Back to Journals » Journal of Multidisciplinary Healthcare » Volume 12
Plummer-Vinson syndrome: improving outcomes with a multidisciplinary approach
Authors Lo KB , Albano J, Sandhu N, Candelario N
Received 7 January 2019
Accepted for publication 20 May 2019
Published 19 June 2019 Volume 2019:12 Pages 471—477
DOI https://doi.org/10.2147/JMDH.S180410
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Kevin Bryan Lo,1 Jeri Albano,1 Naemat Sandhu,2 Nellowe Candelario1
1Department of Medicine, Einstein Medical Center, Philadelphia, PA, USA; 2Department of Gastroenterology and Hepatology, Einstein Medical Center, Philadelphia, PA, USA
Abstract: Plummer-Vinson syndrome is a rare condition associated with dysphagia, iron deficiency, and esophageal webs. Data regarding this condition is limited to mostly case reports and a few small cohort studies. Although most cases have a benign and indolent course, the risk of malignancy warrants long-term surveillance. A multidisciplinary approach among healthcare providers is of the utmost importance in the management of this condition.
Keywords: esophageal webs, iron deficiency anemia, dysphagia, malignancy
Introduction
Plummer-Vinson syndrome (PVS) is a rare condition characterized by the triad of iron deficiency anemia, dysphagia, and esophageal webs. It is also known as Paterson-Brown-Kelly syndrome and sideropenic dysphagia. Although it was first described as early as 1912, there is still limited knowledge about it due to the low and progressively decreasing incidence of the syndrome. It is more common among middle-aged Caucasian females. It is also important to recognize the association of Plummer-Vinson Syndrome with esophageal and hypopharyngeal cancer.
History
PVS was first reported in 1912 by an endocrinologist from Mayo Clinic, Henry Stanley Plummer. He described it as hysterical dysphagia from “cardiospasm” or upper esophageal spasms without anatomic stenosis. In 1919, a surgeon, Porter Paisley Vinson described the dysphagia to be due to an “angulation” of the esophagus and treated it with esophageal dilation. Hence, the disease entity was named after them.1–4 The disease is known in the United Kingdom as Paterson-Brown-Kelly syndrome. The term comes from two British laryngologists who in 1919 independently reported a fuller description of the condition. Donal Ross Paterson described the esophageal web and dysphagia and suggested it may be precancerous while Adam Brown-Kelly described the classic triad of the syndrome.3,4
Epidemiology
PVS is rare. Most clinical data gathered come from case reports and case series. It is more common in females, accounting for 90% of the cases. It is also more common at ages 40–70 years old, although it has been reported to occur in children as young as six years old.5,6 There appears to be a predilection for whites especially from Scandinavia.7 In the latter half of the 20th century, there was a decline in the number of cases which coincided with the improvement in nutrition and decreased cases of iron deficiency anemia.8
Clinical presentation
PVS often presents with symptoms of iron deficiency anemia and dysphagia, hence the name sideropenic dysphagia. Iron deficiency is more important than the presence of anemia and is not necessarily hypochromic and microcytic.3 There are various gastrointestinal manifestations, the main features of which are dysphagia and upper esophageal webs (Figure 1). The dysphagia is often intermittent and painless, but in some cases it could be progressive from solid to liquid food. The dysphagia is most likely from the upper esophageal web, although it may occur without it and is theorized to be from muscular changes and hypomotility from the iron deficiency.3 PVS is also associated with an increased risk for upper gastrointestinal tract cancers, the most common being squamous cell carcinoma of the hypopharynx and cervical esophagus. There have also been reports of gastric cancer.9,10 Malignancies occur at an incidence of 3–15%, but has also decreased with the decreasing incidence of PVS.11,12
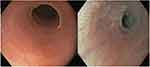 | Figure 1 Endoscopic views of esophageal web thin membranes of esophageal mucosa found either anterior, posterior or circumferential. |
Pathophysiology
