reasons. One is the safety issue, as PEI is widely known for its toxicity. The other is the complicated microenvironment in vivo.61,69 In the microenvironment, every immune-active material has its corresponding targeting immune cells. These cells secrete various chemokines, attracting monocytes, granulocytes and other immune-promoting cells, while the attracted cells secrete more cytokines to form a positive feedback. As frequently depicted for many traditional adjuvants, the monocytes ultimately uptake the complexes, differentiate into dendritic cells (DCs) or phagocytes and then migrate to secondary lymphoid tissues.70,71 Intriguingly in PEI, the local inflammation seems beneficial to the recruitment and activation of APCs.72
Though the general process is somewhat clear, the detailed signal communications of one specific vaccine in in vivo applications are still puzzling. For example, it was found that intramuscularly injected MF59 and aluminum adjuvants targeted different cell types, while all the recruited cells had similar potential to engulf carrier–antigen complexes. All events occurred in draining lymph nodes (DLNs) at certain time intervals, probably due to a bystander effect.70,71 Further research indicated that, different from lipopolysaccharide (LPS), MF59 and aluminum took a TLR-independent mechanism to activate immunization and bias the monocytes differentiation to DCs rather than macrophages.70,71 Yet, as for the PEI-based vaccines, several issues, such as the local activation of cells and the roles of PEI-based vaccines in it, the secretion of cytokines and the uptake and differentiation processes, along with the lymph drainage situations, need further investigation.
Progress of in vivo applications of PEI-based vaccines
Different types of PEI, antigens, constructed strategies and immune routes are chosen in constructing the PEI-based vaccines in vivo. Frequently researched diseases include AIDS,24,73–76 Listeria monocytogenes infection,56,77 respiratory diseases,54,78–80 B cell lymphoma,38 herpes simplex virus attacks,81 viral B hepatitis,82 renal cell carcinoma,83 melanoma84 and others.85 The PEI-based vaccines work through almost all the known immune routes, and usually mimic the pathogenesis of the diseases themselves. For example, in respiratory infection, intranasal administration was prone to elicit mucosal secretory antibodies, as well as more serum antibodies.54 A similar trend was found for the HIV vaccines which elicited more antibodies in the vagina.74
The detected markers prove the efficacy of these vaccines usually through two main aspects: (1) intermediate indicators, such as antigen DNA expression, APCs maturation rates, humoral and cellular immunity responses in serum, lymphoid organs or tissues, the T helper type 1 (Th1)/T helper type 2 (Th2) response bias, the memory T and B cells production and secretion levels of the related cytokines;56,76,86 (2) comprehensive effects, such as higher animal survival rates, less weight loss and less tumor metastasis rates.38,54,77 The vaccines have demonstrated positive results in a series of in vivo experiments, while only a few clinical trials have been performed.40 The only satisfactory example was the DermaVir (mannosylated-PEI/plasmid Simian HIV DNA). Lisziewicz et al performed the ex vivo DC-based vaccination of DermaVir and found that DermaVir-transduced autologous monocyte-derived DCs induced HIV-specific T cells in rhesus macaques.87 Moreover, the same group improved the function of the ex vivo DC vaccination strategy with topical administration. Compared with the previous ex vivo DC-based vaccinations, the vaccination strategy proposed by Lisziewicz et al resulted in a similar number of transduced DCs in the lymph node and induced a similar quantity and quality of HIV-specific Th1-type T cell responses. The DermaVir Patch has been tested in Phase I/II clinical trials at present, and is the new promising agent in clinical applications.73
Intrinsic immune-activating function of PEI
Though many studies showed the performance of PEI-based vaccines in vitro and in vivo, the immune-activating properties of PEI still need to be revisited. It is not clear from these studies whether the improved immune efficiency was solely due to antigen protection (vaccine delivery vehicle) or due to the intrinsic adjuvant property (immunostimulator) of PEI.25,56,78,95 Moreover, in the case of PEI-coated polyplex, PEI together with the NPs/MPs should be regarded as a whole to take effects. When encapsulated inside, it was even more difficult to prove the adjuvanticity of PEI. Thus, the observed immunoactivation effects of PEI need further confirmation, and the revelation of the possible molecular pathways of the PEI-based vaccines would help us in understanding and developing new PEI-based vaccines.
Notably, some biomaterials have been regarded as well-known immunostimulants. Such immunologically active materials have been continuously discovered, and the underlying mechanisms were partially explained.35,96 Among them, the clinically approved adjuvants (aluminum and MF59) are good examples.97 Besides, 2,000-kDa poly(γ-glutamic acid) (γ-PGA) NPs were proved to activate DCs through TLR4 pathway, similar to γ-PGA-Phenol NPs.98–100 The activation by polyanhydride NPs and fullerene NPs was mediated by multiple TLRs on DCs.101–104 Inspiring in vitro and in vivo results have also suggested the immunoactivating functions of PEI, especially in DNA vaccines. With the understanding of the above-stated characteristics of PEI and PEI-based polyplex, it is necessary to provide an overview of the adjuvants’ properties and their underlying mechanisms.
