Back to Journals » OncoTargets and Therapy » Volume 11
Polydatin exerts anti-tumor effects against renal cell carcinoma cells via induction of caspase-dependent apoptosis and inhibition of the PI3K/Akt pathway
Authors Jin YL , Xin LM, Zhou CC, Ren Y
Received 19 July 2018
Accepted for publication 12 October 2018
Published 16 November 2018 Volume 2018:11 Pages 8185—8195
DOI https://doi.org/10.2147/OTT.S180785
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Carlos E Vigil
This paper has been retracted
Yi-Li Jin,1 Li-Min Xin,2 Chang-Chun Zhou,1 Yu Ren2
1Department of Urology, Dongyang People’s Hospital, Wenzhou Medical University, Dongyang, Zhejiang 322100, People’s Republic of China; 2Laboratory of kidney Carcinoma, Ningbo Urology and Nephrology Hospital, Urology and Nephrology Institute of Ningbo University, Ningbo 315000, Zhejiang Province, People’s Republic of China
Purpose: Polydatin, a stilbenoid glucoside of a resveratrol derivative, has many biological functions, including antitumor effects. However, the antitumor effects of polydatin in renal cell carcinoma (RCC) have not been investigated.
Materials and methods: In the current study, MTT assays, transwell invasion assays and wound healing assays were performed to examine cell proliferation, invasion and migration. An apoptosis nucleosome ELISA was used to measure apoptosis. Caspase activity assays were applied to measure the activities of caspase-3/9. A Western blot assay was used to measure the change in protein levels.
Results: Our data demonstrated that polydatin inhibited the proliferation of RCC cells but not normal renal epithelial cells in a time- and dose-dependent manner. Polydatin also triggered apoptosis in a caspase-dependent manner. Moreover, polydatin treatment also led to the downregulation of Bcl-2 and Mcl-1 and to activation of Bax. Ectopic expression of Bcl-2 and Mcl-1 or silencing of Bax could repress the apoptosis that was induced by polydatin. Moreover, incubation with polydatin also suppressed the PI3K/Akt signaling pathway in RCC cells.
Conclusion: Taken together, our data indicated that polydatin may be applied as a potent agent against RCC.
Keywords: polydatin, renal cell carcinoma, apoptosis, PI3K, Akt
Introduction
Renal cell carcinoma (RCC), a common kidney malignancy, accounts for ~3% of all malignancies in adults.1 RCC is characterized by a lack of early symptoms, diverse clinical manifestations and insensitivity to radiation and chemotherapy.2 Currently, surgical intervention is the main strategy for the treatment of localized RCC. However, over 30% of patients with localized RCC who underwent nephrectomy subsequently developed metastases, and the 5-year overall survival rate was less than 10%.3 Although great therapeutic progress has been made in recent years, the long-term prognosis for RCC still remains poor. Therefore, it is necessary to develop novel therapeutic strategies for RCC.
Due to their relatively low toxicity, natural compounds of plant origin are receiving increasing attention as promising antitumor agents.4 Polydatin (PD) is a stilbenoid compound that is isolated from the root of Polygonum cuspidatum, a traditional Chinese herb that has a long history of use as a medication.5 Previous studies indicated that PD possesses a variety of biological activities such as protecting against congestive heart failure, ischemia/reperfusion injury, endometriosis and shock.6–9 Recently, PD has also been found to produce antitumor effects against various cancers. For instance, PD could induce apoptosis and cell cycle arrest in lung cancer cells.10 Treatment with PD also resulted in apoptosis and inhibition of growth in acute monocytic leukemia cells.11 Moreover, PD also induced apoptosis in human osteosarcoma cells by upregulating the ratio of Bax/Bcl-2 and inhibiting cell proliferation.12 However, the role of PD in RCC has not been investigated.