There are multiple theories as to the cause of PVS. Iron deficiency seems to be the critical feature to its etiopathogenesis. Others include other vitamin deficiencies, autoimmune factors, and genetic predisposition.8 Low iron levels lead to reduction in the activity of iron-dependent oxidative enzymes which in turn may lead to myasthenic changes in muscles, atrophy of the mucosa, and eventual development of webs. This is further supported by evidence of mitochondrial damage similar to progressive muscular dystrophy in pharyngeal muscles of mice with iron deficiency anemia. This mitochondrial change also contributes to neurodegeneration and aging of the mucosa. Additional evidence includes the improvement of dysphagia after iron supplementation.13,14 However, not everyone improves with supplementation. Countries like Africa which have high prevalence of iron deficiency anemia do not have a proportional increase in the number of PVS cases.13 The degree of iron deficiency does not correlate with the severity of other symptoms as well. Web formation only occurs in the upper esophagus which does not fit with iron deficiency alone.4,15 Another proposed theory is that despite the mucosal degeneration and atrophy of the esophagus associated with iron deficiency, it is the upper esophagus, surrounded by skeletal elements, that receives the most trauma from swallowing food boluses and thus more prone to web formation. Acid secretion from a heterotropic gastric mucosa in the upper esophagus causing inflammation and web formation has also been hypothesized. However, the lower esophagus is also exposed to gastric acid and seldom forms webs from it. Additionally, no gastric metaplasia has been noted in the biopsy of the samples taken from PVS cases.4,15 Vitamin deficiency, specifically of riboflavin, pyridoxine and thiamine, has also been looked into due to similar manifestations of glossitis and cheilosis. However, vitamin testing in red blood cells (RBCs) have proven to be within normal for this population.4,16 Autoimmunity is also a consideration because the syndrome has at times been associated with conditions such as celiac disease, rheumatoid arthritis, and thyroiditis4,17 (Figure 2).
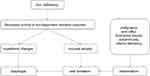 | Figure 2 Summary of pathophysiology for Plummer-Vinson syndrome. |
Diagnosis
With iron deficiency anemia and dysphagia as the most common presenting features of PVS, workup should be directed to these two presentations. Iron deficiency anemia is first suspected on clinical grounds. The most common presenting signs and symptoms of iron deficiency anemia include fatigue, paleness, dyspnea, and headache. Other associated symptoms are atrophic glossitis, alopecia, dry skin and hair, tachycardia, koilonychia and sometimes even angina, vertigo, and syncope.5 Laboratory evaluation of the red cell indices including the mean cell volume (MCV) and mean cell hemoglobin (MCH) may be decreased in long standing anemia. This contributes to the microcytic hypochromic picture in the peripheral blood smear of iron deficiency anemia.18 The most accurate test for iron deficiency anemia is serum ferritin levels. However, this test can be altered by inflammatory conditions and various threshold levels have been used to interpret it more accurately.19 Other indices such as transferrin saturation, together with the overall clinical picture and the use of different thresholds adjusted for inflammation, may also be used as clues to point towards accurate diagnosis of iron deficiency anemia.20 Thalassemia is one potential differential diagnosis that can present as a microcytic hypochromic anemia picture similar to iron deficiency anemia. However, since thalassemia tends to have more microcytic cells and iron deficiency anemia tends to have more hypochromic cells, various RBC to MCV, MCV to MCH ratios have been used to help screen and discriminate between these two conditions.21 After establishing the diagnosis of iron deficiency, the underlying cause should also be determined. In the middle to elderly age group, the most common cause is still blood loss specifically from the gastrointestinal tract.5 Another potential source of blood loss especially for women of premenopausal age is menstrual bleeding.22 Meanwhile, in the younger age group, physiologic anemia and bleeding disorders should also be entertained.23 In the absence of bleeding, iron malabsorption due to a history of bariatric surgery, celiac disease, atrophic gastritis, and chronic inflammation can lead to iron deficiency as well.24 In relation to inflammation, concomitant iron deficiency anemia can occur together with anemia of chronic disease especially in patients with chronic kidney disease and in heart failure.25,26
