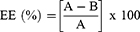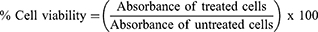Back to Journals » International Journal of Nanomedicine » Volume 19
PLGA-Chitosan Encapsulated IL-10 Nanoparticles Modulate Chlamydia Inflammation in Mice
Authors Yilma AN, Sahu R , Subbarayan P, Villinger F, Coats MT, Singh SR, Dennis VA
Received 29 July 2023
Accepted for publication 12 December 2023
Published 8 February 2024 Volume 2024:19 Pages 1287—1301
DOI https://doi.org/10.2147/IJN.S432970
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor R.D.K. Misra
Abebayehu N Yilma,1,2,* Rajnish Sahu,1,* Praseetha Subbarayan,1,* Francois Villinger,3 Mamie T Coats,4 Shree R Singh,1 Vida A Dennis1
1Center for NanoBiotechnology Research (CNBR), Alabama State University, Montgomery, AL, USA; 2Department of Public Health Sciences, Pennsylvania State University College of Medicine, Hershey, PA, USA; 3Department of Biology, University of Louisiana at Lafayette, New Iberia, LA, USA; 4Department of Clinical and Diagnostics Sciences, School of Health Professionals, The University at Alabama at Birmingham (UAB), Birmingham, AL, USA
*These authors contributed equally to this work
Correspondence: Vida A Dennis, Center for NanoBiotechnology Research (CNBR), Alabama State University, 1627 Harris Way, Montgomery, AL, USA, Tel +1 334-604-9205, Email [email protected]
Introduction: Interleukin-10 (IL-10) is a key anti-inflammatory mediator in protecting host from over-exuberant responses to pathogens and play important roles in wound healing, autoimmunity, cancer, and homeostasis. However, its application as a therapeutic agent for biomedical applications has been limited due to its short biological half-life. Therefore, it is important to prolong the half-life of IL-10 to replace the current therapeutic application, which relies on administering large and repeated dosages. Therefore, not a cost-effective approach. Thus, studies that aim to address this type of challenges are always in need.
Methods: Recombinant IL-10 was encapsulated in biodegradable nanoparticles (Poly-(Lactic-co-Glycolic Acid) and Chitosan)) by the double emulsion method and then characterized for size, surface charge, thermal stability, cytotoxicity, in vitro release, UV–visible spectroscopy, and Fourier Transform-Infrared Spectroscopy as well as evaluated for its anti-inflammatory effects. Bioactivity of encapsulated IL-10 was evaluated in vitro using J774A.1 macrophage cell-line and in vivo using BALB/c mice. Inflammatory cytokines (IL-6 and TNF-α) were quantified from culture supernatants using specific enzyme-linked immunosorbent assay (ELISA), and significance was analyzed using ANOVA.
Results: We obtained a high 96% encapsulation efficiency with smooth encapsulated IL-10 nanoparticles of ~100– 150 nm size and release from nanoparticles as measurable to 22 days. Our result demonstrated that encapsulated IL-10 was biocompatible and functional by reducing the inflammatory responses induced by LPS in macrophages. Of significance, we also proved the functionality of encapsulated IL-10 by its capacity to reduce inflammation in BALB/c mice as provoked by Chlamydia trachomatis, an inflammatory sexually transmitted infectious bacterium.
Discussion: Collectively, our results show the successful IL-10 encapsulation, slow release to prolong its biological half-life and reduce inflammatory cytokines IL-6 and TNF production in vitro and in mice. Our results serve as proof of concept to further explore the therapeutic prospective of encapsulated IL-10 for biomedical applications, including inflammatory diseases.
Keywords: Chlamydia, delivery system, chitosan, nanoparticles, IL-10, inflammation
Introduction
Acute or chronic inflammation is recognized as a major trigger of multiple diseases causing havoc in humans, including infectious pathogens, cardiovascular, arthritis, diabetes, and cancer, to name a few. Inflammation sets the motion for the chemoattractant of immune cells into tissues. Acute inflammation can occur due to infectious pathogens or tissue injury, followed by activating leukocytes and secretion of reactive oxygen species (ROS) to eradicate pathogens or control inflammation in the injurious tissue. As underscored by many human diseases, failure to control acute inflammation can result in chronic inflammation.1–3
Immune response cells (ie, monocytes/macrophages, granulocytes, and natural killer cells), once exposed to external stimuli, participate in inflammatory outcomes. In addition, during a tissue injury or infection, resident macrophages are activated in response to components of dead pathogens, damaged cells, or debris to cause diverse reactions in the inflamed host by secreting devastating proinflammatory mediators such as ROS and proteases, which can damage other neighboring cells.4 Several inflammatory mediators mediate the inflammation process,5,6 chief amongst which interleukin (IL)-1 (α and β), tumor necrosis factor-alpha (TNF-α), IL-6, and IL-8 are involved in acute inflammation in animals due to bacterial and viral infections.7 Thus, a need exists for anti-inflammatory therapeutics to prevent and treat inflammatory diseases.8–11 Wang et al 2018 showed that nanocomposites of collagen and silica-containing IL-10 inhibited proinflammatory cytokines and promoted wound-healing cytokines.12
IL-10 is secreted by various cells, comprising, but not limited, to lymphocytes, monocytes, and macrophages, in response to diverse stimuli.13 IL-10 displays multiple biological activities that modulate the expression of numerous inflammatory mediators,14 suppress monocytes/macrophages functional activities,15–17 and exert immune-stimulatory action on T-18 and B-lymphocytes.19 Thus, IL-10 has robust anti-inflammatory effects, which have been explored against various infection models for possible therapeutic purposes.13,20
It is well known that IL-10 is immunoregulatory and has been proposed for usage in vaccines,21 treatment of allergies,22 infectious diseases,23 as well as acute and chronic inflammatory diseases.20 Producing cytokines on a large scale is laborious and expensive and is prone to denaturation thus losing biological activity, which directly results in a shortened half-life. Therefore, developing strategic delivery systems to effectively deliver therapeutics at a minimal dose but provide optimum efficacy is warranted to advance preclinical testing of therapeutics. Polymeric nanoparticles are promising encapsulation delivery platform for molecules, such as proteins, to minimize premature denaturation concomitant with an effective release dose for a prolonged time period.24–26
Chlamydia trachomatis (Ct) is a bacterium that causes sexually transmitted diseases in developed countries and blindness in developing countries.1,2,27 Evidence suggests that blindness (also known as trachoma) and reproductive sequelae by Ct infections ensue from inflammation-induced pathology.16,17 Ct infection can cause severe and persistent inflammation with necrosis, epithelium damage, and the formation of scars.28 As the infection persists, macrophages and other cells from innate and adaptive immune systems are continuously recruited to halt inflammation.29,30 Consequences of Ct infection appear after many years in many cases, ultimately leading to blindness and infertility in infected hosts.31
Recently, we encapsulated IL-10 in PEGylated poly lactic acid (PLA) and showed that it modulated inflammatory responses induced by Ct in vitro.32 Here, we chose Poly(Lactic-co-Glycolic) (PLGA) and chitosan polymers due to their biocompatibility and bio-adhesive properties that are ideally suited for nano-encapsulation and delivery. Such properties allow the accumulation of biomaterials in intimate contact with the epithelium and mucosal membranes. Previously, PLGA was shown to deliver biomaterials for therapeutic applications.33 Similarly, chitosan was also investigated as a delivery vehicle for various biomaterials for possible applications in medicine.34–36 It is clear that the use of these polymers can simplify the delivery system, especially in delivering enzymatically sensitive proteins such as IL-10 to their target sites such as the genital tract to control Ct-induced inflammation.