Effect of immune activation of PEI
A well-established mode of an adjuvant taking effects is the direct activation of APCs, resulting in costimulatory molecules expression and cytokines secretion in vitro.63 Compared to bare Au nanorods (AuNRs), PEI-coated AuNRs induced a higher level of phenotypic maturation of DCs (Figure 5) and cytokines release. The immune-promoting effects almost matched LPS to some extent, while the subsequent activation of specific immunization and the interferon (IFN)-γ production were strictly Env-dependent.105 Similarly, when conjugating hepatitis B surface antigen (HBsAg), cationic chitosan and PEI-coated poly(lactic acid) (PLA) MPs also had a stronger capability than PLA MPs or bare antigens to promote macrophages internalization and maturation, followed by stronger humoral immune and Th1 responses in vivo.82 The anti-B cell lymphoma vaccines made of 70-kDa BPEI and 25-kDa LPEI-functionalized PLGA MPs also resulted in higher CD80 and MHC II expression in RAW264.7 cells in vitro than bare MPs.38 Moreover, the immunoactivating effects of PEI should also be assessed to understand if better in vivo effect can be achieved without adding PEI in polyplex compared to control groups.
  | Figure 5 Significant increase in the percentage of mature DCs (CD11c+MHCII+CD80+CD86+DCs) when DCs were treated with PEI-coated AuNRs polyplex. *P<0.05. Reprinted with permission from Xu L, Liu Y, Chen Z, et al. Surface-engineered gold nanorods: promising DNA vaccine adjuvant for HIV-1 treatment. Nano Lett. 2012;12(4):2003–2012. Copyright 2012 American Chemical Society.105 |
Immune-activating mechanisms of PEI
To reveal the immunoactivating property of PEI, gene expression profiles of in vivo immunization of mice with PEI were investigated.86 Compared with the control group, immuno-related genes of two injected groups were activated at considerably high levels regardless of the antigens (Figure 6). Based on the results and relations analyzed by PubGene, Regnström et al speculated that PEI has important immune-activating effects, presumably through the granulocyte colony-stimulating factor, and the next step will be the exploration of their interplay.86
  | Figure 6 Immune-related gene expressions in spleen cells of bare polyethylenimine (PEI)-treated mice as compared to reporter plasmid-conjugated PEI-immunized mice. The mRNA levels are shown as adjusted intensities for each gene. (A) Upregulated markers for the Th1 and Th2 responses. (B) Genes of the adaptive and innate immune responses. (C) Upregulated genes involved in immunogenicity and immunostimulation. Adapted by permission from Macmillan Publishers Ltd: Gene Ther. Regnström K, Ragnarsson EG, Köping-Höggård M, Torstensson E, Nyblom H, Artursson P. PEI – a potent, but not harmless, mucosal immuno-stimulator of mixed T-helper cell response and FasL-mediated cell death in mice. Gene Ther. 2003;10(18):1575–1583. copyright 2003.86 |
TLR pathways
With many immune-related genes being upregulated in the treatment of PEI, it is interesting to reveal the precise molecular pathways behind this upregulation. The TLRs and their downstream myeloid differentiation primary response gene 88 (MyD88) or TIR-domain-containing adapter-inducing interferon-β (TRIF) pathways are the most classical immunoactivation routes, which stimulate the nuclear transcription factors, nuclear factor-κB (NF-κB), to activate the expression of a series of cytokines and maturation markers (such as B7 molecules).57 Shokouhi et al studied a series of biomaterials and found that many of them modulated the maturation and cytokine secretion of DCs to different extents in vitro. The TLRs cascade effects may play an important role in sensing the existence of these materials, and the hydrophobic domains may be the “danger signals” which start the APCs activation, which are similar to “pattern antigens”.106 Huang et al and Chen et al found that cationic polymers (such as cationic dextrans and PEI) promoted the maturation of macrophages via TLR4, and secreted type-I cytokines (Figure 7). Surprisingly, further investigation revealed that PEI reversed the differentiation of tumor-associated macrophages and modulated the activity of natural killers (NKs) to kill the tumor cells.107,108 Similarly, cationic dextran and PEI have also been found to repolarize myeloid-derived suppressor cells (MDSCs) into the antitumor phenotype. Knock-out mice experiments further proved the requirement of TLR4 signaling.109 Other results found that in in vitro investigation, LPEI produced the TLR5-inducible cytokines in wild-type mice instead of TLR5-/- littermates. Therefore, LPEI was considered as a TLR5 agonist, and it is speculated that LPEI structurally resembled the flagellin.110 Ma et al found that the PEI/DNA vaccines were much more effective than naked DNA vaccines in antitumor trial. The co-cultivation of PEI with BMDCs in vitro revealed the activation and expression of important nuclear translocation factors (such as NF-κB p50 and p65), which were important nuclear transcription molecules of TLRs. Therefore, the enhanced type I-mediated cytotoxic T lymphocyte activation, type II-mediated Th1/Th2 response and interferon-γ-activated NKs might relate to the NF-κB-dependent inflammation and apoptosis induced by PEI.94
  | Figure 7 PEI-induced IL-12 secretion of macrophages was inhibited by TLR4 antibody. In contrast, the addition of isotype control antibody did not inhibit such IL-12 production significantly. Reprinted from Biomaterials, 2010;31(32), Chen H, Li P, Yin Y, et al, The promotion of type 1 T helper cell responses to cationic polymers in vivo via toll-like receptor-4 mediated IL-12 secretion. 8172–8180, Copyright (2010), with permission from Elsevier.108 |
NLR family pyrin domain-containing 3 (NLRP3) inflammasome pathways
Wegmann et al also supported the adjuvanticity of PEI, which promoted DCs trafficking to DLNs and led to cytokines secretion.25 They proved the immunoactivation mechanisms of PEI related to the interferon regulatory factor 3 (IRF3)-dependent signaling and NLRP3 inflammasome activation, especially the in vivo process.25 The NLRP3 inflammasome is among the nod-like receptors (NLRs), a kind of pattern recognition receptors found on APCs. The NLRP3 inflammasome was known to be activated by various stresses, including K+ efflux, reactive oxygen species (ROS) generation and lysosome rupture.111 Thus, the NLRP3 inflammasome activation by PEI-antigen NPs in the experiment is potentially through the lysosomal-destabilizing activity or other damage-associated molecular patterns (DAMPs) caused by the toxicity of PEI. Interestingly, NLRP3 inflammasome function only biased adaptive immunity toward a Th2 response, instead of affecting the overall PEI adjuvant activity.25
ROS
ROS generation is always related to tissue or cell damage, which is a kind of “danger signal”. The ROS and innate-mediated pro-inflammatory cytokines (such as the tumor necrosis factor-α and interleukin-1β) were signals in arming the adaptive immunity, sufficiently demonstrating the adjuvant effects of PEI.112 Mulens-Arias coated superparamagnetic iron oxide NPs (SPIONs) with PEI and used them to stimulate macrophages. They found that the TLR4 and ROS signaling were involved. Intriguingly, though PEI-coated SPIONs and LPS both induced macrophages activation and M1 phenotype genes upregulation, the gene expression profiles were different.113 It was also reported that the OVA-loaded PLGA MPs with a PEI-DS polyelectrolyte multilayer induced ROS in attachment with APCs. More positive charges on the outermost layer and higher molecular weight resulted in higher ROS production.114 Many nonpathogenic adjuvants, including PEI, are toxic to tissues, under which circumstances ROS is generated. Although ROS are of extensive interest in the exploration of PEI-based vaccine mechanisms, the specific role and relationship with other immunity-related molecules still merit further investigations.