In the present study, we examined the antitumor effects of PD in two RCC cell lines. Our results demonstrated that PD significantly inhibited proliferation, triggered apoptosis and repressed the migration and invasion of RCC cells. Furthermore, mechanistic investigations revealed that PD induced apoptosis in a caspase-dependent manner. Treatment with PD led to downregulation of Bcl-2 and Mcl-1 and activation of Bax. Ectopic expression of Bcl-2 or Mcl-1 decreased the apoptosis that was induced by PD. In addition, silencing of Bax also repressed PD-induced apoptosis. Furthermore, treatment with PD leads to inhibition of the PI3K/Akt signaling pathway. Taken together, our data demonstrated the potential for the use of PD against RCC.
Materials and methods
Cell culture and reagents
The RCC cell lines, ACHN, Caki-1 and 786-O, were purchased from the Shanghai Cell Bank (Shanghai, China). Human embryonic kidney cells 293T, which were approved by the ethics committee of Wenzhou Medical University, were a generous gift from Dr Chao Pan, Wenzhou Medical University. Cells were cultured in RPMI 1640 medium (No. 11875093; Gibco, NY, USA) supplemented with 10% FBS (No. 26400044), 100 U of penicillin and 100 μg of streptomycin (No. SV30010; Gibco). Cells were maintained in a humidified incubator with 5% CO2 at 37°C. PD (No. 15721) was purchased from Sigma-Aldrich Co., St Louis, MO, USA. PD was prepared as a 100 mM stock solution in DMSO (No. D2650; Sigma-Aldrich Co.). The stock solution was stored at −20°C. All other routine chemicals were purchased from Sigma-Aldrich Co. unless indicated otherwise.
Cell viability assays
Cell viability was evaluated by an MTT assay kit (No. 11465007001; Sigma-Aldrich Co.) according to the manufacturer’s instructions. Briefly, cells (2×103/well) were seeded into 96-well plates and cultured for 24 hours and then treated with various doses of PD for different times. The culture medium was removed and MTT (20 μL, 5 mg/mL) was added to each well and incubated for another 4 hours at 37°C. The medium was then discarded, and 200 μL of DMSO (0.01%) was added to each well and incubated for 20 minutes. The absorbance was measured at 490 nm by a microplate reader (BioTek, Winooski, VT, USA).
Apoptosis nucleosome ELISA assay
As described previously, the apoptosis rates were determined using a nucleosome ELISA assay (No. 11544675001; Hoffman-La Roche Ltd., Basel, Switzerland). Briefly, after treatment for 24 hours, the induction of apoptosis was assessed by measuring the enrichment of nucleosome in the cytoplasm according to the manufacturer’s instructions.
Caspase activity assay
The activities of caspase-3 and caspase-9 were determined by caspase-3 and caspase-9 assay kits, respectively (No. ab219915; Abcam, Cambridge, MA, USA) according to the manufacturer’s instructions. After the treatments, 100 μL of caspase-3 or caspase-9 reagent were added to each well and incubated for 1 hour at room temperature. Luminescence was measured using a BioTek 312e microplate reader (BioTek Instruments, Winooski, VT, USA). Caspase-3/9 activities were recorded as a percentage of the untreated control.
Transfection
The pcDNA3.1-Mcl-1, pcDNA3.1-Bcl-2 and control pcDNA 3.1 vectors were purchased from PharmaGene (Hangzhou, China). The myr-Akt1 plasmid was purchased from Addgene. The siRNA against Bax (5′-GGUGCCGGAACUGAUCAGA-3′) and the negative control siRNA (5′-UUCUCCGAACGUGUCACGU-3′) were purchased from Invitrogen (Shanghai, China). The transfection was performed using Lipofectamine 2000 (No. 11668027; Life Technologies, Carlsbad, CA, USA) according to the manufacturer’s instructions.
Transwell invasion assay
A transwell assay (Costar, Washington, DC, USA) was used to determine invasion capacities. 786-O or Caki-1 cells (2×104) were seeded into the upper chamber, which was coated with Matrigel (No. 354230; BD Biosciences, San Jose, CA, USA), and 600 μL of medium containing 10% FBS was added to the lower chamber. Different doses of PD were added to both chambers. After treatment for 24 hours, cells that did not migrate through the pore of the filter were removed. Then, the migrated cells were fixed with 95% ethanol and stained with 0.5% crystal violet (Sigma-Aldrich Co.) and counted using an inverted microscope (Olympus Corporation, Tokyo, Japan).