Dysphagia is a prominent symptom of PVS to which further workup should be directed. It is also the basis on most of the differential diagnoses to consider when considering PVS in the setting of dysphagia and iron deficiency anemia. Dysphagia in PVS is usually attributed to the presence of the esophageal web which usually presents as oropharyngeal dysphagia localized in the neck area up to above the suprasternal notch.4 Symptoms may include a sensation of localized lump or sticking in the throat. Esophageal webs themselves may be associated with inflammatory conditions such as autoimmune bullous diseases such as epidermolysis bullosa, bullous pemphigoid, and pemphigus vulgaris.27–29 Esophagitis from chronic graft versus host disease following bone marrow transplant may also lead to formation of esophageal webs.30 The most common cause of oropharyngeal dysphagia is usually neurologic including stroke, brain and neck surgery or radiation.31 Other commonly overlooked cases of oropharyngeal dysphagia include causes of myopathy and autoimmune diseases such as scleroderma, polymyositis, and dermatomyositis. Medications that can also cause oropharyngeal dysphagia include antihistamines, benzodiazepines, statins, and other drugs that can cause impairment in salivation such as anticholinergics, antidepressants, antipsychotics, anti-Parkinsons, anti-hypertensives, and diuretics.32 An anatomic cause of oropharyngeal dysphagia can be a Zenker’s diverticulum which occurs above the upper esophageal sphincter (similar to where esophageal webs are commonly found) at a point of weakness between the muscle fibers leading to the formation of a diverticulum.33 However, in patients with truly symptomatic Zenker’s diverticulum, there is usually regurgitation of food a couple of hours after the last meal which classically points to this specific pathology. Another closely related structural variation that can cause dysphagia is the cricopharyngeal bar. It is a prominence of the upper esophageal sphincter usually incidentally seen on radiograph which might be related to a diverticulum and most often asymptomatic.33 More serious causes of oropharyngeal dysphagia may be from oropharyngeal tumors, laryngeal tumors and other head and neck tumors. This can be from direct invasion by the tumors themselves or as sequelae of their treatment which might include surgery or radiation that may bring about inflammatory changes such as esophageal strictures.34 Very rarely, oropharyngeal dysphagia can be caused by degenerative bony changes called osteophytes that may impinge on the esophagus most especially in the cervical area which can be from diffuse idiopathic skeletal hyperostosis or DISH.35 While the perceived level of obstruction or dysphagia might assist us in coming up with the diagnosis or differential diagnosis, it should be noted that some distal esophageal lesions might give a perceived proximal referred sensation of obstruction.36,37 As a result, other common causes of lower obstruction or esophageal dysphagia can be considered achalasia, diffuse esophageal spasm, eosinophilic esophagitis, and gastroesophageal reflux. Meanwhile, other mechanical causes include caustic or radiation injury, strictures, malignancy, vascular rings, and Schatzki ring.38 Schatzki rings are usually found on the lower esophageal area. Most are asymptomatic but are considered to be the most common cause of episodic dysphagia in adults. Usually it is associated with other esophageal disorders such as gastroesophageal reflux and which may actually be causally related to it.39
The initial test for oropharyngeal dysphagia is the use of barium swallow or videofluoroscopy.38,40 It offers a real time image of the swallowing phase and the transit of food or liquid down the esophagus which will help distinguish obvious anatomic causes of dysphagia such as stricture, webs, rings, and masses or malignancy. In addition, it is non-invasive but may miss subtle abnormalities and has exposure to radiation.38 Esophageal webs are usually transiently seen for only a fraction of a time wherein the cervical esophagus is filled with barium and can be potentially missed.41 That is why full esophageal column distention and proper patient positioning in both anteroposterior and lateral views should be obtained. In addition, the suspicion for esophageal webs should be shared with the radiologist to help in optimizing study conditions which can aid in diagnosis.42 The majority of esophageal webs are single and either circumferential or anterior in position, but multiple webs or posterior in location may also occur.43 Another classic radiologic finding indicative of esophageal webs in the barium study is the “jet phenomenon.“ It refers to the abrupt narrowing of the barium column at the level of the web with the jet of barium below.44 Although this finding was first described in esophageal webs, it can also be seen in cases of stricture, adenocarcinoma, and cricopharyngeal bar.45 While the majority of esophageal webs can be easily identified with a barium study or videofluoroscopy, smaller webs of 1–2 mm of projection may not be detected and up to 14% may be missed and confirmed in subsequent endoscopy.43
