Back to Journals » International Journal of Nanomedicine » Volume 18
Platelet-Based Nanoparticles with Stimuli-Responsive for Anti-Tumor Therapy
Authors Yang L, Zhang K, Zheng D, Bai Y, Yue D, Wu L, Ling H, Ni S, Zou H, Ye B, Liu C, Deng Y, Liu Q, Li Y, Wang D
Received 21 August 2023
Accepted for publication 25 October 2023
Published 6 November 2023 Volume 2023:18 Pages 6293—6309
DOI https://doi.org/10.2147/IJN.S436373
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor R.D.K. Misra
Linlan Yang,1,* Kaijiong Zhang,2,* Dongming Zheng,1 Yuxin Bai,1 Daifan Yue,1 Lichun Wu,2 Han Ling,2 Sujiao Ni,2 Haimin Zou,2 Bo Ye,2 Chang Liu,2 Yao Deng,2 Qiancheng Liu,3 Yan Li,1 Dongsheng Wang1,2
1College of Medical Technology, Chengdu University of Traditional Chinese Medicine, Chengdu, People’s Republic of China; 2Department of Clinical Laboratory, Sichuan Clinical Research Center for Cancer, Sichuan Cancer Hospital & Institute, Sichuan Cancer Center, Affiliated Cancer Hospital of University of Electronic Science and Technology of China, Chengdu, People’s Republic of China; 3Department of Clinical Laboratory of Mianyang People’s Hospital, Mianyang, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Dongsheng Wang, Department of Clinical Laboratory, Sichuan Clinical Research Center for Cancer, Sichuan Cancer Hospital & Institute, Sichuan Cancer Center, Affiliated Cancer Hospital of University of Electronic Science and Technology of China, Chengdu, People’s Republic of China, Email [email protected] Yan Li, College of Medical Technology, Chengdu University of Traditional Chinese Medicine, Chengdu, People’s Republic of China, Email [email protected]
Abstract: In addition to hemostasis and coagulation, years of studies have proved that platelets are involved in the whole process of tumor progression, including tumor invasion, intravasation, extravasation, and so on. It means that this property of platelets can be used in anti-tumor therapy. However, traditional platelet-based antitumor drugs often cause autologous platelet damage due to lack of targeting, resulting in serious side effects. Therefore, the researchers designed a variety of anti-tumor drug delivery systems based on platelets by targeting platelets or platelet membrane coating. The drug delivery systems have special response modes, which is crucial in the design of nanoparticles. These modes enhance the targeting and improve the anti-tumor effect. Here, we present a review of recent discoveries in the field of the crosstalk between platelets and tumors and the progress of platelet-based anti-tumor nanoparticles.
Keywords: platelet, tumor, targeted platelet, platelet membranes, stimuli-responsive drug delivery system, nanoparticle
Introduction
After the discovery of platelets in 1865, these anucleated cells once regarded as metabolic fragments of megakaryocytes, have progressively emerged as a compelling subject of research.1 Extensive investigations over the years have yielded greater insights into the functions of platelets, particularly their interaction with tumor development.2 The interaction between tumors and platelets serves as a prerequisite for hematogenous metastasis of tumors.3 Upon entering the bloodstream, tumor cells are the first to recruit and activate platelets, which subsequently facilitate the completion of the invasion-metastasis cascade by supporting the tumor cells.3,4 The study of tumor-platelet interactions plays a pivotal role in the development of platelet-associated anti-tumor drugs, while identifying the key targets between these entities offers valuable insights for anti-tumor therapy.5,6 However, common platelet inhibitors such as aspirin, ticlopidine, clopidogrel and prasugrel can attack platelets indifferently in vivo, causing autologous platelet damage and bleeding.7
Obviously, the defect of traditional platelet inhibitors is related to their lack of targeting. To this end, researchers designed nano-drug delivery systems. Drugs are carried in the shell assembled by excipients and modified by targeted peptides, so that the nano-drug delivery system can be actively or passively delivered to the target organ or target tissue. The nano-drug delivery system uses targeting and strong permeability retention effect to enrich the drug in the target organ / tissue, which not only enhances the anti-tumor effect, but also reduces the systemic side effects of the drug.8 Inspired by this, some teams designed platelet-based drug delivery systems using the natural targeting of platelets to tumors such as (i) targeted platelet nanoparticles and (ii) platelet membrane-coated anti-tumor nanoparticles. Nevertheless, Shortcomings such as uncontrolled release of the drug make this antineoplastic drug do not achieve the desired effect.9
The development of stimuli-responsive nano-drug delivery system can solve the problem of random drug release in the complex background of human body. On the basis of the original nano-drug delivery system, the research teams add related peptides, photothermal agents, sound sensitizers and other substances to the system, or use materials that can be degraded by oxides, acids and other substances to make the shell of the system. Then the drug delivery system was stimulated by a variety of special conditions in vivo and in vitro (in vivo factors: enzyme, ROS, pH, H2O2, glutathione (GSH), etc., and in vitro factors: temperature, light and ultrasound (US), etc.) to unload the drug in specific release sites such as tumors.