Herein, we aimed to encapsulate IL-10 in polymeric nanoparticles to develop an effective anti-inflammatory therapeutic delivery system for mucosal pathogens and other diseases of inflammatory etiologies. Our hypothesis is that nano-encapsulation of IL-10 will extend its biological half-life while bolstering its anti-inflammatory actions as an immunotherapeutic prospective for biomedical applications. To test this hypothesis, IL-10 was encapsulated in PLGA-chitosan nanoparticles and characterized and then evaluated for therapeutic bioactivity using mouse macrophages in vitro and BALB/c mice infected with Ct. Here, our results are discussed emphasizing the broader applicability of nano-encapsulated IL-10 for controlling many infectious and non-infectious inflammatory diseases.
Materials and Methods
Materials
PLGA 85/15 (lactic: glycolic acid ratio), chitosan, polyvinyl alcohol (PVA), concanavalin-A (ConA) and lipopolysaccharide (LPS) were obtained from Sigma Aldrich (St. Louis, MO). Mouse J774A.1 macrophages and Chlamydia muridarum (Cm) MoPn Nigg II were purchased from American Type Culture Collection (Manassas, VA). Fetal bovine serum (FBS), Dulbecco’s modified eagle medium (DMEM), and antibiotic-antimycotic were from Life Technologies (Carlsbad, CA). Mouse IL-6 and TNF OptEIA sets were from BD-Biosciences (San Diego, CA). Monkey recombinant IL-10 (rIL-10) was obtained as previously published by us.37 The CellTiter 96 MTT (3-(4,5-Dimethyliazol-2-yl)-2,5-diphenyltetrazoium bromide) Cell Proliferation Assay kit was from Promega (Madison, WI). Dichloromethane (DCM) was obtained from Fisher Scientific (Pittsburgh, PA).
Nanoparticles Fabrication
rIL-10 was encapsulated in PLGA-chitosan nanoparticles (PLGA+chitosan+IL-10) by using W/O/W double emulsion technique, as previously reported by us38–41 (depicted in Figure 1). Briefly, The PLGA (1% w/v) was dissloved in dichloromethane and rIL-10 (250 ug) was added and vortexed and homogenized. The emulsion was then added to PVA (1% aqueous solution) and stirred overnight at room temperature to allow evaporation of organic solvent. Nanoparticles were collected by centrifugation and washed with phosphate buffered saline (PBS). Chitosan coating was apllied to nanoparticles by resuspending in purified chitosan (1% w/v in 0.01% acetic acid solution) and stirred for 30 minutes. Nanoparticles were collected by centrifugation and washed with water. Nanoparticles were resuspended in 5% trehalose to serve as a stabilizer were lyophilized and stored in a sealed container at −80°C. PLGA-chitosan nanoparticles encapsulating phosphate buffered saline (PBS) served as a negative control (PLGA+chitosan+PBS).
Encapsulation Efficiency (EE)
The percent EE was determined as reported previously.37,39–42 Briefly, 20 mg of PLGA+chitosan+IL-10 nanoparticles were mixed with NaOH and sodium dodecyl-sulfate (SDS) overnight on a rocker at room temperature (RT). Nanoparticles were centrifuged, and supernatants were obtained to measure the released IL-10 using the microBCA protein kit (ThermoFisher, Waltham, MA). EE calculation is shown below with total IL-10 (A) and non-encapsulated IL-10 (B).
Ultra Violet (UV)-Visible Spectroscopy
Nanoparticles and rIL-10 for UV–vis spectroscopy were suspended in deionized water (0.5 mg/mL), and the visible spectra were acquired using a spectrophotometer Beckman DU800 (Beckman Coulter, Brea, CA, USA) as previously described.37,39,41,42
IL-10 Release from Encapsulated Nanoparticles
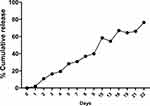
The release of IL-10 from nanoparticles was exactly as we reported.37,39,41,42 Briefly, 5 mg PLGA+chitosan+IL-10 or PLGA+chitosan+PBS nanoparticles were suspended in 500 µL of PBS (pH 7.4) and 0.1% sodium azide and incubated at 37°C for 1, 2, 4 and 6 hr, and then 1–22 days. The supernatants were collected by centrifugation (14000 rpm, 20 min, 4 °C) and stored at −80 °C until used. Protein concentration in the supernatants was determined by MicroBCA protein assay, and PLGA-chitosan+PBS was used for substracting the background reading. The data are shown as a percent (%) cumulative release for 22 days.