Cell wounding assay
Cells were seeded in 6-well plates at 70% confluence. When the cells reached ~90% confluence, the monolayer cells were scratched with pipette tips (200 μL) and treated with different doses of PD. After 24 hours, the wound healing status was recorded with an inverted microscope. The average gap widths were measured from at least ten low-power field images for each assay condition using cellSens Digital Imaging software (Olympus).
Western blot analysis
After treatment, the cells were collected and lysed in RIPA buffer. The protein concentrations were measured by a Bradford protein assay kit (Sigma-Aldrich). Equal amounts of protein were subjected to SDS-PAGE and then transferred to PVDF membranes (Millipore, Boston, MA, USA). After blocking with 5% skimmed milk for 1 hour at room temperature, the PVDF membranes were incubated with primary antibodies overnight at 4°C. The following antibodies were used: caspase-3 (No. 14214), Bcl-2 (No. 4223), Bcl-xl (No. 2762), Mcl-1 (No. 94296), Bax (No. 2774), cytochrome c (No. 4272), Smac/DIABLO (No. 15108), p-mTOR (No. 5536) and mTOR (No. 2972) and were purchased from Cell Signalling Technology (Danvers, MA, USA). Bax (6A7) (No. ab5714), p-Akt (No. ab81283, Ser 473), Akt (No. ab8805), p-PI3K (No. ab182651, Y607), PI3K (No. ab191606) and GAPDH (No. ab8245) were obtained from Abcam (San Diego, CA, USA). The membrane was then incubated with the secondary antibody and visualized by ECL (No. 32106) (Thermo Fisher Scientific, Rockford, IL, USA). Purification of cytosolic fractions and Bax immunoprecipitation was performed as described by Yu et al 2016.13
Statistical analyses
Statistical analyses were performed using SPSS 14.0 software (SPSS Inc., Chicago, IL, USA). Data are expressed as the mean ± SD. Differences among groups were determined by a one-way ANOVA followed by a Tukey’s HSD (Honestly Significant Difference) test. A P<0.05 was considered significantly different.
Results
PD inhibited the viabilities of RCC cells but not normal human embryonic kidney cells
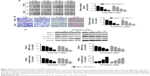
First, we investigated the effects of PD on the viability of RCC cells. We found that PD decreased the viabilities of ACHN, Caki-1 and 786-O cells in a time- and dose-dependent manner (Figure 1A–C). Then, we tested the effects of PD on 293 T cells, which are normal human embryonic kidney cells. Interestingly, PD had little effect on the viability of 293 T cells (Figure 1D). Taken together, these findings suggest that PD selectively inhibited the viability of RCC cells but not normal cells.
PD inhibited the migration, invasion and Epithelial-mesenchymal transition (EMT) of RCC cells
We next investigated whether PD has any effects on cell migration and invasion of RCC cells. As indicated in Figure 2A, treatment with PD significantly inhibited cell migration of both 786-O and Caki-1 cells in a dose-dependent manner. Similarly, exposure to PD also dramatically repressed the invasive ability of 786-O and Caki-1 cells (Figure 2B). Furthermore, the protein levels of MMP-7 and MMP-9, both of which play essential roles in tumor metastasis, were inhibited by PD in a dose-dependent manner (Figure 2C). We also examined the effects of PD on the EMT process. As shown in Figure 2C, treatment with PD significantly decreased the protein levels of N-cadherin, which is a marker of mesenchymal cells. In contrast, the protein levels of E-cadherin, which is a typical epithelial cell protein, were upregulated by PD in a dose-dependent manner (Figure 2C). Taken together, these data suggest that PD represses migration, invasion and the EMT process of RCC cells.