The next important diagnostic test to consider is upper endoscopy or esophagoscopy. This procedure has the advantage of offering both diagnostic and therapeutic measures at the same time. An upper endoscopy is still considered less sensitive in detecting esophageal webs compared to the barium contrast study.42 The esophageal webs are very thin and close to the upper esophageal sphincter which is usually missed. They can also be accidentally ruptured as the endoscope is passed. The endoscopist must be made aware of the suspicion of an esophageal web to increase sensitivity of this study.4 The treatment for esophageal webs can be done using Savary Gilliard dilators wherein a metallic guidewire is first introduced under endoscopy through the web and into the stomach. The endoscope is then withdrawn, and dilators of increasing sizes are gradually introduced.46 In addition to the utility of treatment, endoscopy also allows surveillance for other potential associated malignancies such as post cricoid, buccal, pharyngeal, esophageal, stomach and colonic malignancies, the incidence of which can reach as high as 16% in patients with PVS.9,13,47–50
Treatment
The evidence regarding the successful treatment of PVS has only been documented in case reports and a few small retrospective studies and a single prospective study.9,47,50,51 The treatments so far have addressed two clinically significant presentations namely iron deficiency anemia and dysphagia. Various reports have shown that treatment of iron deficiency anemia alone may resolve the symptoms of dysphagia.51–53 In fact, in one retrospective review, 8 out of 50 cases presenting with iron deficiency anemia, dysphagia and esophageal webs were treated successfully with just iron supplementation with resolution of symptoms in 6-months follow-up.54 While the mechanism underlying the efficacy of iron supplementation in the resolution of symptoms in PVS is unclear, iron deficiency has been implicated to reduce iron-dependent oxidative enzymes leading to degradation of pharyngeal muscles as evidenced by a moth eaten appearance in histopathology.55 Another potential explanation is that the repletion of hemoglobin levels which act as scavengers of nitric oxide which in turn influences esophageal tone and peristalsis leads to stabilization of the appropriate nitric oxide levels and symptomatic improvement.52 However, in cases of clinically significant dysphagia and concern for other serious causes of dysphagia like malignancy, endoscopic examination and treatment might be warranted.
As mentioned above, treatment of dysphagia can be done by esophageal dilators. In a small retrospective study of ten patients only three needed multiple sessions of dilatations while all were treated successfully with endoscopic dilatation.57 Another retrospective study of 50 patients who underwent endoscopic dilatation showed similar results where 72% of cases underwent dilatation, 12% had spontaneous rupture of web on passage of endoscope and 8% were treated with iron supplements alone, 6-month follow-up showed resolution of symptoms in all cases.54 In a larger subsequent retrospective study of 135 patients, 97% were successfully treated with endoscopic dilatation with the remaining 3% treated spontaneously upon passage of the endoscope through the esophageal web.46 The only recent prospective study was done by Goel et al,43in 2015 where they included 37 patients, 31 presented with dysphagia and iron deficiency anemia who were subsequently evaluated using barium swallow and upper endoscopy. The remaining six patients were diagnosed with iron-deficiency anemia and subsequent workup of the anemia with endoscopy revealed esophageal webs. All patients had the web dilated using a controlled radial expansion (CRE) balloon. At the time of discharge all but two patients had complete response with the remaining two patients achieving complete response after another dilatation session with no reports of complications. There was a 10% recurrence rate of dysphagia with a median follow-up of 10 months, this was limited by the relative short duration of follow-up compared to other studies.43 Recurrence rates in other studies may reach as high as 20–30%.46,54,56 In some rare instances of failure of therapy or recurrence, laser lysis of esophageal webs might be a viable alternative.57
Prognosis and multidisciplinary nature of care