Characterization of Nanoparticles
Nanoparticles were suspended in deionized water to measure the zeta-sizing and zeta-potential using Nano-ZS (Malvern Instruments Ltd., Malvern, UK) and reported as the mean size in diameter (nm) and zeta-potential as (mV).37,39–42 Scanning electron microscopy (SEM) was employed to determine the morphology and size of encapsulated nanoparticles according to our published procedures.37,39,41,42 Fourier-transform infrared spectroscopy (FT-IR) was employed to acquire the spectra of nanoparticles as published.41,42 For the Differential Scanning Calorimetry (DSC), samples were heated at the rate of 20°C per minutes from 20°C to 150°C under nitrogen and then cooled at 54% relative humidity and the melting point process was assessed by DSC as described.37,39,41
Toxicity of Nanoparticles to Cells
The cytotoxicity of nanoparticles was measured using the MTT assay. Mouse J774A.1 macrophages were grown in complete DMEM media (glutamine, heat-inactivated FBS and antibiotic and antimycotic) as described previously.11,37,40–42 Briefly, each well of a 96-well plate was seeded with 1×105 viable cells and incubated in a humidified 37°C incubator. PLGA+chitosan+IL-10 nanoparticles (0–1000 ng/mL) were pipetted into wells and toxicity was evaluated after 24, 48, and 72 hr. MTT dye was added at each time point and incubated in a humidified 37°C incubator for 1 hour to allow formation of formazan crystals. After the incubation, solubilization/stop solution was added to dissolve the formazan crystals and absorbance was recorded at 570 nm using a micro-well plate reader (BioTek Epoch). PLGA+chitosan+PBS nanoparticles were used negative control. Cells alone were used as positive control to compute the % cell viability as follows:
Bioactivity of Nano-Encapsulated IL-10 in vitro
The bioactivity of nano-encapsulated IL-10 in vitro was investigated by exposing murine J774A.1 macrophages to LPS, a potent inducer of proinflammatory mediators in macrophages.43 Cells (106 /well) were propagated in 12-well plates using DMEM and incubated at 37°C. After 24 hrs, the media were replaced with DMEM containing nanoparticles at concentrations of 0–1000 ng/mL with and without LPS (1 µg/mL) and incubated for 24, 48 or 72 hrs. Culture supernatants collected after centrifugation were used to quantify cytokine.
Bioactivity of Nano-Encapsulated IL-10 in vivo
Six- to eight-week-old female BALB/c mice (Charles River Laboratories, Wilmington, MA) studies were approved by the Institutional Animal Care and Use Committee (IACUC) at Alabama State University and under the guidelines of Office of Laboratory Animal Welfare (OLAW). Mice were housed under standard pathogen-free environmental conditions at ambient temperatures of 25°C and provided with sterile food and water ad libitum. Three groups of mice (n=5) were used to determine the in vivo bioactivity of the IL-10 encapsulated nanoparticles. The groups were as follows: PBS, PLGA+chitosan+IL-10 and PLGA+chitosan+PBS. All animals were injected subcutaneously38–40,42,44 on day 0 and 7 with the nanoparticles 2 mg of encapsulated IL-10 (containing 2 µg of IL-10), and 2 mg of encapsulated PBS. Mice injected with sterile PBS served as the controls. With the exception of the PBS group, all other groups were infected intravaginally with live Cm (105 inclusion forming unit; IFU/30µL/mouse) on day 14. The animals were sacrificed on day 28 and spleen cells were collected for subsequent in vitro stimulation. Viable spleen cells (3 × 106 cells/mL) were stimulated with ConA (5 µg/mL) as positive control, the recombinant major outer membrane protein (rMOMP) of Cm (20 µg/mL) or live Cm (3 × 105 IFU/mL) and incubated for 24 and 48 hr at 37°C with 5% CO2. Supernatants from cell cultures were used to measure cytokines as described below.
Cytokines Measurement
Cytokines (IL-6 and TNF) were quantified from culture supernatants using specific enzyme-linked immunosorbent assay (ELISA) as described previously.11,32,37,38,40–42,44,45 Samples were run in triplicates and repeated at least twice.
Statistical Analysis
Comparisons between various concentrations, and different time intervals were performed by the single-factor Analysis of Variance (ANOVA) followed by Sidak’s multiple-test comparisons test to compare IL-6 and TNF secretion levels using GraphPad Prism 10 (San Diego, CA, USA). The significance was considered at P < 0.05.
Results
EE and IL-10 Release from Encapsulated Nanoparticles
The encapsulation method yielded ~96% EE of IL-10 within nanoparticles. We observed a sustained IL-10 cumulative slow-release pattern over 22 days (Figure 2), suggesting that smaller concentrations of IL-10 have the possibility of being released at the in vivo target sites.
UV-Visible Spectra of Nanoparticles
The burst effect and proteins absorbed on the surface of nanoparticles during fabrication process are some of the undesirable characteristics. To rule out the burst effect possibility, we acquired the comparative absorbance spectra of encapsulated IL-10 and naked IL-10. Naked IL-10 peak absorbance spectrum was at a 285 nm wavelength (spectrum for protein) (Figure 3, green line). On the contrary, encapsulated IL-10 had no protein peak absorbance, further validating complete encapsulation of IL-10 in PLGA+chitosan nanoparticles (Figure 3, blue line).
 |
Figure 3 UV visible spectra of encapsulated IL-10. Nanoparticles (5 mg dissolved with 1 mL of DNase RNA free H2O) and 2 mg/mL of free IL-10 were analyzed by UV visible spectrum. |
Zeta Size and Zeta-Potential of Nanoparticles
The particle size is vital in determining cellular uptake. Thus, we employed zeta-sizing to assess PLGA+chitosan+PBS and PLGA+chitosan+IL-10 sizes, which were 164 and 153.9 nm, respectively (Table 1 and Supplement Figure 1). Next, we measured their zeta-potential, a parameter that affects particle stability, and observed 31.7 mV and 17.3 mV surface charge, respectively, for PLGA+chitosan+PBS and PLGA+chitosan+IL-10 (Table 1 and Supplement Figure 1) suggesting their stability. The lower polydispersity index (PDI) values of PLGA+chitosan+PBS (0.205) and PLGA+chitosan+IL-10 (0.143) were indicative of their closely uniformed nanoparticle sizes (Table 1 and Supplement Figure 1). Collectively, these findings suggest that the encapsulation of IL-10 and Chitosan coating did not adversely change the characteristics of nanoparticles.
 |
Table 1 Properties of Nanoparticles |
SEM Analyses of Encapsulated Nanoparticles
Given that the size of nanoparticles is critical for cellular and functional, we next employed SEM to determine the size and morphology of and size of nanoparticles. SEM images of PLGA+chitosan+PBS (Figure 4A and C) and PLGA+chitosan+IL-10 (Figure 4B and D) appeared to disclose the homogeneity and uniform particle size distribution which were spherical. The estimated size of the nanoparticles was ~100–150 nm (Figure 4).