Interaction Between Tumor and Platelets
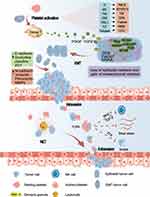
The interaction between tumor and platelet in vivo has been widely studied, and a large number of studies have found that platelets play a key role in the process of tumor invasion, intravasation and extravasation. Firstly, platelets are activated by platelet activation mediators such as CD40, thrombin and thromboxane A2 (TXA2) secreted by tumor cells or stromal cells. Activated platelets secrete transforming growth factor-beta (TGF- β), platelet derived growth factor(PDGF) and other factors to assist the epithelial-mesenchymal transition (EMT) of tumor cells and promote tumor entry into the blood. Subsequently, platelets will pass through the physical barrier, inhibit NK cells, inhibit apoptosis and other processes to protect the tumor and make it metastasize smoothly. Platelets play an important role in the cascade of tumor invasion and metastasis, so it is of great significance to study this interaction and related targets for the research and development of anti-tumor drugs.
Tumor Actives Platelets
Tumors are often regarded as ‘non-healing wounds’, which play a crucial role in platelet activation. Tumors activate platelets through diverse mechanisms, with thrombin continuing to be the most effective platelet activator in the platelet activation process. Tissue factor (TF) is expressed on the cell membranes of some cancer cells, triggering the plasma coagulation cascade, resulting in thrombotic reactions and thrombin generation, which is activated primarily through protease-activated receptors (PARs).10–12 Among the PARs (PAR1, PAR2, PAR3, and PAR4), platelet activation is mainly associated with PAR1 and PAR4, while PAR-2 is not expressed on platelets, and PAR-3 acts solely as a cofactor for PAR-4.13,14 Tumor cells can also secrete soluble mediators, including adenosine diphosphate (ADP) and TXA2, to activate platelets.15,16 Furthermore, activated platelets release ADP and enhance their own activation through autocrine and paracrine mechanisms.17 ADP acts as an agonist for two purinergic G protein-coupled receptors (P2Y1, P2Y12) in platelets, contributing to platelet activation and aggregation.18 TXA2, through its receptor TXR, also influences platelet activation and aggregation.19 When interacting with CD40 factors produced by tumors, platelets secrete the CD40 receptor (CD40L), a vital source of CD40L in the blood, resulting in broad platelet activation.20,21
In recent years, CLEC-2 has been shown to be a platelet receptor for podoplanin proteins on tumor cells,22 and podoplanin proteins, which are widely expressed in tumor cells, can activate platelets through the immunoreceptor tyrosine-based activation motif (ITAM) and downstream kinase, thereby cross-linking platelet CLEC-2.6 Additionally, receptors for advanced glycosylation end product, including S100, amyloid β, and high mobility group frame 1 (HMGB1), can also stimulate local platelet activation, with HMGB1 predominantly binding to Toll-like receptors on the surface of tumor cells.23 P-selectin on platelets can also induce platelet activation by interaction with the P-selectin receptor (PSGL-1) on tumor cells.24 Furthermore, CD97, an adhesive G protein-coupled receptor expressed by cancer cells, was discovered by Ward et al to activate platelets.25 Other tumor patients lack the enzyme that cleaves von Willebrand factor (vWf), leading to platelet activation by a highly polymerized form of vWf in the circulation.26 Platelets contain matrix metalloproteinases (MMPs), which, when triggered by malignancies, can activate platelets as well.27,28 Glycoprotein VI can direct ITAM signaling to activate platelets, thereby controlling several processes including platelet adhesion, aggregation, and procoagulant activity.29 To enhance the effects of platelet activation, Tumor cells also stimulate platelet aggregation (TCIPA).30 Depending on the type of stimulation, aggregated platelets through TCIPA can secrete proangiogenic and tumor-promoting factors that are essential for tumor migration and growth.31,32
Interaction Between Platelets and Immunocytes