As mentioned previously, the prognosis of PVS is good in terms of symptom control. A significant number of cases have been reported to respond with correction of iron deficiency alone and the dysphagia from esophageal webs has been managed successfully with endoscopic techniques as well.43,51,54 However, it is also well known that PVS is associated with malignancy.9,13,47–50 Sometimes, it is even considered to be a precancerous condition.4 At present, there is no formal recommendation for cancer surveillance for patients with PVS but most experts recommend yearly surveillance and a tailored approach depending on risk factors and symptoms.4,43,58 This is where the role of the primary care provider is central in coordination of multidisciplinary care. It has been shown in some studies that delay or prolongation in the diagnostic process especially in malignancy can negatively impact treatment outcomes.59 There are various steps in the natural history of the disease in patients with PVS where multidisciplinary care and communication is essential. For the evaluation of unexplained iron deficiency anemia, malignancy is considered especially in the correct age group and the presence of risk factors, inevitably, both upper and lower endoscopy might be warranted.60 This entails coordination of care between the primary care providers and gastroenterologists. In certain cases where other causes of anemia aside from iron deficiency anemia are suspected, or in cases of suspected bleeding disorders especially in the younger population, collaboration with a hematologist might be a reasonable option. This is vital as mentioned above, correction of the anemia alone and improvement in hemoglobin levels may improve symptoms of dysphagia in patients with PVS.52 In the evaluation of dysphagia as one of the most common presenting symptoms, primary care providers need to coordinate with radiologists in order to communicate the suspicion of the esophageal web. This is to ensure that proper patient positioning and technique for upper GI series or barium swallow or videofluoroscopy can be done to increase the diagnostic yield of these tests. In upper endoscopic evaluation, gastroenterologists should be informed of the suspicion of esophageal webs as they are easily missed and sometimes, as discussed above, just passage of the endoscope itself can cause rupture of the esophageal web. In addition to that, they need to be followed up regularly with cancer surveillance especially of the upper gastrointestinal tract on top of their regular age appropriate cancer screening. The multidisciplinary nature expands even more once a patient is diagnosed with malignancy on subsequent follow-up. Now coordination of care must be done between the patient’s primary care provider, oncologist, radiation oncologist, surgeon, and other members of the health care team. Over the years, research has shown some indication that good multidisciplinary approach to cancer care may improve patient satisfaction, improve time to treatment and even suggest improvement in overall survival.61 However, studies also show that there is much room for improvement in terms of primary care and cancer specialist care coordination in terms of better communication and in better delineation of roles or responsibilities.62
Conclusions and key points
PVS is a complex disease process. Current evidence points to good responses to treatment of iron deficiency and esophageal dilatation for dysphagia from esophageal webs. There is a need for continued cancer surveillance as the disease is a risk factor for subsequent malignancy. Careful coordination of care between the primary care physician and the various medical specialties is needed in every step in the management of the disease process to ensure better patient outcomes.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Plummer HS. Diffuse dilatation of the esophagus without anatomic stenosis (cardiospasm): a report of 91 cases. JAMA. 1912;58:2013–2015. doi:10.1001/jama.1912.04260060366003
2. Vinson PP. Hysterical dysphagia. Minn Med. 1922;5:107–108.
3. Novacek G. Plummer-vinson syndrome. Orphanet J Rare Dis. 2006;1(1):36. doi:10.1186/1750-1172-1-36
4. Goel A, Bakshi SS, Soni N, Chhavi N. Iron deficiency anemia and Plummer–Vinson syndrome: current insights. J Blood Med. 2017;8:175. doi:10.2147/JBM.S127801
5. Lopez A, Cacoub P, MacDougall IC, Peyrin-Biroulet L. Iron deficiency anaemia. Lancet. 2016;387(10021):907–916. doi:10.1016/S0140-6736(15)60865-0
6. Anthony R, Sood S, Strachan DR, Fenwick JD. A case of Plummer-Vinson syndrome in childhood. J Pediatr Surg. 1999;34(10):1570–1572.