Other factors in the “danger signals”
The “danger signals” elicited from PEI seem to be the main cause of its adjuvant effects. Regnström et al explored the gene expression profiles of PEI/DNA bronchial vaccines and found that bare PEI upregulated the expressions of genes involved in cell cycle regulation, oncogenesis and differentiation. These findings indicated the cytotoxicity and risks involved with PEI-based vaccines.86 The hypothesis went along with the previous explanations of aluminum adjuvants and that the immunoactivation potency may be caused by the physiological reaction toward the sensed danger signals.115 Meanwhile, the toxicity from adjuvants would affect the cell states to exhibit various activities, which will impair the outcome of overall effects of PEI. Recently, Palumbo et al constructed an in vitro co-culture system and demonstrated the feasibility to transfect the PEI/DNA vaccine via fibroblasts. The vaccination surprisingly resulted in the cross-presentation of antigens and DC maturation. By comparing the high- and less-toxic PEI/DNA compositions and corresponding DC maturation states, as well as MHC I-restricted OVA presentation, the results highlighted that polymer-induced cytotoxicity probably benefited the immune activation.116 Many endogenous antigens from the apoptotic or necrotic cells (such as the dsDNA, HMGB1, heat shock proteins and uric acids) have long been known to recruit and activate immune systems.116 In Wegmann et al’s report, they proved the strong dependence of double-stranded DNA (dsDNA)-mediated IRF3-triggered adjuvant effect of PEI. The free cellular dsDNA was released from the apoptotic or dead cells.25 It is a good representative of the DAMPs signal to investigate an immune response.
Summary of the immunoactivation mechanisms of PEI
Generally, PEI or PEI-based particles can stimulate the immune system, accompanied by the induction of various sorts of cell stress and immune response-related transcription factors.117 Yet, it is notoriously difficult to elucidate the mechanisms clearly. The evidence for adjuvant effect of PEI or PEI-based particles in different research is generalized and drawn out by our own understanding (Figure 8). Most current evidence supports the “danger signals” or “damage-associated molecular patterns” theory. Both TLRs and NLRs are receptors in APCs that recognize the danger signals. PEI-associated cytotoxicity may be one manifested pattern of such danger signals.24,25 Some nontoxic biomaterials also have profound effects on immune cells.106 In some experiments, PEI was also found to be nontoxic in vivo, but still possessed the adjuvanticity.25 Thus, other mechanisms may exist to stimulate immunity in addition to the direct toxicity of PEI. Some products reflecting the danger or damage signals have been tested continuously, such as the ROS and the uric acids, while the roles involved have not been clarified.97,116,118 Moreover, all the above-explained factors are actually not independent but work as a network that affects mutually. For example, the activation of NLRP3 inflammasome may be caused by the uric acids, which formed under necrosis or ROS.25,97 Besides, ROS can be induced by the primary generation, or the product from cell stress, while NF-κB is the key suppressor of ROS-induced apoptosis.117 Other intermediates (such as the dectin-1) intermediate bioactive cytokines and chemokines (such as the CCL family), and accessory immune cells (such as neutrophils and invariant NK T cells) are also involved in the network.119
Conclusion and future perspectives
Although adjuvants are capable of increasing the efficacy of clinical vaccines and have been extensively studied, the pace of their development is relatively slow. Till now, only five adjuvants have been licensed for use in clinical applications by the US Food and Drug Administration and European Medicines Agency, including aluminum (1926), MF59 (1997), virosome (2000), AS04 (2005) and AS03 (2009). Among them, even aluminum, the most widely used adjuvant in vaccination for almost one century with many advantages such as good safety profile, antigen stabilization and augmentation of high-titer antibody production, does not have the ability to elicit CMI. Furthermore, only a few adjuvants have been licensed for cancer immunotherapy, indicating a need for novel adjuvants that can induce robust antitumor CMI. Therefore, it is desirable to develop new immunostimulant adjuvants in vaccine formulations to induce robust immune responses including both humoral immunity and CMI.