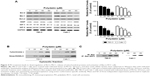
PD-induced caspase-dependent apoptosis in RCC cells
We next investigated whether apoptosis was responsible for the PD-induced cytotoxicity in RCC cells. An apoptosis nucleosome ELISA was performed as previously described.14 After treatment with various doses of PD for 24 hours, it was shown that PD induced apoptosis in 786-O and Caki-1 cells in a dose-dependent manner (Figure 3A). To elucidate the molecular mechanisms underlying the apoptosis induced by PD, a Western blot analysis was performed. As indicated in Figure 3B and C, PD treatment leads to the upregulation of cleaved caspase-3/9. Furthermore, caspase-3/9 activity assays also revealed that treatment with PD increased the activation of caspase-3/9 in a dose-dependent manner (Figure 3D and E). To determine whether the activation of caspases is critical for apoptosis that is induced by PD, zVAD.fmk, a pan-caspase-inhibitor, was used. As shown in Figure 3F, zVAD.fmk fully inhibited the apoptosis that was induced by PD. Taken together, these findings suggest that apoptosis that was induced by PD relies on the activation of caspases.
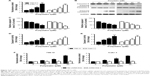  | Figure 3 PD induces caspase-activation and caspase-dependent apoptosis in RCC cells. |
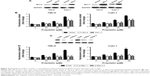
PD treatment leads to the downregulation of Bcl-2 and Mcl-1, activation of Bax, and release of mitochondrial proteins in RCC cells
It is well documented that the process of apoptosis is subjected to regulation by various proteins. Therefore, we analyzed the protein levels of Bcl-2 and IAP members after PD treatment by Western blotting. We found that the protein levels of Bcl-2 and Mcl-1 were repressed by PD in a dose-dependent manner (Figure 4A). In addition, the expression of Bcl-xl, XIAP, IAP-1 and IAP-2 was not affected by PD in RCC cells (Figure 4A). The release of cytochrome c and Smac/DIABLO from mitochondria into the cytosol is an essential step during the process of apoptosis.15 Therefore, we measured the release of cytochrome c and Smac/DIABLO into the cytosol after treatment with PD. As shown in Figure 4B, the release of cytochrome c and Smac/DIABLO into the cytosol was increased in a dose-dependent manner after treatment with PD. The release of mitochondrial proteins into the cytosol relies partly on the activation of Bax.15 Therefore, we examined whether exposure to PD results in the activation of Bax. To address this question, we immunoprecipitated Bax using a conformation-specific antibody that specifically detects the active form. We found that PD treatment leads to the activation of Bax in a dose-dependent manner in RCC cells (Figure 4C).
Overexpression of Bcl-2 and Mcl-1 or silencing of Bax repressed the apoptosis induced by PD
Since we observed downregulation of Mcl-1 and Bcl-2 and activation of Bax after treatment with PD in RCC cells, we examined the role of Mcl-1 and Bcl-2 and Bax in PD-induced apoptosis. First, we forced the expression of Bcl-2 and Mcl-1 in RCC cells (Figure 5A). We observed that ectopic expression of Bcl-2 or Mcl-1 significantly repressed the apoptosis induced by PD in RCC cells (Figure 5B). Then, we used two siRNAs to knockdown Bax, as confirmed by Western blot analysis (Figure 5C). Interestingly, silencing of Bax markedly impaired the apoptosis induced by PD (Figure 5D).
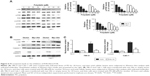
PD treatment leads to inhibition of the PI3K/Akt signaling pathway
Next, we examined whether PD had any effects on the PI3K/Akt/mTOR signaling pathway, which is involved in the progression of various cancers, including RCC.16 As shown in Figure 6A, PD treatment led to the downregulation of phosphorylated PI3K, Akt and mTOR, while it had little effect on total PI3K, Akt and mTOR. To further elucidate the role of PI3K/Akt/mTOR in PD-induced apoptosis, we transfected 786-O and Caki-1 cells with a plasmid encoding constitutively active Akt (Myr-Akt) (Figure 6B). Twenty-four hours after the transfection, the cells were treated with PD for another 24 hours, and apoptosis was determined. We found that apoptosis induced by PD was significantly inhibited by myr-Akt (Figure 6C); furthermore, cleavage of caspase-3 was inhibited by myr-AKt as well (Figure 6B). Taken together, these data suggest that PD treatment led to the inhibition of PI3K/Akt/mTOR, which is involved in apoptosis that is induced by PD.