FT-IR Analyses
Variations in chemical functional groups within PLGA, PLGA+chitosan+PBS and PLGA+chitosan+IL-10 nanoparticles (blue and red lines) were assessed by FT-IR as further validation of IL-10 encapsulation. Peak shifting (black arrow) for various functional groups was observed at ~1807, 1720, 1600, 1421, 1368, 1222, and 1080 cm−1 for nanoparticles tested (Figure 5). The positions of some peaks differed in the spectra appearance of PLGA+chitosan+IL-10 and the PLGA+chitosan+PBS nanoparticles due to encapsulation (Figure 5). The broader band at ~3300 cm−1 (purple circle and green arrow) is due to the –OH group of H2O. The peaks at ~1833, 1720, 1600, and 1421 cm−1 are the amine group of the protein (–NH and –NH). The various peaks at 1222, 1000, and 500 cm−1 are attributed to C–C of the aromatic ring. The peak ~1801 cm−1 shift (green arrow) in the PLGA+chitosan+IL-10 spectrum, indicating a chemical interaction between IL-10 and PLGA+chitosan or alternatively the entrapment of IL-10 in the PLGA+chitosan nanoparticles. Overall, the FT-IR data further justified encapsulating IL-10 in PLGA+chitosan nanoparticles.
DSC Analysis
The stability of the IL-10 in PLGA+chitosan, which could impact its release profile, was evaluated employing DSC. As depicted in Figure 6, the thermograms of the various nanoparticles show that the melting endothermic peaks for PLGA+chitosan+IL-10 and PLGA+chitosan+PBS appeared at 45°C and 50°C respectively, suggesting that the PLGA+chitosan+IL-10 nanoparticles were thermally stable. These results further show that the nano-encapsulation IL-10 is less thermostable when compared with PLGA+chitosan+PBS alone.
Cytotoxicity of Nano-Encapsulated IL-10 on Mouse J774A.1 Macrophages
A major concern when employing nanoparticles for biomedical applications is their cellular toxicity. Since macrophages are very important for innate immunity, we verified the cytotoxicity of encapsulated nanoparticles in vitro to macrophages prior to the in vivo studies. Cells were stimulated with nanoparticles (0.1 to 1 µg/mL) for 24, 48 and 72 hr to evaluate the dose and time-dependency effect. As shown, PLGA+chitosan+IL-10 had minimal adverse effect on cell viability (>80% cell viability for all concentrations and time-points tested) (Figure 7), indicating the safety of PLGA+chitosan+IL-10 in eukaryotic cells.
In vitro Bioactivity of IL-10 Nano-Encapsulation
LPS triggers the release of proinflammatory mediators from stimulated macrophages. Furthermore, LPS directly, or via cytokines, activates the transcription of many proinflammatory genes.4 Therefore, we evaluated whether nano-encapsulated IL-10 would modulate TNF production by mouse J774A.1 macrophages when stimulated with LPS. Our data demonstrate that encapsulated IL-10 significantly abrogated (P < 0.05) TNF production by macrophages at all examined time-points (Figure 8). Interestingly, the IL-10 inhibitory effect increased with time, where complete inhibition was observed at 72 hr (Figure 8), implying that IL-10 was slowly released from nanoparticles during its interaction with macrophages. Overall, this data provide resounding evidence of the immune-modulatory capacity of the nano-encapsulated IL-10 in eukaryotic cells.
In vivo Bioactivity of IL-10 Nano-Encapsulation
Several reports provide in vitro evidence that IL-10 is effective in controlling diseases with inflammatory and autoimmune etiologies including Ct.11,20 However, in vivo application of IL-10 is problematic because of its short-lived bioactivity. Thus, IL-10 must be administered continually at a very high dose. Here, we demonstrated the bioactivity of encapsulated IL-10 in vivo in controlling inflammatory responses as induced by a Ct infection in mice. Spleen cells from treated and infected animals (injected with nanoparticles and inoculated with Ct) as compared with the PBS group exhibited significant (P < 0.05 to P < 0.01) low level of IL-6 and TNF-α for all stimulants used (Con A, rMOMP and Ct) for both the 24 and 48 hr time-points (Figure 9A and B). However, animals receiving PLGA+chitosan+PBS along with Ct infection exhibited significant (P < 0.05 to P < 0.0001) IL-6 and TNF-α secretion levels in response to all stimulants (Figure 9C and D). In animals administered with PLGA+chitosan+IL-10 and Ct infection, IL-6 and TNF-α were reduced for all stimulants, but significant (P < 0.05 to P < 0.01) reduction was seen when Ct was used as a stimulant (Figure 9E and F). The data suggest that IL-10 is released in vivo and maintains its anti-inflammatory activity against cytokines induced by a Ct infection.
Discussion
The therapeutic potential could be enhanced for many pharmaceutical molecules by the development of well-characterized and reliable delivery systems. Here, we enacted the controlled release strategy for IL-10 release by using PLGA+chitosan nanoparticles. IL-10 has immunotherapeutic potential in acute inflammatory diseases, including Ct, amongst many, but it has a short biological half-life,46 thus limiting its in vivo usage. Therefore, it is of major significance to develop delivery systems to prevent its rapid degradation, facilitate its controlled release, and enhance its in vivo application. Polymeric biodegradable nanoparticles are being exploited as delivery system for a variety of biomaterials, including proteins.33–35,41,47–51 Our recent nano-encapsulation of IL-10 in PEGylated PLA provided novel information on reducing in vitro inflammation induced by chlamydial stimulants and some of its potential mechanistic actions.32 Here in this paper, to target mucosal surfaces such as the genital tract, we encapsulated IL-10 in PLGA+chitosan and evaluated its anti-inflammatory effect against Ct-induced inflammation.