Interestingly, PEI confers the ability to induce the “danger signals” of immune cells and the production of pro-inflammatory cytokines, which forms the basis of its adjuvanticity. Given limited research and publications related to the effect of PEI on immunity, an in-depth understanding of its immune mechanism of action, especially the relationship with the cytotoxicity, is of great significance. Moreover, the biodistribution and biodegradation of PEI-based vaccines in vivo, with different sizes and shapes, different administration routes and different modification strategies, still need further investigation.85,120,121 With breakthrough advances in multidisciplinary fields including chemistry, materials science, immunology and nanotechnology, it is believed that PEI-based vaccines, as well as other new adjuvants that improve the potency of the vaccine with precise modulations, will appear on the list of licensed adjuvants in the near future.
Acknowledgments
The authors gratefully acknowledge funding for this work provided by the National Natural Science Foundation of China (81573047 and 81502367) and Clinical Research Physician Program of Tongji Medical College, HUST (5001530014).
Disclosure
The authors report no conflicts of interest in this work.
References
Neu M, Fischer D, Kissel T. Recent advances in rational gene transfer vector design based on poly(ethylene imine) and its derivatives. J Gene Med. 2005;7(8):992–1009. | ||
Boussif O, Lezoualc’h F, Zanta MA, et al. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proc Natl Acad Sci U S A. 1995;92(16):7297–7301. | ||
Listner K, Bentley L, Okonkowski J, et al. Development of a highly productive and scalable plasmid DNA production platform. Biotechnol Prog. 2006;22(5):1335–1345. | ||
Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature. 2007;449(7161):419–426. | ||
Jäger M, Schubert S, Ochrimenko S, Fischer D, Schubert US. Branched and linear poly(ethylene imine)-based conjugates: synthetic modification, characterization, and application. Chem Soc Rev. 2012;41(13):4755–4767. | ||
Merdan T, Kunath K, Fischer D, Kopecek J, Kissel T. Intracellular processing of poly(ethylene imine)/ribozyme complexes can be observed in living cells by using confocal laser scanning microscopy and inhibitor experiments. Pharm Res. 2002;19(2):140–146. | ||
Incani V, Lavasanifar A, Uludağ H. Lipid and hydrophobic modification of cationic carriers on route to superior gene vectors. Soft Matter. 2010;6(10):2124–2138. | ||
Bolto BA. Soluble polymers in water purification. Prog Polym Sci. 1995;20:987–1041. | ||
Kumar P, Kim S, Ida J, Guliants VV. Polyethyleneimine-modified MCM-48 membranes: effect of water vapor and feed. Ind Eng Chem Res. 2008;47(1):201–208. | ||
Beyth N, Houri-Haddad Y, Baraness-Hadar L, Yudovin-Farber I, Domb AJ, Weiss EI. Surface antimicrobial activity and biocompatibility of incorporated polyethylenimine nanoparticles. Biomaterials. 2008;29(31):4157–4163. | ||
Vancha AR, Govindaraju S, Parsa KV, Jasti M, González-García M, Ballestero RP. Use of polyethyleneimine polymer in cell culture as attachment factor and lipofection enhancer. BMC Biotechnol. 2004;4:23. | ||
Sahiner N, Sagbas S, Sahiner M, Ayyala RS. Polyethyleneimine modified poly(hyaluronic acid) particles with controllable antimicrobial and anticancer effects. Carbohydr Polym. 2017;159:29–38. | ||
Lee SY, Huh MS, Lee S, et al. Stability and cellular uptake of polymerized siRNA (poly-siRNA)/polyethylenimine (PEI) complexes for efficient gene silencing. J Control Release. 2010;141(3):339–346. | ||
Urban-Klein B, Werth S, Abuharbeid S, Czubayko F, Aigner A. RNAi-mediated gene-targeting through systemic application of polyethylenimine (PEI)-complexed siRNA in vivo. Gene Ther. 2005;12(5):461–466. | ||
Moffatt S, Wiehle S, Cristiano RJ. Tumor-specific gene delivery mediated by a novel peptide-polyethylenimine-DNA polyplex targeting aminopeptidase N/CD13. Hum Gene Ther. 2005;16(1):57–67. | ||
Hayek A, Ercelen S, Zhang X, et al. Conjugation of a new two-photon fluorophore to poly(ethylenimine) for gene delivery imaging. Bioconjug Chem. 2007;18(3):844–851. | ||
Chertok B, David AE, Yang VC. Polyethyleneimine-modified iron oxide nanoparticles for brain tumor drug delivery using magnetic targeting and intra-carotid administration. Biomaterials. 2010;31(24):6317–6324. | ||
Cho HJ, Han SE, Im S, et al. Maltosylated polyethylenimine-based triple nanocomplexes of human papillomavirus 16L1 protein and DNA as a vaccine co-delivery system. Biomaterials. 2011;32(20):4621–4629. | ||
Parhiz H, Shier WT, Ramezani M. From rationally designed polymeric and peptidic systems to sophisticated gene delivery nano-vectors. Int J Pharm. 2013;457(1):237–259. | ||