It is worth noting that platelets also interact with immunocytes, which is also extremely important in tumor progression. In previous studies, it has been found that platelets are involved in regulating the function of immunocytes such as natural killer (NK) cells, dendritic cells, neutrophils, macrophages and lymphocytes.3 In tumor progression, platelets play an important role in extracellular neutrophil trapping (NET) and NK cell inhibition.33
NET is a unique immune response of neutrophils to neutrophil elastin and DNA excretion substances in pathogens. The DNA network in NET can also be used as a chemokine to attract and capture circulating tumor cells (CTC), but this capture is not harmful, NET will enhance the proliferation and invasion of CTC.34 In recent years, it has been found that NET can also enhance the permeability of local blood vessels and facilitate the extravasation of tumor cells.35 In addition, substances such as elastin released by NET can also break down laminin and provide help for tumor invasion.36 NET also captures platelets and activates platelets to form thrombus, which in turn promotes the formation of NET and amplifies this effect.37 In tumors, activated platelets immediately bind to neutrophils through P-selectin, and induce Net production through thrombin, TLR4 and HMGB1 produced by platelets, thus enhancing platelet activation and aggregation, thus promoting tumor progression.38,39
NK cells are deadly to CTC, but platelets can help CTC survive the killing of NK cells. Platelets can transfer histocompatibility class I molecules (MHC-1) to the surface of tumor cells, shielding tumor cells from immune recognition and diminishing NK cell function, and platelet-derived TGF-β can also reduce NK cell activity.40 Platelet-derived RGS18 can also protect CTC from NK-mediated immune surveillance by participating in immune checkpoint HLA-E: CD94-NKG2A.41 In addition, platelets can induce the exfoliation of NKG2D33 and NKG2DL42 ligands to enhance the immune escape of CTC to NK cells.
Platelets Assist in Tumor Invasion
Tumor invasion is associated with tumor EMT, and crucial soluble mediators such as platelet-derived growth factors and matrix metalloproteinases, which are released by activated platelets are essential for the induction of EMT.19 EMT confers mesenchymal cell competence to epithelial cells, significantly increases cell motility, contributes to tumor cell invasiveness and facilitates tumor invasion by degrading the extracellular matrix (ECM) of metastasis.43,44
EMT is a biological process through which epithelial cells undergo a specific program to acquire a mesenchymal phenotype. EMT is a biological process that is regulated by genes such as the SNAIL family, the TWIST family, and the zinc finger E-box binding homology box (ZEB) family. These genes, in turn, are influenced by signaling pathways such as TGF-β, Smad, P38, Wnt, NF-κB, and PI3K-AKT. The expression of epithelial genes such as E-cadherin, Occludins, claudins, and ZO1 is suppressed, while the expression of mesenchymal phenotype-related genes such as N-cadherin, Vimentin, and Fibronectin is enhanced. Additionally, increased degradation of the extracellular matrix by MMPs is a significant feature of EMT.45,46
In addition to gene regulation, platelets are also involved in the regulation of EMT-related signaling pathways to promote the invasive-metastatic cascade response of tumor cells. For instance, platelet-secreted TGFβ1 can activate EMT through the TGFβ/Smad pathway. Platelets can also activate the NF-κB pathway upon contact with tumors and thus enhance TGFβ1 secretion from tumor cells, promoting EMT activation.47 Platelets in breast cancer can also activate EMT by inducing the NF-κB pathway, conferring an aggressive phenotype.48 Moreover, a study on cholangiocarcinoma found that platelet-released PDGF activates EMT via the P38/MAPK pathway.49 In hepatocellular and breast cancers, platelets can also promote EMT through PAR activation of TWIST.50
Tumor cells that complete EMT are also vulnerable to anoikis, a programmed cell death that occurs when cells detach from the extracellular matrix.51 Resistance to anoikis is a crucial prerequisite for tumor cells to metastasize. Platelets frequently assist tumor cells in resisting evading by producing autotoxin (ATX) during tumor growth, mainly through the conversion of lysophosphatidylcholine to lysophosphatidic acid (LPA), followed by LPA binding to LPA receptor (LPAR-1) on tumor cells via RhoA-Gα12/ 13-YAP-1 signaling pathway promoting resistance to anoikis and facilitating tumor progression.52
