Back to Journals » Clinical Ophthalmology » Volume 8
Pneumatic displacement with intravitreal bevacizumab for massive submacular hemorrhage due to polypoidal choroidal vasculopathy
Authors Kitahashi M, Baba T, Sakurai M, Yokouchi H, Kubota-Taniai M, Mitamura Y , Yamamoto S
Received 4 October 2013
Accepted for publication 19 November 2013
Published 3 March 2014 Volume 2014:8 Pages 485—492
DOI https://doi.org/10.2147/OPTH.S55413
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Masayasu Kitahashi,1 Takayuki Baba,1 Madoka Sakurai,1 Hirotaka Yokouchi,1 Mariko Kubota-Taniai,1 Yoshinori Mitamura,2 Shuichi Yamamoto1
1Department of Ophthalmology and Visual Science, Chiba University Graduate School of Medicine, Chiba, 2Institute of Health Biosciences, University of Tokushima Graduate School, Tokushima, Japan
Background: The purpose of this study was to compare the effectiveness of pneumatic displacement combined with intravitreal bevacizumab (IVB) with that of pneumatic displacement (PD) alone to treat massive submacular hemorrhage (SMH) secondary to polypoidal choroidal vasculopathy (PCV).
Methods: Thirty-two eyes of 32 patients with massive SMH secondary to PCV were studied. Twenty-two eyes were treated with a combination of PD and 1.25 mg of intravitreal bevacizumab (PD + IVB group), and ten eyes with pneumatic displacement alone (PD group).
Results: Pretreatment, the differences in best-corrected visual acuity and size of the SMH between the two groups were not significant (P=0.59 and P=0.72, respectively). Complete displacement of the hemorrhage from under the fovea was achieved in 19 of 22 eyes (86.4%) in the PD + IVB group and in five of ten eyes (50%) in the PD group. The best-corrected visual acuity in the PD + IVB group was significantly better than that in the PD group at one, 3, and 6 months after treatment (P<0.001, P<0.001, and P<0.001, respectively). Improvement in best-corrected visual acuity by >0.3 logMAR units was obtained in 18 eyes (81.8%) in the PD + IVB group and two eyes (20%) in the PD group (P<0.001). The number of eyes that required additional treatments was significantly fewer in the PD + IVB group than in the PD group (P=0.0001).
Conclusion: The combination of PD and IVB may be a better therapeutic procedure for eyes with massive SMH due to PCV in the short term because of the better visual outcome and less need for additional treatments.
Keywords: pneumatic displacement, intravitreal bevacizumab, submacular hemorrhage, polypoidal choroidal vasculopathy
Introduction
Massive submacular hemorrhage (SMH) is commonly found in eyes with the neovascular type of exudative age-related macular degeneration, polypoidal choroidal vasculopathy (PCV), retinal arterial macroaneurysm, trauma, and choroidal neovascularization of any cause.1–6 SMH results in an acute and severe decrease of vision, especially if the blood clot is thick and the fovea is involved.3,4,7
One of the most common procedures used to treat SMH is intravitreal injection of sulfur hexafluoride (SF6) for pneumatic displacement (PD) of the hemorrhage with or without recombinant tissue plasminogen activator (rTPA).8–12 After displacement of the blood clot from the central fovea, the choroidal neovascularization can be treated by either intravitreal injection of anti-vascular endothelial growth factor (VEGF) drugs or photodynamic therapy.2
Bevacizumab (Genentech, San Francisco, CA, USA), a humanized monoclonal antibody that blocks all VEGF isoforms, has had good results in the treatment of choroidal neovascularization associated with age-related macular degeneration.13–15 It has been reported that intravitreal bevacizumab (IVB) is a good therapeutic option for eyes with neovascular age-related macular degeneration with SMH, leading to stable visual acuity and anatomic improvements.16 Combination therapy of IVB injection, rTPA, and PD has been reported to be effective for massive subretinal hemorrhage caused by choroidal neovascularization associated with age-related macular degeneration.17,18
However, it has been reported that the results of intravitreal gas without rTPA are not significantly different from those of gas combined with rTPA for massive SMH in the final visual outcome.19 In addition, there have been concerns about the toxicity of rTPA to the neural retina, and this has led us to stop using rTPA for the treatment of SMH.20,21 Recently, two case series reported successful treatment of SMH using a combination of IVB and PD without rTPA.22,23
The purpose of this study was to compare the effects of combining IVB and an intravitreal injection of an expandable gas with that of PD only in eyes with massive SMH due to PCV.