Murata H, Futami J, Kitazoe M, et al. Intracellular delivery of glutathione S-transferase-fused proteins into mammalian cells by polyethylenimine-glutathione conjugates. J Biochem. 2008;144(4):447–455. | ||
Zhang S, Kucharski C, Doschak MR, Sebald W, Uludağ H. Polyethylenimine-PEG coated albumin nanoparticles for BMP-2 delivery. Biomaterials. 2010;31(5):952–963. | ||
Wang F, Chatterjee DK, Li Z, Zhang Y, Fan X, Wang M. Synthesis of polyethylenimine/NaYF4 nanoparticles with upconversion fluorescence. Nanotechnology. 2006;17(23):5786–5791. | ||
Feng M, Lee D, Li P. Intracellular uptake and release of poly(ethyleneimine)-co-poly(methyl methacrylate) nanoparticle/pDNA complexes for gene delivery. Int J Pharm. 2006;311(1–2):209–214. | ||
Sheppard NC, Brinckmann SA, Gartlan KH, et al. Polyethyleneimine is a potent systemic adjuvant for glycoprotein antigens. Int Immunol. 2014;26(10):531–538. | ||
Wegmann F, Gartlan KH, Harandi AM, et al. Polyethyleneimine is a potent mucosal adjuvant for viral glycoprotein antigens. Nat Biotechnol. 2012;30(9):883–888. | ||
Deng S, Ting YP. Polyethylenimine-modified fungal biomass as a high-capacity biosorbent for Cr(VI) anions: sorption capacity and uptake mechanisms. Environ Sci Technol. 2005;39(21):8490–8496. | ||
Li Y, Tian H, Xiao C, Ding J, Chen X. Efficient recovery of precious metal based on Au–S bond and electrostatic interaction. Green Chem. 2014;16(12):4875–4878. | ||
Xu X, Song C, Andresen JM, Miller BG, Scaroni AW. Novel polyethylenimine-modified mesoporous molecular sieve of MCM-41 type as high-capacity adsorbent for CO2 capture. Energy Fuels. 2002;16(6):1463–1469. | ||
Greene G, Radhakrishna H, Tannenbaum R. Protein binding properties of surface-modified porous polyethylene membranes. Biomaterials. 2005;26(30):5972–5982. | ||
Chen F, Wan D, Chang Z, Pu H, Jin M. Highly efficient separation, enrichment, and recovery of peptides by silica-supported polyethylenimine. Langmuir. 2014;30(41):12250–12257. | ||
Beyth N, Yudovin-Farber I, Bahir R, Domb AJ, Weiss EI. Antibacterial activity of dental composites containing quaternary ammonium polyethylenimine nanoparticles against Streptococcus mutans. Biomaterials. 2006;27(21):3995–4002. | ||
Zhang S, Doschak MR, Uludağ H. Pharmacokinetics and bone formation by BMP-2 entrapped in polyethylenimine-coated albumin nanoparticles. Biomaterials. 2009;30(28):5143–5155. | ||
Xiang SD, Scholzen A, Minigo G, et al. Pathogen recognition and development of particulate vaccines: does size matter? Methods. 2006;40(1):1–9. | ||
Champion JA, Katare YK, Mitragotri S. Particle shape: a new design parameter for micro- and nanoscale drug delivery carriers. J Control Release. 2007;121(1–2):3–9. | ||
Dobrovolskaia MA, McNeil SE. Immunological properties of engineered nanomaterials. Nat Nanotechnol. 2007;2(8):469–478. | ||
Pack DW, Hoffman AS, Pun S, Stayton PS. Design and development of polymers for gene delivery. Nat Rev Drug Discov. 2005;4(7):581–593. | ||
Nishikawa M, Huang L. Nonviral vectors in the new millennium: delivery barriers in gene transfer. Hum Gene Ther. 2001;12(8):861–870. | ||
Pai Kasturi S, Qin H, Thomson KS, et al. Prophylactic anti-tumor effects in a B cell lymphoma model with DNA vaccines delivered on polyethylenimine (PEI) functionalized PLGA microparticles. J Control Release. 2006;113(3):261–270. | ||
Champion JA, Mitragotri S. Role of target geometry in phagocytosis. Proc Natl Acad Sci U S A. 2006;103(13):4930–4934. | ||
Jilek S, Merkle HP, Walter E. DNA-loaded biodegradable microparticles as vaccine delivery systems and their interaction with dendritic cells. Adv Drug Deliver Rev. 2005;57(3):377–390. | ||
Pack DW. Timing is everything. Nat Mater. 2004;3(3):133–134. | ||
Read ML, Logan A, Seymour LW. Barriers to gene delivery using synthetic vectors. Adv Genet. 2005;53:19–46. | ||
Joubran S, Zigler M, Pessah N, et al. Optimization of liganded polyethylenimine polyethylene glycol vector for nucleic acid delivery. Bioconjug Chem. 2014;25(9):1644–1654. | ||
Li D, Ping Y, Xu F, et al. Construction of a star-shaped copolymer as a vector for FGF receptor-mediated gene delivery in vitro and in vivo. Biomacromolecules. 2010;11(9):2221–2229. | ||
Hu Y, Xu B, Ji Q, et al. A mannosylated cell-penetrating peptide-graft-polyethylenimine as a gene delivery vector. Biomaterials. 2014;35(13):4236–4246. | ||
Blander JM, Medzhitov R. On regulation of phagosome maturation and antigen presentation. Nat Immunol. 2006;7(10):1029–1035. | ||
Park IY, Kim IY, Yoo MK, Choi YJ, Cho MH, Cho CS. Mannosylated polyethylenimine coupled mesoporous silica nanoparticles for receptor-mediated gene delivery. Int J Pharm. 2008;359(1–2):280–287. | ||