The PLGA and chitosan polymers are extensively investigated for developing nano-encapsulated therapeutic materials. This is due to some inherent advantages such as controlled-release applications.34,52,53 Recently, we have shown that PLGA provides sustained release of a recombinant Ct MOMP peptide over a period of several days.39–41 Furthermore, we also reported the capacity of chitosan, a mucoadhesive polymer to serve as a delivery vehicle for a Respiratory syncytial virus (RSV) DNA vaccine.54 Our results in the current study reveal that PLGA+chitosan nanomaterials are a reliable and safe delivery platform IL-10. This platform was very effective in prolonging the short-lived bioactivity of IL-10 by sustaining its slow release concomitant with an enhanced anti-inflammatory effect in vitro and in mice.
PLGA and chitosan have been widely used for encapsulation of cytokines and other proteins. Park et al showed the encapsulation and bioactivity of IL-6 and LIF (leukemia inhibitory factor) in PLGA, ultimately affected cell growth by inhibiting differentiation.47 PLGA was also used to encapsulate a clinically relevant tumor antigen tyrosinase-related protein 2 (TRP2) where PLGA-TRP2 enhanced the bioactivity of TRP2 with EE of 87%.55 Also, successful encapsulation of IL-2 in chitosan improved humoral and cellular immunity in paratyphoid vaccination studies.50 Other investigators have shown that encapsulation of Mycobacterium tuberculosis proteins in chitosan stimulated immune responses similar to that induced by natural infections.56 IL-10 also has been encapsulated in dextrin nanogels, which provided slow release of IL-10 in vitro.57,58 Recently, Chitosan-PLGA nanoparticles were efficient to deliver a Streptococcus vaccine.59 Here we show for the first time, to our knowledge, the encapsulation of IL-10 in PLGA+chitosan nanomaterial, its characterization and successful bioactivity in vitro and in mice.
Successful encapsulation of proteins within PLGA and chitosan nanomaterials depends on the charge/molecular weight of polymers, co-emulsifier and protein size.60 The IL-10 in PLGA+chitosan nanoparticles in the present study had a 96% EE with a homogeneous morphology and smooth spherical shape. The high EE of IL-10 may have resulted from the increased sonication time of IL-10 in PLGA+chitosan, which contributed to IL-10 stability during the synthesis. EE of 89–95% and with similar morphology as observed here in our study have been reported for IL-6 and IL-2 encapsulated within PLGA and chitosan, respectively;47,50 therefore, these studies are congruent with our findings.
Our nano-encapsulated IL-10 is desirable in size to facilitate uptake by immune cells61 being of ~200 nm. Such a difference in size between the cells and nanoparticle will provide the opportunity for the nanoparticles to be used without introducing too much interference, if the IL-10 is in a stable state once encapsulated. Stability of nanoparticles is also a critical parameter for efficient delivery of biomaterials into cells. One study revealed that certain proteins encapsulated in PLGA suffered from denaturation.34 We demonstrate in the current study that encapsulated IL-10 and encapsulated PBS were thermally stable exhibiting endothermic peaks between 45°C and 60°C ensuring their stability during in vivo administration.
The release profile of biomaterials from nano-encapsulation is a predictive measure of their effective delivery to a eukaryotic system.62 Previously, Sales-Junior and colleagues reported that PLGA encapsulated SBm74632, a vaccine candidate for Boophilus microplus, exhibited a slow-release pattern for up to 37 days.63 Others have shown the small burst release of bovine serum albumin (BSA) from chitosan, and then a slow constant release with time.64 Wang et al 2018 study revealed the modulatory potential of IL-10-encapsulated nanocomposites on TNF-α and IL-1β production in a chronic wound model12 indicating the therapeutic potential of IL-10 released from nanocomposites.
The encapsulated IL-10 release profile in our study revealed that IL-10 was released at a constant rate until 21 days followed by a rapid decline. Perhaps, a longer release of IL-10 is needed to potentially limit high usage of IL-10 in vivo. It is well documented that LPS, a “microbial product” of Gram-negative bacteria, promotes the expression of major proinflammatory mediators such as TNF-α and IL-6 that are produced during the acute inflammation process.65,66 Various studies have reported that uncontrolled and excessive production of these mediators will lead to chronic inflammation to induce immune-pathological diseases.3 Herein, we observed that the nano-encapsulated IL-10 as released from nanoparticles reduced the levels of TNF-α in macrophages, as produced in response to LPS stimulation, thus providing evidence that the released IL-10 is functional by modulating inflammatory responses in macrophages. TNF-α production decreased with increased incubation time with an observed inhibition pattern of 72 > 48 >24 hr (Figure 8). One plausible reason for such inhibition may be that more IL-10 is released as the nanoparticles remain in contact with cells. Our cytotoxicity studies further support that the inhibition is due to IL-10 presence rather than cell death. Encapsulated IL-10 was relatively not toxic at concentrations as high as 1000 ng/mL (>80% cell viability). Moreover, the toxicity of the nano-encapsulated IL-10 to macrophages was not affected by time since all examined time-points exhibited similar cytotoxicity profiles, indicating the safety of this delivery system in eukaryotic systems.
Finally, we present evidence that spleen cells from mice administered with nano-encapsulated IL-10 and infected with live Cm produced lower TNF-α and IL-6 after these cells were stimulated in vitro with either Con A, live Cm or its rMOMP as compared to mice injected with PLGA+chitosan and infected with live Cm; suggesting that encapsulated IL-10 exhibited marked immunotherapeutic actions in vivo. Additionally, this anti-inflammatory effect was observed until 28 days after the initial injection of mice with the encapsulated IL-10, indicating that the half-life of IL-10 is prolonged in vivo as compared to its short-term biological half-life.57 Besides showing the in vivo anti-inflammatory activities of encapsulated IL-10, our studies show that the PLGA+chitosan delivery system prolonged the biological half-life of IL-10. Overall, our data show the functional capacity of the encapsulated IL-10 and its modulatory effects, and holds promise for IL-10 as a therapeutic agent in biomedical applications. Despite the limitation of testing only inflammatory cytokine, the study provides evidence in potential of PLGA+chitosan encapsulated-IL-10 for therapeutic application.