Discussion
RCC, an aggressive solid tumor with an increasing incidence, has imposed huge economic and social pressures worldwide. Treatment of RCC remains a therapeutic challenge due to its resistance to conventional chemotherapy. Despite great progress in the diagnosis and treatment of RCC, the overall survival remains poor, and no effective systemic chemotherapy exists for patients with advanced RCC. Therefore, an urgent need exists for the development of novel therapeutic strategies for RCC.
In recent years, natural products have received great attention as antitumor agents due to their high efficiency and relatively low toxicity. Mounting evidence has demonstrated that PD is cytotoxic against various types of cancers. For instance, PD could inhibit proliferation and induce apoptosis of breast cancer cells.17 PD also induced cell cycle arrest and apoptosis in leukemia, lung cancer and colorectal cancer cells.10,18,19 However, little is known about the effects of PD on RCC cells.
In the present study, we evaluated the antitumor effects of PD in two human renal cancer cell lines. MTT assays were performed to examine the antiproliferation efficacy of PD in vitro. The results of MTT assays indicated that PD exerted cytotoxic effects on renal cancer cells. In addition to the antiproliferation effect of PD, we also measured the effects of PD on cancer cell invasion and migration, which are believed to account largely for cancer metastasis. Our results showed that PD also inhibited cell invasion and migration of RCC cells. Our findings are in agreement with a very recent study in which PD inhibited the invasion and migration of human liver cancer cells.20 The degradation of basement membranes and stromal extracellular matrix is an essential step that leads to invasion and metastasis. MMPs are a family of human zinc-dependent endopeptidases that are responsible for degradation of the extracellular matrix.21 We also observed downregulation of MMP-7/9, which may account for the inhibition of migration and invasion of RCC cells after PD treatment. EMT is a process that contributes to cancer progression, particularly as it relates to invasion and metastasis.22 During the EMT process, cancer cells lose epithelial markers such as E-cadherin and acquire mesenchymal markers such as N-cadherin.23 In our study, we found that treatment with PD inhibited the EMT transition of RCC cells. This finding is in agreement with a previous study that found that PD could inhibit EMT in lung tissues.24 Considering that EMT may contribute to chemoresistance, it would be interesting to test whether PD could overcome chemoresistance in cancer cells.
Apoptosis, also known as programmed cell death, plays an essential role in the initiation and progression of cancer. Induction of apoptosis is still considered to be the first choice for antitumor treatment. An early event in apoptosis is DNA fragmentation followed by the release of nucleosomes into the cytoplasm.25 In our study, we detected an enrichment of nucleosomes after exposure to PD in RCC cells. Mechanistic investigations revealed that PD treatment triggers caspase-dependent cell death in RCC cells. This conclusion is supported by the finding that the pan-caspase inhibitor zVAD.fmk rescued cell death from PD. There are two pathways leading to apoptosis, namely, the extrinsic pathway and the intrinsic/mitochondrial pathway.26 The latter pathway is subjected to regulation by Bcl-2 proteins and is characterized by the activation of Bax and the release of mitochondrial proteins into the cytosol.27 In this study, we observed the downregulation of Bcl-2 and Mcl-1, the activation of Bax and the release of mitochondrial proteins. These data suggest that the activation of the intrinsic/mitochondrial pathway is required for PD-mediated apoptosis, as overexpression of Bcl-2 and Mcl-1 or silencing of Bax markedly protects cells from cell death.
The constitutive activation of PI3K/Akt/mTOR is frequently detected in human malignancies and is often associated with chemoresistance in tumors, including RCC.16,28 The results demonstrate that treatment with PD significantly reduced the levels of phosphorylated PI3K, Akt and mTOR in RCC cells. We hypothesized that PD induced cell death at least partially via the inhibition of PI3k/Akt/mTOR. To address this, we transfected cells with the myr-Akt vector, which can mimic the constitutive activation of Akt. We found that overexpression of myr-Akt could reduce the apoptosis induced by PD. Thus, we concluded that the inhibition of PI3K/Akt/mTOR was part of the mechanism underlying the antitumor effects of PD. Similar to our findings, it has been reported that PD inhibited PI3K/Akt in leukemia cells.29 Interestingly, another study reported that treatment with PD leads to the activation of PI3K/Akt in renal ischemia/reperfusion injured mice. This discrepancy indicates that the effects of PD on PI3K/Akt may be tissue specific, and further investigation is required to resolve this issue.