7. Wynder EL, Hultberg S, Jacobsson F, Bross IJ. Environmental factors in cancer of the upper alimentary tract. A Swedish study with special reference to Plummer-Vinson (Paterson-Kelly) syndrome. Cancer. 1957;10(3):470–487.
8. Chen TS, Chen PS. Rise and fall of the Plummer-Vinson syndrome. J Gastroenterol Hepatol. 1994;9(6):654–658.
9. Kim KH, Kim MC, Jung GJ. Gastric cancer occurring in a patient with Plummer-Vinson syndrome: a case report. World J Gastroenterol. 2005;11(44):7048. doi:10.3748/wjg.v11.i44.7048
10. Hellara, o., Hammami, A., Njim, N., et al. Gastric cancer occurring in a patient with Plummer-Vinson syndrome: an unusual association about one case. Afr J Cancer. 2013;5(4):224–227. doi:10.1007/s12558-013-0278-5
11. Larsson LG, Sandströn A, Westling P. Relationship of Plummer-Vinson disease to cancer of the upper alimentary tract in Sweden. Cancer Res. 1975;35(11 Part 2):3308–3316.
12. Messmann H. Squamous cell cancer of the oesophagus. Best Prac Res Clin Gastroenterol. 2001;15(2):249–265. doi:10.1053/bega.2000.0172
13. Chisholm M. The association between webs, iron and post-cricoid carcinoma. Postgrad Med J. 1974;50(582):215–219. doi:10.1136/pgmj.50.582.215
14. Dantas RO, Villanova MG. Esophageal motility impairment in Plummer-Vinson syndrome. Dig Dis Sci. 1993;38(5):968–971.
15. Gude D, Bansal DP, Malu A. Revisiting Plummer Vinson syndrome. Ann Med Health Sci Res. 2013;3(1):119–121. doi:10.4103/2141-9248.109476
16. Thakur K, Tomar SK, Singh AK, Mandal S, Arora S. Riboflavin and health: a review of recent human research. Crit Rev Food Sci Nutr. 2017;57(17):3650–3660. doi:10.1080/10408398.2016.1145104
17. Dickey W, McConnell B. Celiac disease presenting as the Paterson-Brown Kelly (Plummer-Vinson) syndrome. Am J Gastroenterol. 1999;94(2):527–529. doi:10.1111/j.1572-0241.1999.889_r.x
18. Massey AC. Microcytic anemia. Differential diagnosis and management of iron deficiency anemia. Med Clin North Am. 1992;76(3):549–566.
19. Guyatt GH, Oxman AD, Ali M, Willan A, McIlroy W, Patterson C. Laboratory diagnosis of iron-deficiency anemia. J Gen Intern Med. 1992;7(2):145–153. doi:10.1007/bf02598003
20. Camaschella C. Iron-deficiency anemia. N Eng J Med. 2015;372(19):1832–1843. doi:10.1056/NEJMra1401038
21. Hoffmann JJ, Urrechaga E, Aguirre U. Discriminant indices for distinguishing thalassemia and iron deficiency in patients with microcytic anemia: a meta-analysis. Clin Chem Lab Med. 2015;53(12):1883–1894. doi:10.1515/cclm-2015-0179
22. Estadella J, Villamarín L, Feliu A, Perelló J, Calaf J. Characterization of the population with severe iron deficiency anemia at risk of requiring intravenous iron supplementation. Eur J Obstetrics Gynecol Reprod Biol. 2018;224:41–44. doi:10.1016/j.ejogrb.2018.03.005
23. O’Brien SH. Evaluation and management of heavy menstrual bleeding in adolescents: the role of the hematologist. Blood. 2018;132(20):2134–2142. doi:10.1182/blood-2018-05-848739
24. Dahlerup JF, Eivindson M, Jacobsen BA, Jensen NM, Jorgensen SP, Laursen SB. Diagnosis and treatment of unexplained anemia with iron deficiency without overt bleeding. Dan Med J. 2015;62:C5072.