Conclusion
In conclusion, we show that the PLGA+chitosan nanomaterial is an effective delivery system for enhancing the short-lived IL-10 and prolonging its controlled slow release. Of significance, PLGA+chitosan encapsulated IL-10 was safe in mice and bioactive by exerting its anti-inflammatory actions against inflammatory cytokines induced by LPS and C. trachomatis. The encapsulated IL-10 described herein provides evidence for its potential application as an immunotherapeutic agent. The study lays a foundation for further studies to be conducted using encapsulated IL-10 in treating various infectious pathogens (especially mucosal pathogens due to the much-adhesiveness of chitosan) and noninfectious diseases of inflammatory nature or those with autoimmune etiologies. Furthermore, encapsulating IL-10 in PLGA+chitosan is a promising concept not only for IL-10 delivery but perhaps has a broader impact for other biomolecules of biomedical significance.
Acknowledgments
The authors acknowledge the excellent administrative assistance of Yvonne Williams and LaShaundria Lucas of CNBR, and Golden Muse (http://www.golden-muse.com/scientific-illustrations) (Figure 1 diagram). This research was supported by the National Institutes of Health under Award NIH-NIGMS-RISE (2R25GM106995-06A1), the National Science Foundation (NSF)-CREST (HRD-1241701), NSF-EiR (2200529), NSF-HBCU-RISE (HRD-1646729) and NSF-HBCU-UP (HRD1911660) grants.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Gonzalez-Chavez A, Elizondo-Argueta S, Gutierrez-Reyes G, Leon-Pedroza JI. Pathophysiological implications between chronic inflammation and the development of diabetes and obesity. Cir Cir. 2011;79(2):209–216.
2. Harvey AE, Lashinger LM, Hursting SD. The growing challenge of obesity and cancer: an inflammatory issue. Ann N Y Acad Sci. 2011;1229:45–52. doi:10.1111/j.1749-6632.2011.06096.x
3. Chen L, Deng H, Cui H, et al. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget. 2018;9(6):7204–7218. doi:10.18632/oncotarget.23208
4. Wortmann M, Skorubskaya E, Peters AS, Hakimi M, Bockler D, Dihlmann S. Necrotic cell debris induces a NF-kappaB-driven inflammasome response in vascular smooth muscle cells derived from abdominal aortic aneurysms (AAA-SMC). Biochem Biophys Res Commun. 2019;511(2):343–349. doi:10.1016/j.bbrc.2019.02.051
5. Abdulkhaleq LA, Assi MA, Abdullah R, Zamri-Saad M, Taufiq-Yap YH, Hezmee MNM. The crucial roles of inflammatory mediators in inflammation: a review. Vet World. 2018;11(5):627–635. doi:10.14202/vetworld.2018.627-635
6. Slaats J, Ten Oever J, van de Veerdonk FL, Netea MG. IL-1beta/IL-6/CRP and IL-18/ferritin: distinct inflammatory programs in infections. PLoS Pathog. 2016;12(12):e1005973. doi:10.1371/journal.ppat.1005973
7. Lin L, Curtin JA, Regis E, et al. A systems immunology approach to investigate cytokine responses to viruses and bacteria and their association with disease. Sci Rep. 2022;12(1):13463. doi:10.1038/s41598-022-16509-4
8. Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473(7347):317–325. doi:10.1038/nature10146
9. Charo IF, Taub R. Anti-inflammatory therapeutics for the treatment of atherosclerosis. Nat Rev Drug Discov. 2011;10(5):365–376. doi:10.1038/nrd3444
10. Delbaere Q, Chapet N, Huet F, et al. Anti-inflammatory drug candidates for prevention and treatment of cardiovascular diseases. Pharmaceuticals. 2023;16(1):78. doi:10.3390/ph16010078
11. Yilma AN, Singh SR, Fairley SJ, Taha MA, Dennis VA. The anti-inflammatory cytokine, interleukin-10, inhibits inflammatory mediators in human epithelial cells and mouse macrophages exposed to live and UV-inactivated chlamydia trachomatis. Mediators Inflamm. 2012;2012:520174. doi:10.1155/2012/520174
12. Wang X, Coradin T, Helary C. Modulating inflammation in a cutaneous chronic wound model by IL-10 released from collagen-silica nanocomposites via gene delivery. Biomater Sci. 2018;6(2):398–406. doi:10.1039/c7bm01024a
13. Saraiva M, Vieira P, O’Garra A. Biology and therapeutic potential of interleukin-10.. J Exp Med. 2020;217(1). doi:10.1084/jem.20190418
14. Trifunovic J, Miller L, Debeljak Z, Horvat V. Pathologic patterns of interleukin 10 expression–a review. Biochem Med. 2015;25(1):36–48. doi:10.11613/BM.2015.004
15. Watanabe S, Alexander M, Misharin AV, Budinger GRS. The role of macrophages in the resolution of inflammation. J Clin Invest. 2019;129(7):2619–2628. doi:10.1172/JCI124615
16. Minton K. IL-10 targets macrophage metabolism. Nat Rev Immunol. 2017;17(6):345. doi:10.1038/nri.2017.57
17. Mittal SK, Cho KJ, Ishido S, Roche PA. Interleukin 10 (IL-10)-mediated Immunosuppression: MARCH-I INDUCTION REGULATES ANTIGEN PRESENTATION BY MACROPHAGES BUT NOT DENDRITIC CELLS. J Biol Chem. 2015;290(45):27158–27167. doi:10.1074/jbc.M115.682708
18. Perez-Hernandez J, Chiurchiu V, Perruche S, You S. Regulation of T-cell immune responses by pro-resolving lipid mediators. Front Immunol. 2021;12:768133. doi:10.3389/fimmu.2021.768133
19. de Gruijter NM, Jebson B, Rosser EC. Cytokine production by human B cells: role in health and autoimmune disease. Clin Exp Immunol. 2022;210(3):253–262. doi:10.1093/cei/uxac090
20. Wang X, Wong K, Ouyang W, Rutz S. Targeting IL-10 family cytokines for the treatment of human diseases. Cold Spring Harb Perspect Biol. 2019;11:2.