To our knowledge, PD has potential value that may facilitate its application in the future. Clinical usage indicates that PD is relatively safe and has low toxicity in human.30 Moreover, PD can also alleviate inflammation.31 It would be intriguing to test PD in combination with other antitumor reagents, and further investigation is required to determine the full value of PD in the treatment of various cancers.
Conclusion
We evaluated the antitumor effects of PD in human RCC cells. Mechanistic investigations revealed that PD induced apoptosis in a caspase-dependent manner via the intrinsic apoptotic pathway and by inhibition of the PI3K/Akt pathway. Furthermore, our data demonstrated that PD significantly suppressed the migration and invasion of RCC cells. In addition, PD inhibited the PI3K/Akt/mTOR signaling pathway. The mechanisms were indicated in Figure 7. Collectively, our results provide evidence that PD may be a potential anticancer agent for RCC therapy.
Acknowledgment
We thank the American Journal Experts (Durham, NC, USA) for improving the language quality of the manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
Ljungberg B, Cowan NC, Hanbury DC, et al. EAU guidelines on renal cell carcinoma: the 2010 update. Eur Urol. 2010;58(3):398–406. | ||
Mosillo C, Ciccarese C, Bimbatti D, et al. Renal cell carcinoma in one year: going inside the news of 2017 – a report of the main advances in RCC cancer research. Cancer Treat Rev. 2018;67:29–33. | ||
Breda A, Konijeti R, Lam JS. Patterns of recurrence and surveillance strategies for renal cell carcinoma following surgical resection. Expert Rev Anticancer Ther. 2007;7(6):847–862. | ||
Lucas DM, Still PC, Pérez LB, Grever MR, Kinghorn AD. Potential of plant-derived natural products in the treatment of leukemia and lymphoma. Curr Drug Targets. 2010;11(7):812–822. | ||
Zhang H, Li C, Kwok ST, Zhang QW, Chan SW. A Review of the pharmacological effects of the dried root of Polygonum cuspidatum (Hu Zhang) and its constituents. Evid Based Complement Alternat Med. 2013;2013:1–13. | ||
Gao Y, Chen T, Lei X, et al. Neuroprotective effects of polydatin against mitochondrial-dependent apoptosis in the rat cerebral cortex following ischemia/reperfusion injury. Mol Med Rep. 2016;14(6):5481–5488. | ||
Ling Y, Chen G, Deng Y, et al. Polydatin post-treatment alleviates myocardial ischaemia/reperfusion injury by promoting autophagic flux. Clin Sci. 2016;130(18):1641–1653. | ||
Di Paola R, Fusco R, Gugliandolo E, et al. Co-micronized palmitoylethanolamide/polydatin treatment causes endometriotic lesion regression in a rodent model of surgically induced endometriosis. Front Pharmacol. 2016;7:382. | ||
Wang X, Song R, Chen Y, Zhao M, Zhao KS. Polydatin – a new mitochondria protector for acute severe hemorrhagic shock treatment. Expert Opin Investig Drugs. 2013;22(2):169–179. | ||
Zhang Y, Zhuang Z, Meng Q, Jiao Y, Xu J, Fan S. Polydatin inhibits growth of lung cancer cells by inducing apoptosis and causing cell cycle arrest. Oncol Lett. 2014;7(1):295–301. | ||
Wang C, Luo Y, Lu J, Wang Y, Sheng G. Polydatin induces apoptosis and inhibits growth of acute monocytic leukemia cells. J Biochem Mol Toxicol. 2016;30(4):200–205. | ||
Xu G, Kuang G, Jiang W, Jiang R, Jiang D. Polydatin promotes apoptosis through upregulation the ratio of Bax/Bcl-2 and inhibits proliferation by attenuating the β-catenin signaling in human osteosarcoma cells. Am J Transl Res. 2016;8(2):922–931. | ||