Materials and methods
This was a nonrandomized clinical trial in 32 eyes from 32 consecutive patients (seven women and 25 men) who received PD combined with IVB or PD only for massive SMH due to PCV. The study was conducted at Chiba University Hospital between January 2004 and December 2010. The diagnosis of PCV was based on characteristic indocyanine green angiographic (ICGA) findings. The criteria for making a diagnosis of PCV were hyperfluorescent aneurysm-like dilatations and polyps, with or without a dilated network of inner choroidal vessels in the images obtained by ICGA. Inclusion criteria were the presence of a large subretinal hemorrhage with a size ≥2 disc diameters or an SMH located in the macular region and a duration of symptoms <15 days. The blood clot had to be thick enough to block the view of fluorescein leakage and the borders of the complete vascular lesion could not be seen on fluorescein angiography. Exclusion criteria included: presence of choroidal neovascularization secondary to typical age-related macular degeneration, pathologic myopia, or retinal artery macroaneurysm; tears of the retinal pigment epithelium (RPE); and other maculopathies, such as diabetic maculopathy or retinal vascular occlusion.
The procedures conformed to the tenets of the Declaration of Helsinki, and approval was obtained from the institutional review board of Chiba University. An informed consent was obtained from each patient.
Among the 32 eyes, ten were treated with intravitreal SF6 (0.5 mL) between January 2004 and September 2007 (PD group), and 22 eyes were treated with a combination of intravitreal SF6 (0.3–0.5 mL) and 1.25 mg of bevacizumab between August 2007 and March 2010 (PD + IVB group). There were two women and eight men in the PD group and five women and 17 men in the PD + IVB group.
All patients underwent a standard ophthalmologic examination including best-corrected visual acuity (BCVA) measurement, slit-lamp biomicroscopy, indirect ophthalmoscopy, and color fundus photography before and 3 months after treatment. BCVA was measured using a standard Japanese Landolt visual acuity chart and converted to logarithm of the minimum angle resolution (logMAR) units for the statistical analyses.
In all subjects, 0.3–0.5 mL of expandable SF6 gas were injected intravitreally to displace the macular hemorrhage as described by Ohji et al.9 The bevacizumab was prepared by the hospital pharmacy as sterile filled and packed tuberculin syringes containing 0.1 mL of bevacizumab. Intravitreal injection of bevacizumab 1.25 mg/0.05 mL was followed by intravitreal injection of SF6 gas. An anterior chamber paracentesis was performed immediately after the injection in eyes with high intraocular pressure. All patients were instructed to maintain a prone position for 2–3 days.
Central foveal thickness was measured manually on the images obtained by optical coherence tomography (OCT; Stratus III OCT, Carl Zeiss, Dublin, CA, USA, and RTVue-100, Optovue, Fremont, CA, USA) using 5 mm horizontal scans before, and one and 3 months after treatment. The Stratus III OCT was used from 2004 to 2007 and the RTVue from 2008 to 2010. Central foveal thickness was measured by placing calibrated calipers at the vitreous-retina interface and the inner border of the choroid, and included the thickness of the subretinal fluid or hemorrhage. Huang et al reported that central foveal thickness measured by RTVue frequency domain OCT was thicker by 7.7% than that measured with the Stratus OCT. Therefore, we corrected the central foveal thickness with RTVue by reducing the values by 7.7%.24 All OCT images were acquired through a dilated pupil. When fixation was poor, the scans were centered on the fovea under video observation.
The disc diameter of the SMH was measured on the fundus photographs. The degree of leakage from the polypoidal lesions in the ICGA images before treatment was compared with those at 1 month after treatment. The degree of blood displacement was determined by comparing fundus photographs taken before and 1 month after the procedure. Complete displacement was defined as no blood or only a thin layer of blood within one disc area of the fovea.25
The indications and procedures for additional treatment were as follows. First was the presence of a rhegmatogenous retinal detachment (RRD) with vitreous hemorrhage or dense vitreous hemorrhage, and these were treated by pars plana vitrectomy (PPV). Second was the presence of exudative changes from polypoidal lesions or classic choroidal neovascularizations located in the subfoveal or juxtafoveal area, and these were treated after August 2007 by anti-VEGF therapy, eg, intravitreal ranibizumab or IVB. Patients with persistent exudation after anti-VEGF therapy or with strong leakage from polypoidal lesions with ICGA were treated with photodynamic therapy. Before July 2007, patients with residual polypoidal lesions with ICGA were treated with photodynamic therapy because we were not permitted to use any anti-VEGF therapy before August 2007. Third was recurrence of an SMH with clear vitreous body, and these eyes were treated by IVB + PD or PD only. Fourth was the presence of polypoidal lesions located as extrafoveal lesions, and these eyes were treated by direct photocoagulation. Patients who needed additional photocoagulation were treated before ICGA.