21. Silva JR, Sales NS, Silva MO, et al. Expression of a soluble IL-10 receptor enhances the therapeutic effects of a papillomavirus-associated antitumor vaccine in a murine model. Cancer Immunol Immunother. 2019;68(5):753–763. doi:10.1007/s00262-018-02297-2
22. Wang SB, Deng YQ, Ren J, Xiao BK, Liu Z, Tao ZZ. Exogenous interleukin-10 alleviates allergic inflammation but inhibits local interleukin-10 expression in a mouse allergic rhinitis model. BMC Immunol. 2014;15(1):9. doi:10.1186/1471-2172-15-9
23. Rojas JM, Avia M, Martin V, Sevilla N. IL-10: a multifunctional cytokine in viral infections. Review J Immunol Res. 2017;2017:6104054. doi:10.1155/2017/6104054
24. Sung YK, Kim SW. Recent advances in polymeric drug delivery systems. Biomater Res. 2020;24(1):12. doi:10.1186/s40824-020-00190-7
25. Leonard M, De Boisseson MR, Hubert P, Dalencon F, Dellacherie E. Hydrophobically modified alginate hydrogels as protein carriers with specific controlled release properties. J Control Release. 2004;98(3):395–405. doi:10.1016/j.jconrel.2004.05.009
26. McClements DJ. Encapsulation, protection, and delivery of bioactive proteins and peptides using nanoparticle and microparticle systems: a review. Adv Colloid Interface Sci. 2018;253:1–22. doi:10.1016/j.cis.2018.02.002
27. Maskrey BH, Megson IL, Whitfield PD, Rossi AG. Mechanisms of resolution of inflammation: a focus on cardiovascular disease. Arterioscler Thromb Vasc Biol. 2011;31(5):1001–1006. doi:10.1161/ATVBAHA.110.213850
28. Xiang W, Yu N, Lei A, et al. Insights into host cell cytokines in chlamydia infection. Front Immunol. 2021;12:639834. doi:10.3389/fimmu.2021.639834
29. Redpath SA, Fonseca NM, Perona-Wright G. Protection and pathology during parasite infection: IL-10 strikes the balance. Parasite Immunol. 2014;36(6):233–252. doi:10.1111/pim.12113
30. Kumar R, Ng S, Engwerda C. The role of IL-10 in Malaria: a double edged sword review. Front Immunol. 2019;10:229. doi:10.3389/fimmu.2019.00229
31. Yilma AN, Singh SR, Dixit S, Dennis VA. Anti-inflammatory effects of silver-polyvinyl pyrrolidone (Ag-PVP) nanoparticles in mouse macrophages infected with live Chlamydia trachomatis. Int J Nanomed. 2013;8:2421–2432. doi:10.2147/IJN.S44090
32. Duncan SA, Sahu R, Dixit S, Singh SR, Dennis VA. Suppressors of Cytokine Signaling (SOCS)1 and SOCS3 proteins are mediators of interleukin-10 modulation of inflammatory responses induced by Chlamydia muridarum and Its Major Outer Membrane Protein (MOMP) in mouse J774 macrophages. Mediators Inflamm. 2020;2020:7461742. doi:10.1155/2020/7461742
33. Pirooznia N, Hasannia S, Lotfi AS, Ghanei M. Encapsulation of alpha-1 antitrypsin in PLGA nanoparticles: in vitro characterization as an effective aerosol formulation in pulmonary diseases. J Nanobiotechnology. 2012;10:20. doi:10.1186/1477-3155-10-20
34. Jarudilokkul S, Tongthammachat A, Boonamnuayvittaya V. Preparation of chitosan nanoparticles for encapsulation and release of protein. Korean J Chem Eng. 2011;28(5):1247–1251. doi:10.1007/s11814-010-0485-z
35. Amidi M, Mastrobattista E, Jiskoot W, Hennink WE. Chitosan-based delivery systems for protein therapeutics and antigens. Adv Drug Deliv Rev. 2010;62(1):59–82. doi:10.1016/j.addr.2009.11.009
36. de Sousa Victor R, Marcelo da Cunha Santos A, Viana de Sousa B, de Araujo Neves G, Navarro de Lima Santana L, Rodrigues Menezes R. A review on chitosan’s uses as biomaterial: tissue engineering, drug delivery systems and cancer treatment. Materials. 2020;13:21.
37. Duncan SA, Dixit S, Sahu R, et al. Prolonged Release and Functionality of Interleukin-10 Encapsulated within PLA-PEG Nanoparticles. Nanomaterials. 2019;9:8.
38. Sahu R, Dixit S, Verma R, et al. Encapsulation of Recombinant MOMP in Extended-Releasing PLGA 85:15 nanoparticles confer protective immunity against a chlamydia muridarum genital challenge and re-challenge. Original Res Front Immunol. 2021;12(1197):660932. doi:10.3389/fimmu.2021.660932
39. Sahu R, Dixit S, Verma R, et al. A nanovaccine formulation of Chlamydia recombinant MOMP encapsulated in PLGA 85:15 nanoparticles augments CD4(+) effector (CD44(high) CD62L(low)) and memory (CD44(high) CD62L(high)) T-cells in immunized mice. Nanomedicine. 2020;29:102257. doi:10.1016/j.nano.2020.102257
40. Fairley SJ, Singh SR, Yilma AN, et al. Chlamydia trachomatis recombinant MOMP encapsulated in PLGA nanoparticles triggers primarily T helper 1 cellular and antibody immune responses in mice: a desirable candidate nanovaccine. Int J Nanomed. 2013;8:2085–2099. doi:10.2147/IJN.S44155
41. Taha MA, Singh SR, Dennis VA. Biodegradable PLGA85/15 nanoparticles as a delivery vehicle for Chlamydia trachomatis recombinant MOMP-187 peptide. Nanotechnology. 2012;23(32):325101. doi:10.1088/0957-4484/23/32/325101
42. Dixit S, Singh SR, Yilma AN, Agee RD, Taha M, Dennis VA. Poly(lactic acid)-poly(ethylene glycol) nanoparticles provide sustained delivery of a Chlamydia trachomatis recombinant MOMP peptide and potentiate systemic adaptive immune responses in mice. Nanomedicine. 2014;10(6):1311–1321. doi:10.1016/j.nano.2014.02.009
43. Tucureanu MM, Rebleanu D, Constantinescu CA, et al. Lipopolysaccharide-induced inflammation in monocytes/macrophages is blocked by liposomal delivery of G(i)-protein inhibitor. Int J Nanomed. 2018;13:63–76. doi:10.2147/IJN.S150918
44. Verma R, Sahu R, Dixit S, et al. The Chlamydia M278 major outer membrane peptide encapsulated in the poly(lactic acid)-Poly(ethylene glycol) nanoparticulate self-adjuvanting delivery system protects mice against a chlamydia muridarum genital tract challenge by stimulating robust systemic and local mucosal immune responses. Front Immunol. 2018;9:2369. doi:10.3389/fimmu.2018.02369
45. Dixit S, Sahu R, Verma R, et al. Caveolin-mediated endocytosis of the Chlamydia M278 outer membrane peptide encapsulated in poly(lactic acid)-Poly(ethylene glycol) nanoparticles by mouse primary dendritic cells enhances specific immune effectors mediated by MHC class II and CD4(+) T cells. Biomaterials. 2018;159:130–145. doi:10.1016/j.biomaterials.2017.12.019
46. Saxena A, Khosraviani S, Noel S, Mohan D, Donner T, Hamad AR. Interleukin-10 paradox: a potent immunoregulatory cytokine that has been difficult to harness for immunotherapy. Research support, N.I.H., Extramural Research Support, Non-U.S. Gov’t Review. Cytokine. 2015;74(1):27–34. doi:10.1016/j.cyto.2014.10.031
47. Park J, Gao W, Whiston R, Strom TB, Metcalfe S, Fahmy TM. Modulation of CD4+ T lymphocyte lineage outcomes with targeted, nanoparticle-mediated cytokine delivery. Mol Pharm. 2011;8(1):143–152. doi:10.1021/mp100203a
48. Akagi T, Baba M, Akashi M. Biodegradable Nanoparticles as Vaccine Adjuvants and Delivery Systems: regulation of Immune Responses by Nanoparticle-Based Vaccine. In: Kunugi S, Yamaoka T, editors. Polymers in Nanomedicine. Springer Berlin Heidelberg; 2011.