25. Fishbane S, Pollack S, Feldman HI, Joffe MM. Iron indices in chronic kidney disease in the National Health and Nutritional Examination Survey 1988–2004. Clin J Am Soc Nephrol. 2009;4(1):57–61. doi:10.2215/CJN.01670408
26. Enjuanes C, Klip IT, Bruguera J, et al. Iron deficiency and health-related quality of life in chronic heart failure: results from a multicenter European study. Int J Cardiol. 2014;174(2):268–275. doi:10.1016/j.ijcard.2014.03.169
27. Ergun GA, Lin AN, Dannenberg AJ, Carter DM. Gastrointestinal manifestations of epidermolysis bullosa. A study of 101 patients. Medicine. 1992;71(3):121–127.
28. Foroozan P, Enta T, Winship DH, Trier JS. Loss and regeneration of the esophageal mucosa in pemphigoid. Gastroenterology. 1967;52(3):548–558.
29. Kaplan RP, Touloukian J, Ahmed AR, Newcomer VD. Esophagitis dissecans superficialis associated with pemphigus vulgaris. J Am Acad Dermatol. 1981;4(6):682–687.
30. McDonald GB, Sullivan KM, Schuffler MD, Shulman HM, Thomas ED. Esophageal abnormalities in chronic graft-versus-host disease in humans. Gastroenterology. 1981;80(5):914–921.
31. Carucci LR, Turner MA. Dysphagia revisited: common and unusual causes. Radiographics. 2015;35(1):105–122. doi:10.1148/rg.351130150
32. Cook IJ. Oropharyngeal dysphagia. Gastroenterol Clin. 2009;38(3):411–431. doi:10.1016/j.gtc.2009.06.003
33. Achkar E. Esophageal diverticula. Gastroenterol Hepatol (N Y). 2008;4(10):691.
34. Granell J, Garrido L, Millas T, Gutierrez-Fonseca R. Management of oropharyngeal dysphagia in laryngeal and hypopharyngeal cancer. Int J Otolaryngol. 2012;2012:1–9. doi:10.1155/2012/157630
35. Candelario N, Lo KB, Naranjo M. Cervical diffuse idiopathic skeletal hyperostosis (DISH) causing oropharyngeal dysphagia. BMJ Case Rep. 2017;2017:bcr2016218630. doi:10.1136/bcr-2016-218630
36. Wilcox CM, Alexander LN, Clark WS. Localization of an obstructing esophageal lesion. Dig Dis Sci. 1995;40(10):2192–2196.
37. Ashraf HH, Palmer J, Dalton HR, et al. Can patients determine the level of their dysphagia? World J Gastroenterol. 2017;23(6):1038. doi:10.3748/wjg.v23.i6.1038
38. Kuo P, Holloway RH, Nguyen NQ. Current and future techniques in the evaluation of dysphagia. J Gastroenterol Hepatol. 2012;27(5):873–881. doi:10.1111/j.1440-1746.2012.07097.x
39. Müller M, Gockel I, Hedwig P, et al. Is the schatzki ring a unique esophageal entity? World J Gastroenterol. 2011;17(23):2838.
40. Yeom J, Song YS, Lee WK, Oh BM, Han TR, Seo HG. Diagnosis and clinical course of unexplained dysphagia. Ann Rehabil Med. 2016;40(1):95–101. doi:10.5535/arm.2016.40.1.95