The Student’s t-test for continuous variables or the Fisher’s exact probability test for categorical variables was used to determine the significance of differences or changes in the findings in the two treatment groups. All analyses were conducted using JMP version 8 (SAS Inc, Cary, NC, USA). A P-value<0.05 was considered to be statistically significant.
Results
The mean age (± standard deviation) at initial examination was 69.7±8.7 years in the PD group and 66.9±8.9 years in the PD + IVB group (P=0.41, unpaired t-test; Table 1). The average interval between onset of symptoms of SMH and initial treatment was 8.1±3.7 days in the PD + IVB group and 6.1±4.9 days in the PD group (P=0.23, unpaired t-test; Table 1). The pretreatment diameter of the SMH in the PD + IVB group (4.7±2.8 disc diameters) was not significantly different from that in the PD group (4.4±1.7 disc diameters; P=0.72, unpaired t-test; Table 1).
At baseline, the mean BCVA in the PD + IVB group was 0.84±0.36 logMAR units, and was not significantly different from that in the PD group (0.94±0.69; P=0.59, unpaired t-test; Table 1). In the PD + IVB group, mean BCVA gradually improved during the follow-up period and was significantly better than the baseline value at 3 and 6 months after treatment (P<0.0001, paired t-test; Figure 1). On the other hand, BCVA in the PD group decreased slightly at 1 month, and then improved at 3 and 6 months after treatment. However, there was no significant difference from the baseline value at any point throughout the follow-up period in the PD group (Figure 1). Mean BCVA in the PD + IVB group was significantly better than that in the PD group at one, 3, and 6 months postoperatively (P<0.001, P<0.001, and P<0.001, respectively, paired t-test).
In the PD + IVB group, BCVA improved by >0.3 logMAR units in 18 eyes (81.8%) and remained unchanged in four eyes (18.2%). In the PD group, BCVA improved in two eyes (20%), remained unchanged in four eyes (40%), and worsened in two eyes (20%). The number of eyes in which BCVA improved by >0.3 logMAR units was significantly greater in the PD + IVB group than in the PD group (P<0.001, Fisher’s exact probability test; Table 2).
Central foveal thickness at baseline was 561.0±178.4 μm in the PD + IVB group and 648.4±162.1 μm in the PD group (Table 1). This difference was not significant (P=0.235, unpaired t-test). One month after treatment, mean central foveal thickness was 220.0±122.4 μm in the PD + IVB group, which was significantly thinner than the 402±194.4 μm in the PD group (P=0.0069, unpaired t-test; Table 2 and Figure 2). However, the difference between the two groups at 3 and 6 months after treatment was not significant (P=0.511 and P=0.261, respectively).
Complete displacement of the hemorrhage from under the fovea was achieved after initial treatment in 19 of 22 eyes (86.4%) in the IVB + PD group, and in five of ten eyes (50%) in the PD group (P=0.39, Yates Chi-squared test; Table 2).
The number of eyes with resolution of leakage from the polypoidal lesions or not requiring additional treatment was significantly higher in the PD + IVB group (18 eyes) than in the PD group (four eyes, P=0.037, Fisher’s exact probability test; Table 2).
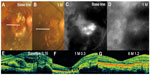
The results for a case of SMH secondary to PCV in which combination therapy with IVB + PD was used successfully are shown in Figure 3.
The difference in incidence of a recurrence of SMH within 1 month in the PD + IVB group was not significantly different from that in the PD group (two eyes, P=0.090, Fisher’s exact probability test; Table 2). The PD + IVB group had fewer recurrences of SMH within 1 month than the PD only group, but the difference was not significant. A recurrence of SMH during the follow-up period was observed in one eye in the PD + IVB group and two eyes in the PD group, and this difference was not significant (P=0.22, Fisher’s exact probability test).