Forrest ML, Meister GE, Koerber JT, Pack DW. Partial acetylation of polyethylenimine enhances in vitro gene delivery. Pharm Res. 2004;21(2):365–371. | ||
Gabrielson NP, Pack DW. Acetylation of polyethylenimine enhances gene delivery via weakened polymer/DNA interactions. Biomacromolecules. 2006;7(8):2427–2435. | ||
Wang H, Chen J, Ying J, Xu Y, Sheng R. Hydrophobic chain modified low molecular weight polyethylenimine for efficient antigen delivery. RSC Adv. 2016;6(17):13636–13643. | ||
Morris VB, Sharma CP. Enhanced in-vitro transfection and biocompatibility of L-arginine modified oligo (-alkylaminosiloxanes)-graft-polyethylenimine. Biomaterials. 2010;31(33):8759–8769. | ||
Ogris M, Steinlein P, Kursa M, Mechtler K, Kircheis R, Wagner E. The size of DNA/transferrin-PEI complexes is an important factor for gene expression in cultured cells. Gene Ther. 1998;5(10):1425–1433. | ||
Delves PJ, Martin SJ, Burton DR, Roitt IM, editors. Roitt’s Essential Immunology. 12th ed. Chichester: Wiley-Blackwell; 2011:79–80. | ||
Shim BS, Park SM, Quan JS, et al. Intranasal immunization with plasmid DNA encoding spike protein of SARS-coronavirus/polyethylenimine nanoparticles elicits antigen-specific humoral and cellular immune responses. BMC Immunol. 2010;11:65. | ||
Sharma AK, Khuller GK. DNA vaccines: future strategies and relevance to intracellular pathogens. Immunol Cell Biol. 2001;79(6):537–546. | ||
Grant EV, Thomas M, Fortune J, Klibanov AM, Letvin NL. Enhancement of plasmid DNA immunogenicity with linear polyethylenimine. Eur J Immunol. 2012;42(11):2937–2948. | ||
Delves PJ, Martin SJ, Burton DR, Roitt IM, editors. Roitt’s Essential Immunology. 12th ed. Chichester: Wiley-Blackwell; 2011:10–12. | ||
Delves PJ, Martin SJ, Burton DR, Roitt IM, editors. Roitt’s Essential Immunology. 12th ed. Chichester: Wiley-Blackwell; 2011:128–131. | ||
Joffre OP, Segura E, Savina A, Amigorena S. Cross-presentation by dendritic cells. Nat Rev Immunol. 2012;12(8):557–569. | ||
Vazquez E, Ferrer-Miralles N, Villaverde A. Peptide-assisted traffic engineering for nonviral gene therapy. Drug Discov Today. 2008;13(23–24):1067–1074. | ||
Gharwan H, Wightman L, Kircheis R, Wagner E, Zatloukal K. Nonviral gene transfer into fetal mouse livers (a comparison between the cationic polymer PEI and naked DNA). Gene Ther. 2003;10(9):810–817. | ||
Akinc A, Thomas M, Klibanov AM, Langer R. Exploring polyethylenimine-mediated DNA transfection and the proton sponge hypothesis. J Gene Med. 2005;7(5):657–663. | ||
Song C, Noh YW, Lim YT. Polymer nanoparticles for cross-presentation of exogenous antigens and enhanced cytotoxic T-lymphocyte immune response. Int J Nanomedicine. 2016;11:3753–3764. | ||
Bolhassani A, Ghasemi N, Servis C, Taghikhani M, Rafati S. The efficiency of a novel delivery system (PEI600-Tat) in development of potent DNA vaccine using HPV16 E7 as a model antigen. Drug Deliv. 2009;16(4):196–204. | ||
Zhang H, Gerson T, Varney ML, Singh RK, Vinogradov SV. Multifunctional peptide-PEG intercalating conjugates: programmatic of gene delivery to the blood-brain barrier. Pharm Res. 2010;27(12):2528–2543. | ||
Tan YX, Chen C, Wang YL, et al. Truncated peptides from melittin and its analog with high lytic activity at endosomal pH enhance branched polyethylenimine-mediated gene transfection. J Gene Med. 2012;14(4):241–250. | ||
Heitz F, Morris MC, Divita G. Twenty years of cell-penetrating peptides: from molecular mechanisms to therapeutics. Br J Pharmacol. 2009;157(2):195–206. | ||
Parhiz H, Hashemi M, Hatefi A, Shier WT, Amel Farzad S, Ramezani M. Arginine-rich hydrophobic polyethylenimine: potent agent with simple components for nucleic acid delivery. Int J Biol Macromol. 2013;60:18–27. | ||
Kircheis R, Wightman L, Wagner E. Design and gene delivery activity of modified polyethylenimines. Adv Drug Delivery Rev. 2001;53(3):341–358. | ||
Seubert A, Monaci E, Pizza M, O’Hagan DT, Wack A. The adjuvants aluminum hydroxide and MF59 induce monocyte and granulocyte chemoattractants and enhance monocyte differentiation toward dendritic cells. J Immunol. 2008;180(8):5402–5412. | ||
Calabro S, Tortoli M, Baudner BC, et al. Vaccine adjuvants alum and MF59 induce rapid recruitment of neutrophils and monocytes that participate in antigen transport to draining lymph nodes. Vaccine. 2011;29(9):1812–1823. | ||
Nguyen DN, Green JJ, Chan JM, Langer R, Anderson DG. Polymeric materials for gene delivery and DNA vaccination. Adv Mater. 2009;21(8):847–867. | ||
Lisziewicz J, Trocio J, Whitman L, et al. DermaVir: a novel topical vaccine for HIV/AIDS. J Invest Dermatol. 2005;124(1):160–169. | ||
Bivas-Benita M, Bar L, Gillard GO, et al. Efficient generation of mucosal and systemic antigen-specific CD8+ T-cell responses following pulmonary DNA immunization. J Virol. 2010;84(11):5764–5774. | ||