49. Han J, Zhao D, Li D, Wang X, Jin Z, Zhao K. Polymer-based nanomaterials and applications for vaccines and drugs. Polymers. 2018;10(1):31. doi:10.3390/polym10010031
50. Yang Y, Chen J, Li H, et al. Porcine interleukin-2 gene encapsulated in chitosan nanoparticles enhances immune response of mice to piglet paratyphoid vaccine. Comp Immunol Microbiol Infect Dis. 2007;30(1):19–32. doi:10.1016/j.cimid.2006.09.006
51. Elmowafy EM, Tiboni M, Soliman ME. Biocompatibility, biodegradation and biomedical applications of poly(lactic acid)/poly(lactic-co-glycolic acid) micro and nanoparticles. J Pharm Invest. 2019;49(4):347–380. doi:10.1007/s40005-019-00439-x
52. Adepu S, Ramakrishna S. Controlled drug delivery systems: current status and future directions. Molecules. 2021;26:19.
53. Kamaly N, Yameen B, Wu J, Farokhzad OC. Degradable controlled-release polymers and polymeric nanoparticles: mechanisms of controlling drug release. Chem Rev. 2016;116(4):2602–2663. doi:10.1021/acs.chemrev.5b00346
54. Boyoglu S, Vig K, Pillai S, et al. Enhanced delivery and expression of a nanoencapsulated DNA vaccine vector for respiratory syncytial virus. Nanomedicine. 2009;5(4):463–472. doi:10.1016/j.nano.2009.02.004
55. Hamdy S, Molavi O, Ma Z, et al. Co-delivery of cancer-associated antigen and Toll-like receptor 4 ligand in PLGA nanoparticles induces potent CD8+ T cell-mediated anti-tumor immunity. Vaccine. 2008;26(39):5046–5057. doi:10.1016/j.vaccine.2008.07.035
56. Verma AK, Pandey RP, Chanchal A, Siddiqui I, Sharma P. Encapsulation of antigenic secretory proteins of mycobacterium tuberculosis in biopolymeric nanoparticles for possible aerosol delivery system. J Bionanosci. 2011;5(1):88–95. doi:10.1166/jbns.2011.1042
57. Carvalho V, Castanheira P, Madureira P, et al. Self-assembled dextrin nanogel as protein carrier: controlled release and biological activity of IL-10. Biotechnol Bioeng. 2011;108(8):1977–1986. doi:10.1002/bit.23125
58. Carvalho V, Castanheira P, Faria TQ, et al. Biological activity of heterologous murine interleukin-10 and preliminary studies on the use of a dextrin nanogel as a delivery system. Int J Pharm. 2010;400(1–2):234–242. doi:10.1016/j.ijpharm.2010.08.040
59. Kole S, Shin SM, Kwak IS, Cho SH, Jung SJ. Efficacy of Chitosan-PLGA encapsulated trivalent oral vaccine against viral haemorrhagic septicemia virus, Streptococcus parauberis, and Miamiensis avidus in olive flounder (Paralichthys olivaceus). Fish Shellfish Immunol. 2022;127:843–854. doi:10.1016/j.fsi.2022.07.029
60. Zielinska A, Carreiro F, Oliveira AM, et al. Polymeric nanoparticles: production, characterization, toxicology and ecotoxicology. Molecules. 2020;25:16.
61. Gustafson HH, Holt-Casper D, Grainger DW, Ghandehari H. Nanoparticle uptake: the phagocyte problem. Nano Today. 2015;10(4):487–510. doi:10.1016/j.nantod.2015.06.006
62. Zhang J, Saltzman M. Engineering biodegradable nanoparticles for drug and gene delivery. Chem Eng Prog. 2013;109(3):25–30.
63. Sales-Junior PA, Guzman F, Vargas MI, et al. Use of biodegradable PLGA microspheres as a slow release delivery system for the Boophilus microplus synthetic vaccine SBm7462. Vet Immunol Immunopathol. 2005;107(3–4):281–290. doi:10.1016/j.vetimm.2005.05.004
64. Aranaz I, Alcantara AR, Civera MC, et al. Chitosan: an overview of its properties and applications. Polymers. 2021;13:19.
65. Hirano T. IL-6 in inflammation, autoimmunity and cancer. Int Immunol. 2021;33(3):127–148. doi:10.1093/intimm/dxaa078
66. Mbongue JC, Vanterpool E, Firek A, Langridge WHR. Lipopolysaccharide-induced immunological tolerance in monocyte-derived dendritic cells. Immuno. 2022;2(3):482–500.
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.