Additional treatments were performed during the 6-month follow-up period in six eyes in the PD + IVB group, comprising: photocoagulation directly to residual PCV for an extrafoveal lesion in two eyes, photodynamic therapy for cases showing strong leakage from residual polypoidal lesions with ICGA in two eyes, intravitreal ranibizumab injection for subretinal detachment from a low active residual polypoidal lesion in one eye, and IVB + PD for recurrent SMH in one eye (Table 2). Repeat treatments were performed for eight eyes in the PD only group; photodynamic therapy with strong leakage from a residual polypoidal lesions with ICGA in four eyes, PPV for RRD after PD in one eye, and PPV for dense vitreous hemorrhage in three eyes. Three patients treated with PPV for vitreous hemorrhage were treated with photodynamic therapy within a month after the PPV. The number of eyes with additional treatments for exudative change, recurrence of SMH, or strong leakage from polypoidal lesions was significantly fewer in the PD + IVB group than in the PD group (P=0.049, Fisher’s exact probability test).
No serious ocular or systemic adverse events were observed to be associated with combination of intravitreal SF6 and bevacizumab during the 6-month follow-up period. One patient in the PD only group developed rhegmatogenous retinal detachment at 1 month after treatment.
Discussion
The prevalence of PCV in eyes with age-related macular degeneration is high in nonwhite populations, and has been reported to be 23%–54.7% in Japanese patients diagnosed with age-related macular degeneration.26,27 The presence of massive SMH is a relatively common complication of PCV.26,28 The natural course of SMH is generally poor, especially in cases where the hemorrhage is thick and involves a large area of the macula.3,7
An early displacement of a massive SMH from the macular area improves the prognosis for visual acuity.9,29 Intravitreal injection of an expandable gas such as SF6 or perfluorocarbon (C3F8) with or without intravitreal injection of rTPA has been shown to displace the blood clot in patients with SMH due to age-related macular degeneration or PCV.2,25,28 After displacement of the blood clot, direct photocoagulation or photodynamic therapy can be considered for persistent polypoidal lesions at the macular area when the bleeding sites can be easily identified.2 In some cases, the bleeding sites might not be identified because massive SMH or active vascular lesions might be covered by a thick blood clot at the sub-RPE level. In such cases, photodynamic therapy or photocoagulation cannot be performed or must be postponed. We had four patients in the PD group on whom photodynamic therapy or photocoagulation was not able to be performed because of vitreous hemorrhage and/or recurrence of SMH.
IVB has been used to treat choroidal neovascularization with large SMH older than 2 weeks in patients with age-related macular degeneration.16 This treatment was shown to achieve anatomic and functional stabilization in both the short term and long term. It has been reported that a combination of anti-VEGF therapy with PD and rTPA is effective as an initial therapy in SMH due to age-related macular degeneration.17,18
Stabilization of visual acuity during the follow-up period was achieved in all eyes, and an improvement of visual acuity of >0.3 logMAR units was found in 81.8% of eyes in the PD + IVB group, which was significantly better than that in the PD only group. On the other hand, there was a significant decrease in mean BCVA at 1 month in the PD group because of recurrence of SMH or vitreous hemorrhage. Subretinal and sub-RPE hemorrhages are typical findings in eyes with PCV. Therefore, preventing recurrence of SMH is also important at the time of initial treatment.
If PD only is used in eyes with SMH, subsequent photodynamic therapy might be postponed for a number of reasons, eg, a vitreous hemorrhage, a large greatest linear dimension size, or a delay in absorption or recurrence of SMH. PD combined with IVB may be used to reduce exudative changes during the early period after treatment.
Another advantage of PD combined with IVB was a reduction in the need for additional treatments for exudative or hemorrhagic changes due to PCV. We found that the number of eyes requiring retreatment for PCV was significantly lower in eyes treated with IVB + PD than those treated with PD only, indicating that IVB at the time of PD successfully suppressed the activity of PCV.
The mechanism by which IVB causes reduction of a polypoidal lesion with SMH was not determined. Gomi et al reported that the mechanism of action of IVB is reduction of fluids from the PCV but not choroidal vascular changes.30 They also reported that polyps were reduced in only one of eleven (9.1%), unchanged in eight of eleven (72.7%), and increased in two of eleven (18.2%) eyes with PCV. In contrast, we found that polyps resolved in nine of 22 (40.9%), decreased in nine (40.9%), and were unchanged in four of 22 (18.2%) eyes. Whether bevacizumab can penetrate deeply into the sub-RPE space has not been determined, but it has been reported that IVB is effective for PCV with RPE atrophy.30 There is a possibility that the presence of an RPE crack is more frequent in PCV cases with massive SMH because an elevated RPE due to sub-RPE hemorrhage might generate tension on the RPE.