Zhou X, Liu B, Yu X, et al. Controlled release of PEI/DNA complexes from PLGA microspheres as a potent delivery system to enhance immune response to HIV vaccine DNA prime/MVA boost regime. Eur J Pharm Biopharm. 2008;68(3):589–595. | ||
Garzón MR, Berraondo P, Crettaz J, et al. Induction of gp120-specific protective immune responses by genetic vaccination with linear polyethylenimine-plasmid complex. Vaccine. 2005;23(11):1384–1392. | ||
Oster CG, Kim N, Grode L, et al. Cationic microparticles consisting of poly(lactide-co-glycolide) and polyethylenimine as carriers systems for parental DNA vaccination. J Control Release. 2005;104(2):359–377. | ||
Qin T, Yin Y, Huang L, Yu Q, Yang Q. H9N2 influenza whole inactivated virus combined with polyethyleneimine strongly enhances mucosal and systemic immunity after intranasal immunization in mice. Clin Vaccine Immunol. 2015;22(4):421–429. | ||
Firdous J, Islam MA, Park SM, et al. Induction of long-term immunity against respiratory syncytial virus glycoprotein by an osmotic polymeric nanocarrier. Acta Biomater. 2014;10(11):4606–4617. | ||
Bivas-Benita M, Lin MY, Bal SM, et al. Pulmonary delivery of DNA encoding Mycobacterium tuberculosis latency antigen Rv1733c associated to PLGA-PEI nanoparticles enhances T cell responses in a DNA prime/protein boost vaccination regimen in mice. Vaccine. 2009;27(30):4010–4017. | ||
Hu K, Dou J, Yu F, et al. An ocular mucosal administration of nanoparticles containing DNA vaccine pRSC-gD-IL-21 confers protection against mucosal challenge with herpes simplex virus type 1 in mice. Vaccine. 2011;29(7):1455–1462. | ||
Chen X, Liu Y, Wang L, et al. Enhanced humoral and cell-mediated immune responses generated by cationic polymer-coated PLA microspheres with adsorbed HBsAg. Mol Pharm. 2014;11(6):1772–1784. | ||
Sun Z, Liu B, Ruan X, Liu Q. An enhanced immune response against G250, induced by a heterologous DNA prime-protein boost vaccination, using polyethyleneimine as a DNA vaccine adjuvant. Mol Med Rep. 2014;10(5):2657–2662. | ||
Velluto D, Thomas SN, Simeoni E, Swartz MA, Hubbell JA. PEG-b-PPS-b-PEI micelles and PEG-b-PPS/PEG-b-PPS-b-PEI mixed micelles as non-viral vectors for plasmid DNA: tumor immunotoxicity in B16F10 melanoma. Biomaterials. 2011;32(36):9839–9847. | ||
Zhang M, Hong Y, Chen W, Wang C. Polymers for DNA vaccine delivery. ACS Biomater Sci Eng. 2016;3(2):108–125. | ||
Regnström K, Ragnarsson EG, Köping-Höggård M, Torstensson E, Nyblom H, Artursson P. PEI – a potent, but not harmless, mucosal immuno-stimulator of mixed T-helper cell response and FasL-mediated cell death in mice. Gene Ther. 2003;10(18):1575–1583. | ||
Lisziewicz J, Gabrilovich DI, Varga G, et al. Induction of potent human immunodeficiency virus type 1-specific T-cell-restricted immunity by genetically modified dendritic cells. J Virol. 2001;75(16):7621–7628. | ||
Li M, Jiang Y, Xu C, Zhang Z, Sun X. Enhanced immune response against HIV-1 induced by a heterologous DNA prime-adenovirus boost vaccination using mannosylated polyethyleneimine as DNA vaccine adjuvant. Int J Nanomedicine. 2013;8:1843–1854. | ||
Cherif MS, Shuaibu MN, Kodama Y, et al. Nanoparticle formulation enhanced protective immunity provoked by PYGPI8p-transamidase related protein (PyTAM) DNA vaccine in Plasmodium yoelii malaria model. Vaccine. 2014;32(17):1998–2006. | ||
Al-Deen FM, Xiang SD, Ma C, et al. Magnetic nanovectors for the development of DNA blood-stage malaria vaccines. Nanomaterials (Basel). 2017;7(2). | ||
Chen YZ, Yao XL, Ruan GX, et al. Gene-carried chitosan-linked polyethylenimine induced high gene transfection efficiency on dendritic cells. Biotechnol Appl Biochem. 2012;59(5):346–352. | ||
Zeng Q, Jiang H, Wang T, Zhang Z, Gong T, Sun X. Cationic micelle delivery of Trp2 peptide for efficient lymphatic draining and enhanced cytotoxic T-lymphocyte responses. J Control Release. 2014;200:1–12. | ||
Mohit E, Bolhassani A, Zahedifard F, et al. Immunomodulatory effects of IP-10 chemokine along with PEI600-Tat delivery system in DNA vaccination against HPV infections. Mol Immunol. 2013;53(1–2):149–160. | ||
Ma YF, Yang YW. Delivery of DNA-based cancer vaccine with polyethylenimine. Eur J Pharm Sci. 2010;40(2):75–83. | ||
Seth A, Oh DB, Lim YT. Nanomaterials for enhanced immunity as an innovative paradigm in nanomedicine. Nanomedicine (Lond). 2015;10(6):959–975. | ||
Babensee JE. Interaction of dendritic cells with biomaterials. Semin Immunol. 2008;20(2):101–108. | ||
Lambrecht BN, Kool M, Willart MA, Hammad H. Mechanism of action of clinically approved adjuvants. Curr Opin Immunol. 2009;21(1):23–29. | ||