Platelets-Based Tumor Intravasation
Tumor cells that have undergone EMT and survived anoikis can infiltrate. Tumor cell invasion into lymphatic systems, blood vessels, and adjacent tissues occur in various ways. This article primarily focuses on the processes that occur when tumor cells enter the bloodstream. Tumor endosmosis and extravasation require the destruction of the endothelium connection to pass through endothelial cells, known as transendothelial cell migration (TEM).53,54 Due to the distinct ways in which tumor cells migrate through endothelial cells, endosmosis and extravasation differ. Tumor cells invade by creating intercellular connections with the body’s blood vessels and producing local blood vessels during intravasation. This process is regulated by angiogenesis-related factors such as vascular endothelial growth factor (VEGF), angiopoietin, and PDGF.55,56 Ward et al discovered that expressed in cancer cells, CD97 adhesion GPCR may activate platelets and cause LPA release from platelets, promoting tumor cell migration across the endothelium.25 Endothelial cells activated by thrombin have a circular appearance and lack adhesion connections, which can encourage tumor cell movement across the endothelium.57 Furthermore, vascular diameter, specific proteases such as uPAR and MMPs can also influence tumor invasion into blood arteries.55,58
The tumor invades blood arteries and transforms into CTC, which enter the circulatory system and prepare for metastasis. However, most CTC is destroyed during this process by blood flow shear stress and NK cells. As a result, CTC must work with platelets to avoid numerous hazards in the circulatory system.
To begin, CTC must conserve enough platelets to create tumor cell immune thrombi by TCIPA. The mechanism of CTC-mediated TCIPA is primarily associated with various platelet activation pathways, including PAR binding to TF, ADP and P2Y12 binding, TXA2 and TXR binding, CD40L and CD40 binding, podoplanin and CLEC-2 binding, HMGB-1 and TLR4 binding and so on. Excessive vWF binding to platelet glycoprotein GPIb IX–V, as well as enhanced synthesis of PAI-1 and MMPs and accelerated platelet aggregation.3 This implies that CTCs stimulate and recruit platelets, enabling them to promptly respond to multiple life-threatening challenges in the circulatory system.
Subsequently, platelets that aggregate around tumor cells adhere to them through the action of adhesion molecules including integrin αIIbβ3, P-selectin, CD97, and GPIb-IX-V/GPIIb-IIIa.59,60 These proteins are also involved in platelet activation and the development of TCIPA.61 Platelets adhering to the surface of tumor cells support the survival of CTCs in the circulatory system, shielding them from blood flow shear, tumor necrosis factor-alpha (TNF-α), and cell death induced by NK cells.62
Tumor Extravasation via Platelets
CTCs that survive in the circulation eventually come to a gradual standstill as peripheral capillary blood flow slows down and adheres to endothelial cells in the presence of P-selectin and integrins, among others, and undergo TEM, similar to the process of endo-osmosis.52 In addition to actively releasing MMPs to break down the ECM to promote extravasation, platelets also release PDGF, 12(S)-hydroxyeicosatetraenoic acid (12-HETE), and LPA to, in turn, stimulate the production of MMPs by CTCs,63 with 12-HETE also promoting contraction of endothelial cells to promote extravasation.64 ATP binds to the P2Y2 receptor on vascular endothelial cells, leading to the disruption of tight junctions and increasing endothelial permeability.65 ATP released from activated platelets has also recently been found to promotes endothelial cell contraction to increase permeability and enhance the expression of endothelial adhesion molecules, which in turn promotes extravasation.66 However, in some cases, CTCs are not required for TEM, and stagnant cancer cells may proliferate within the vascular lumen, leading to the growth of large intraluminal tumor colonies that eventually rupture the nearby endothelial wall, allowing direct access to the tissue parenchyma.67 Figure 1 provides an illustrative overview of this interaction.