Complete displacement of blood from under the fovea was achieved in 19 of 22 eyes (86.4%) in the IVB + PD group. This result is comparable with that of an earlier study using IVB, rTPA, and gas tamponade. In addition, Cakir et al reported that a combination of gas with or without rTPA for massive SMH had no significant effect on the final visual outcome.19
We had one patient with RRD after PD for SMH secondary to PCV. He had lattice degeneration at the superior peripheral retina. Unfortunately, posterior vitreous detachment had not occurred before the PD in his treated eye. We supposed that the PVD occurred because of the gas injection and RRD was complicated. Ophthalmologists should notice the incidence of RRD when PD is performed for SMH in patients who have lattice degeneration of the peripheral retina without posterior vitreous detachment.
The limitations of this study are its small sample size, the relatively short follow-up period, and differences in methodology used in the two groups of patients. In particular, a different period of time between the IVB + PD group and the PD group and the difference in additional treatments given to the two groups could lead to errors in interpretation. The types of additional treatment could have direct implications for final visual acuity, and we should have standardized the period of time for treatment and therapeutic procedures in the two groups. A randomized clinical trial with standardized procedures for additional treatment should be undertaken in the future. Nevertheless, the finding that PD + IVB leads to better functional and morphologic outcomes indicates that it should be strongly considered for patients with SMH secondary to PCV.
In summary, our results show that the combination of IVB and PD is an effective therapy for eyes with massive SMH due to PCV. This therapeutic regimen led to stabilization of both anatomic and functional properties for at least 6 months.
Disclosure
The authors report no conflicts of interest in this work.
References
Bennett SR, Folk JC, Blodi CF, Klugman M. Factors prognostic of visual outcome in patients with subretinal hemorrhage. Am J Ophthalmol. 1990;109(1):33–37. | |
Chan WM, Liu DT, Lai TY, Li H, Tong JP, Lam DS. Extensive submacular haemorrhage in polypoidal choroidal vasculopathy managed by sequential gas displacement and photodynamic therapy: a pilot study of one-year follow up. Clin Experiment Ophthalmol. 2005;33(6):611–618. | |
Avery RL, Fekrat S, Hawkins BS, Bressler NM. Natural history of subfoveal subretinal hemorrhage in age-related macular degeneration. Retina. 1996;16(3):183–189. | |
Berrocal MH, Lewis ML, Flynn HW Jr. Variations in the clinical course of submacular hemorrhage. Am J Ophthalmol. 1996;122(4):486–493. | |
Saito K, Iijima H. [Visual prognosis and macular pathology in eyes with retinal macroaneurysms]. Nippon Ganka Gakkai Zasshi. 1997;101(2):148–151. Japanese. | |
Ibanez HE, Williams DF, Thomas MA, et al. Surgical management of submacular hemorrhage. A series of 47 consecutive cases. Arch Ophthalmol. 1995;113(1):62–69. | |
Chen WL, Liu JH, Lee FL. Natural course of submacular hemorrhage. Zhonghua Yi Xue Za Zhi (Taipei). 1999;62(5):268–277. | |
Heriot W. Futher experience in management of submacular hemorrhage with intravitreal tPA. In: Proceedings of the Update on Macular Surgery. San Francisco, CA, USA: American Academy of Ophthalmology; 1997. | |
Ohji M, Saito Y, Hayashi A, Lewis JM, Tano Y. Pneumatic displacement of subretinal hemorrhage without tissue plasminogen activator. Arch Ophthalmol. 1998;116(10):1326–1332. | |
Hassan AS, Johnson MW, Schneiderman TE, et al. Management of submacular hemorrhage with intravitreous tissue plasminogen activator injection and pneumatic displacement. Ophthalmology. 1999;106(10):1900–1906. | |