41. Ekberg O. Cervical oesophageal webs in patients with dysphagia. Clin Radiol. 1981;32(6):633–641.
42. Smith MS. Diagnosis and management of esophageal rings and webs. Gastroenterol Hepatol (N Y). 2010;6(11):701.
43. Goel A, Lakshmi CP, Bakshi SS, Soni N, Koshy S. Single-center prospective study of Plummer–Vinson syndrome. Dis Esophagus. 2016;29(7):837–841. doi:10.1111/dote.12393
44. Shauffer IA, Phillips HE, Sequeira JO. The jet phenomenon: a manifestation of esophageal web. Am J Roentgenol. 1977;129(4):747–748. doi:10.2214/ajr.129.4.747
45. Taylor AJ, Stewart ET, Dodds WJ. The esophageal” jet” phenomenon revisited. Am J Roentgenol. 1990;155(2):289–290. doi:10.2214/ajr.155.2.2115253
46. Bakari G, Benelbarhdadi I, Bahije L, El feydi Essaid A. Endoscopic treatment of 135 cases of Plummer-Vinson web: a pilot experience. Gastrointest Endosc. 2014;80(4):738–741. doi:10.1016/j.gie.2014.05.332
47. Candelario N, Tiu A. Colon adenocarcinoma presenting a Plummer-Vinson Syndrome. Am J Med. 2018;131:e461–e462. doi:10.1016/j.amjmed.2018.05.017
48. Owen RD. The problem of hypopharyngeal carcinoma [Abridged]. Proc R Soc Med. 1950;43:157–170.
49. Shamma’a MH, Benedict EB. Esophageal webs: a report of 58 cases and an attempt at classification. N Eng J Med. 1958;259(8):378–384. doi:10.1056/NEJM195808212590805
50. Rashid Z, Kumar A, Komar M. Plummer-Vinson syndrome and postcricoid carcinoma: late complications of unrecognized celiac disease. Am J Gastroenterol. 1999;94(7):1991. doi:10.1111/j.1572-0241.1999.01653.x
51. Tahara T, Shibata T, Okubo M, et al. A case of Plummer-Vinson syndrome showing rapid improvement of Dysphagia and esophageal web after two weeks of iron therapy. Case Rep Gastroenterol. 2014;8(2):211–215. doi:10.1159/000364820
52. Sugiura Y, Nakagawa M, Hashizume T, Nemoto E, Kaseda S. Iron supplementation improved dysphagia related to Plummer–Vinson Syndrome. Keio J Med. 2015;64(3):48–50. doi:10.2302/kjm.2014-0011-CR
53. Bredenkamp JK, Castro DJ, Mickel RA. Importance of iron repletion in the management of Plummer-Vinson syndrome. Ann Otol Rhinol Laryngol. 1990;99(1):51–54. doi:10.1177/000348949009900109
54. Fall F, Gning SB, Ndiaye AR, et al. The Plummer-Vinson syndrome: a retrospective study of 50 cases [Le syndrome de Plummer-Vinson: étude rétrospective de 50 cas]. J Afr Hépato-Gastroentérol. 2011;5(4):259–263. doi:10.1007/s12157-011-0340-9
55. Okamura H, Tsutsumi S, Inaki S, Mori T. Esophageal web in Plummer‐Vinson syndrome. Laryngoscope. 1988;98(9):994–998. doi:10.1288/00005537-198809000-00014
56. Hefaiedh R, Boutreaa Y, Ouakaa-Kchaou A, et al. Plummer-Vinson syndrome. Tunis Med. 2010;88(10):721–724.
57. Krevsky B, Pusateri JP. Laser lysis of an esophageal web. Gastrointest Endosc. 1989;35(5):451–453.
58. Hoffman RM, Jaffe PE. Plummer-Vinson syndrome: a case report and literature review. Arch Intern Med. 1995;155(18):2008–2011.
59. Tørring ML, Frydenberg M, Hansen RP, Olesen F, Vedsted P. Evidence of increasing mortality with longer diagnostic intervals for five common cancers: a cohort study in primary care. Eur J Cancer. 2013;49(9):2187–2198. doi:10.1016/j.ejca.2013.01.025
60. Zhu A, Kaneshiro M, Kaunitz JD. Evaluation and treatment of iron deficiency anemia: a gastroenterological perspective. Dig Dis Sci. 2010;55(3):548–559. doi:10.1007/s10620-009-1108-6
61. Jnr GA. The effect of multidisciplinary team care on cancer management. Pan Afr Med J. 2011;9(1):20.
62. Dossett LA, Hudson JN, Morris AM, et al. The primary care provider (PCP)-cancer specialist relationship: a systematic review and mixed-methods meta-synthesis. CA Cancer J Clin. 2017;67(2):156–169. doi:10.3322/caac.21385
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