Uto T, Wang X, Sato K, et al. Targeting of antigen to dendritic cells with poly(γ-glutamic acid) nanoparticles induces antigen-specific humoral and cellular immunity. J Immunol. 2007;178(5):2979–2986. | ||
Uto T, Akagi T, Yoshinaga K, Toyama M, Akashi M, Baba M. The induction of innate and adaptive immunity by biodegradable poly(γ-glutamic acid) nanoparticles via a TLR4 and MyD88 signaling pathway. Biomaterials. 2011;32(22):5206–5212. | ||
Moon JJ, Suh H, Bershteyn A, et al. Interbilayer-crosslinked multilamellar vesicles as synthetic vaccines for potent humoral and cellular immune responses. Nat Mater. 2011;10(3):243–251. | ||
Kipper MJ, Wilson JH, Wannemuehler MJ, Narasimhan B. Single dose vaccine based on biodegradable polyanhydride microspheres can modulate immune response mechanism. J Biomed Mater Res A. 2006;76(4):798–810. | ||
Agueros M, Areses P, Campanero MA, et al. Bioadhesive properties and biodistribution of cyclodextrin-poly(anhydride) nanoparticles. Eur J Pharm Sci. 2009;37(3–4):231–240. | ||
Salman HH, Irache JM, Gamazo C. Immunoadjuvant capacity of flagellin and mannosamine-coated poly(anhydride) nanoparticles in oral vaccination. Vaccine. 2009;27(35):4784–4790. | ||
Xu L, Liu Y, Chen Z, et al. Morphologically virus-like fullerenol nanoparticles act as the dual-functional nanoadjuvant for HIV-1 vaccine. Adv Mater. 2013;25(41):5928–5936. | ||
Xu L, Liu Y, Chen Z, et al. Surface-engineered gold nanorods: promising DNA vaccine adjuvant for HIV-1 treatment. Nano Lett. 2012;12(4):2003–2012. | ||
Shokouhi B, Coban C, Hasirci V, et al. The role of multiple toll-like receptor signalling cascades on interactions between biomedical polymers and dendritic cells. Biomaterials. 2010;31(22):5759–5771. | ||
Huang Z, Yang Y, Jiang Y, et al. Anti-tumor immune responses of tumor-associated macrophages via toll-like receptor 4 triggered by cationic polymers. Biomaterials. 2013;34(3):746–755. | ||
Chen H, Li P, Yin Y, et al. The promotion of type 1 T helper cell responses to cationic polymers in vivo via toll-like receptor-4 mediated IL-12 secretion. Biomaterials. 2010;31(32):8172–8180. | ||
He W, Liang P, Guo G, et al. Re-polarizing myeloid-derived suppressor cells (MDSCs) with cationic polymers for cancer immunotherapy. Sci Rep. 2016;6:24506. | ||
Cubillos-Ruiz JR, Engle X, Scarlett UK, et al. Polyethylenimine-based siRNA nanocomplexes reprogram tumor-associated dendritic cells via TLR5 to elicit therapeutic antitumor immunity. J Clin Invest. 2009;119(8):2231–2244. | ||
Okada M, Matsuzawa A, Yoshimura A, Ichijo H. The lysosome rupture-activated TAK1-JNK pathway regulates NLRP3 inflammasome activation. J Biol Chem. 2014;289(47):32926–32936. | ||
Tse HM, Milton MJ, Schreiner S, Profozich JL, Trucco M, Piganelli JD. Disruption of innate-mediated proinflammatory cytokine and reactive oxygen species third signal leads to antigen-specific hyporesponsiveness. J Immunol. 2007;178(2):908–917. | ||
Mulens-Arias V, Rojas JM, Pérez-Yagüe S, Morales MP, Barber DF. Polyethylenimine-coated SPIONs trigger macrophage activation through TLR-4 signaling and ROS production and modulate podosome dynamics. Biomaterials. 2015;52:494–506. | ||
Yang YW, Hsu PY. The effect of poly(D,L-lactide-co-glycolide) microparticles with polyelectrolyte self-assembled multilayer surfaces on the cross-presentation of exogenous antigens. Biomaterials. 2008;29(16):2516–2526. | ||
Marichal T, Ohata K, Bedoret D, et al. DNA released from dying host cells mediates aluminum adjuvant activity. Nat Med. 2011;17(8):996–1002. | ||
Palumbo RN, Zhong X, Wang C. Polymer-mediated DNA vaccine delivery via bystander cells requires a proper balance between transfection efficiency and cytotoxicity. J Control Release. 2012;157(1):86–93. | ||
Lojk J, Strojan K, Miš K, Bregar BV, et al. Cell stress response to two different types of polymer coated cobalt ferrite nanoparticles. Toxicol Lett. 2017;270:108–118. | ||
Peter C, Wesselborg S, Herrmann M, Lauber K. Dangerous attraction: phagocyte recruitment and danger signals of apoptotic and necrotic cells. Apoptosis. 2010;15(9):1007–1028. | ||
De Gregorio E, D’Oro U, Wack A. Immunology of TLR-independent vaccine adjuvants. Curr Opin Immunol. 2009;21(3):339–345. | ||
Orson FM, Kinsey BM, Hua PJ, Bhogal BS, Densmore CL, Barry MA. Genetic immunization with lung-targeting macroaggregated polyethyleneimine-albumin conjugates elicits combined systemic and mucosal immune responses. J Immunol. 2000;164(12):6313–6321. | ||
Palumbo RN, Zhong X, Panus D, Han W, Ji W, Wang C. Transgene expression and local tissue distribution of naked and polymer-condensed plasmid DNA after intradermal administration in mice. J Control Release. 2012;159(2):232–239. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.