Mizutani T, Yasukawa T, Ito Y, et al. Pneumatic displacement of submacular hemorrhage with or without tissue plasminogen activator. Graefes Arch Clin Exp Ophthalmol. 2011;249(8):1153–1157. | |
Fang IM, Lin YC, Yang CH, Yang CM, Chen MS. Effects of intravitreal gas with or without tissue plasminogen activator on submacular haemorrhage in age-related macular degeneration. Eye (Lond). 2009;23(2):397–406. | |
Rosenfeld PJ, Moshfeghi AA, Puliafito CA. Optical coherence tomography findings after an intravitreal injection of bevacizumab (avastin) for neovascular age-related macular degeneration. Ophthalmic Surg Lasers Imaging. 2005;36(4):331–335. | |
Rich RM, Rosenfeld PJ, Puliafito CA, et al. Short-term safety and efficacy of intravitreal bevacizumab (Avastin) for neovascular age-related macular degeneration. Retina. 2006;26(5):495–511. | |
Bashshur ZF, Bazarbachi A, Schakal A, Haddad ZA, El Haibi CP, Noureddin BN. Intravitreal bevacizumab for the management of choroidal neovascularization in age-related macular degeneration. Am J Ophthalmol. 2006;142(1):1–9. | |
Stifter E, Michels S, Prager F, et al. Intravitreal bevacizumab therapy for neovascular age-related macular degeneration with large submacular hemorrhage. Am J Ophthalmol. 2007;144(6):886–892. | |
Sacu S, Stifter E, Vecsei-Marlovits PV, et al. Management of extensive subfoveal haemorrhage secondary to neovascular age-related macular degeneration. Eye (Lond). 2009;23(6):1404–1410. | |
Guthoff R, Guthoff T, Meigen T, Goebel W. Intravitreous injection of bevacizumab, tissue plasminogen activator, and gas in the treatment of submacular hemorrhage in age-related macular degeneration. Retina. 2011;31(1):36–40. | |
Cakir M, Cekic O, Yilmaz OF. Pneumatic displacement of acute submacular hemorrhage with and without the use of tissue plasminogen activator. Eur J Ophthalmol. 2010;20(3):565–571. | |
Johnson MW, Olsen KR, Hernandez E. Tissue plasminogen activator treatment of experimental subretinal hemorrhage. Retina. 1991;11(2):250–258. | |
Chen CY, Hooper C, Chiu D, Chamberlain M, Karia N, Heriot WJ. Management of submacular hemorrhage with intravitreal injection of tissue plasminogen activator and expansile gas. Retina. 2007;27(3):321–328. | |
Chawla S, Misra V, Khemchandani M. Pneumatic displacement and intravitreal bevacizumab: a new approach for management of submacular hemorrhage in choroidal neovascular membrane. Indian J Ophthalmol. 2009;57(2):155–157. | |
Hasler PW, la Cour M, Villumsen J. Pneumatic displacement and intravitreal bevacizumab in the management of subretinal haemorrhage caused by choroidal neovascularization. Acta Ophthalmol Scand. 2007;85(5):577–579. | |
Huang J, Liu X, Wu Z, Xiao H, Dustin L, Sadda S. Macular thickness measurements in normal eyes with time-domain and Fourier-domain optical coherence tomography. Retina. 2009;29(7):980–987. | |
Kung Y-H, Wu T-T, Hong M-C, Sheu S-J. Intravitreal tissue plasminogen activator and pneumatic displacement of submacular hemorrhage. J Ocul Pharmacol Ther. 2010;26(5):469–474. | |
Sho K, Takahashi K, Yamada H, et al. Polypoidal choroidal vasculopathy: incidence, demographic features, and clinical characteristics. Arch Ophthalmol. 2003;121(10):1392–1396. | |
Maruko I, Iida T, Saito M, Nagayama D, Saito K. Clinical characteristics of exudative age-related macular degeneration in Japanese patients. Am J Ophthalmol. 2007;144(1):15–22. | |
Yoon JS, Lee J, Lee SC, Koh HJ, Kim SS, Kwon OW. Polypoidal choroidal vasculopathy in Korean patients with large submacular hemorrhage. Yonsei Med J. 2007;48(2):225–232. | |
Hesse L, Schmidt J, Kroll P. Management of acute submacular hemorrhage using recombinant tissue plasminogen activator and gas. Graefes Arch Clin Exp Ophthalmol. 1999;237(4):273–277. | |
Gomi F, Sawa M, Sakaguchi H, et al. Efficacy of intravitreal bevacizumab for polypoidal choroidal vasculopathy. Br J Ophthalmol. 2008;92(1):70–73. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.