Yu R, Yu BX, Chen JF, et al. Anti-tumor effects of Atractylenolide I on bladder cancer cells. J Exp Clin Cancer Res. 2016;35:40. | ||
Hsu YL, Kuo YC, Kuo PL, Ng LT, Kuo YH, Lin CC. Apoptotic effects of extract from Antrodia camphorata fruiting bodies in human hepatocellular carcinoma cell lines. Cancer Lett. 2005;221(1):77–89. | ||
Kluck RM, Bossy-Wetzel E, Green DR, Newmeyer DD. The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science. 1997;275(5303):1132–1136. | ||
Guo H, German P, Bai S, et al. The PI3K/AKT pathway and renal cell carcinoma. J Genet Genomics. 2015;42(7):343–353. | ||
Chen S, Tao J, Zhong F, et al. Polydatin down-regulates the phosphorylation level of Creb and induces apoptosis in human breast cancer cell. PLoS One. 2017;12(5):e0176501. | ||
Cao WJ, Wu K, Wang C, Wan DM. Polydatin-induced cell apoptosis and cell cycle arrest are potentiated by Janus kinase 2 inhibition in leukemia cells. Mol Med Rep. 2016;13(4):3297–3302. | ||
De Maria S, Scognamiglio I, Lombardi A, et al. Polydatin, a natural precursor of resveratrol, induces cell cycle arrest and differentiation of human colorectal Caco-2 cell. J Transl Med. 2013;11:264. | ||
Jiao Y, Wu Y, Du D. Polydatin inhibits cell proliferation, invasion and migration, and induces cell apoptosis in hepatocellular carcinoma. Braz J Med Biol Res. 2018;51(4):e6867. | ||
Parks WC, Shapiro SD. Matrix metalloproteinases in lung biology. Respir Res. 2001;2(1):10–19. | ||
Brabletz T, Jung A, Spaderna S, Hlubek F, Kirchner T. Opinion: migrating cancer stem cells – an integrated concept of malignant tumor progression. Nat Rev Cancer. 2005;5(9):744–749. | ||
Klymkowsky MW, Savagner P. Epithelial-mesenchymal transition: a cancer researcher’s conceptual friend and foe. Am J Pathol. 2009;174(5):1588–1593. | ||
Cao K, Lei X, Liu H, et al. Polydatin alleviated radiation-induced lung injury through activation of Sirt3 and inhibition of epithelial-mesenchymal transition. J Cell Mol Med. 2017;21(12):3264–3276. | ||
Salgame P, Varadhachary AS, Primiano LL, Fincke JE, Muller S, Monestier M. An ELISA for detection of apoptosis. Nucleic Acids Res. 1997;25(3):680–681. | ||
Ashkenazi A, Salvesen G. Regulated cell death: signaling and mechanisms. Annu Rev Cell Dev Biol. 2014;30:337–356. | ||
Estaquier J, Vallette F, Vayssiere JL, Mignotte B. The mitochondrial pathways of apoptosis. Adv Exp Med Biol. 2012;942:157–183. | ||
Juengel E, Makarević J, Tsaur I, et al. Resistance after chronic application of the HDAC-inhibitor valproic acid is associated with elevated Akt activation in renal cell carcinoma in vivo. PLoS One. 2013;8(1):e53100. | ||
Ye J, Piao H, Jiang J, et al. Polydatin inhibits mast cell-mediated allergic inflammation by targeting PI3K/Akt, MAPK, NF-κB and Nrf2/HO-1 pathways. Sci Rep. 2017;7(1):11895. | ||
Pace MC, Passavanti MB, Aurilio C, et al. Polydatin administration improves serum biochemical parameters and oxidative stress markers during chronic alcoholism: a pilot study. In Vivo. 2015;29(3):405–408. | ||
Ji H, Zhang X, Du Y, Liu H, Li S, Li L. Polydatin modulates inflammation by decreasing NF-κB activation and oxidative stress by increasing Gli1, Ptch1, SOD1 expression and ameliorates blood-brain barrier permeability for its neuroprotective effect in pMCAO rat brain. Brain Res Bull. 2012;87(1):50–59. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.