Platelet-Based Anti-Tumor Nanoparticles
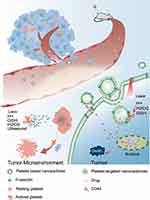
Nanoparticles, as emerging anti-tumor tools in recent years, are designed to transport anti-tumor drugs using unique drug delivery systems, addressing challenges such as limited specificity, high toxicity, and drug resistance encountered by conventional chemotherapeutic drugs like Tegretol and Doxorubicin. Platelets have garnered significant attention in anti-tumor nanoparticles research due to their innate tumor-targeting properties. Anti-tumor nanoparticles with stimulus-responsive drug delivery system designed using platelets have higher specificity, and when combined with radiotherapy and ultrasound, these drugs can be concentrated near the tumor site, minimizing damage to healthy cells. Currently, platelet-based anti-tumor nanoparticles primarily consist of platelet-targeted drugs and platelet membrane-encapsulated anti-tumor nanoparticles designed to specifically target tumors.
Tumor-Based Platelet Targeted Nanoparticles
Platelets are commonly implicated in tumor growth through the interaction of their membrane receptors with tumor cells. Platelet-targeted medicines that frequently exhibit positive imaging and anti-tumor effects have targeted these receptors. In order to inhibit platelet activation, Zhou’s team developed nanoparticles made of albumin that can coat the surface of platelets with ADP, collagen (a GPVI agonist), and a thrombin activation site. Utilizing this nano platform can enhance the effectiveness of anti-tumor immunotherapy by promoting increased permeability of tumor blood vessels and T-cell intravasation.68
Nevertheless, systemic administration of anti-platelet drugs often harms autologous platelets and disrupts functions, including coagulation. Nanoparticles with targeted delivery capabilities circumvent damage to autologous platelets and enable localized dosage at the tumor site for improved anti-tumor effects (Table 1). Initially, researchers altered nanoparticles by incorporating tumor-targeting proteins, such as the CREKA (Cys-Arg-Glu-Lys-Ala) peptide,59 TM33 peptide,60 fucoidan segment,61 and P-selectin-targeting peptide (PSN),62 and coupled them with platelet inhibitors to concentrate them near tumor cells. Subsequently, they employed specific excitation techniques crucial for the anti-tumor effects to facilitate drug release. Currently, two distinct types of excitation techniques exist: internal stimuli, such as an acidic pH, redox reactions, or tumor microenvironment (TME) enzymes, and external stimuli, including laser and ultrasound.
 |
Table 1 Tumor-Based Platelet-Targeted Nanoparticles |
Excessive acidity results from the accumulation of lactate and hypoxia in the TME due to the increased metabolic rate of tumor cells.79 Building upon this knowledge, researchers have successfully achieved drug release by incorporating ionizable groups of the drug into nanomaterials. When the nanoparticles is exposed to an acidic environment, the binding sites between the drug and the nanomaterial become ionized, resulting in drug release. In one study, Zhang’s team prepared an acid-responsive nanoparticles, CREKA-lipo-T. The platelet inhibitor ticagrelor was encapsulated in liposomes and conjugated with the tumor-targeting pentapeptide CREKA, facilitating the enrichment of the nanoparticles in the vicinity of the tumor. In a 4T1 mouse model, CREKA effectively targeted the fibronectin-fibronectin complex in the tumor vessel wall. CREKA-Lipo-T actively bound to tumor vessel microthrombi, leading to local release of ticagrelor, which interacts with the P2Y12 receptor, through an acid response. This mechanism successfully inhibited tumor-associated platelet function and blocked tumor lung metastasis, without inducing significant side effects such as bleeding complications in the experiment.77 Subsequent research will heavily concentrate on acid-responsive nanoparticles as it provides promising approaches for the delivery and release of anti-tumor medications.
Besides its highly acidic nature, the tumor microenvironment exhibits elevated levels of glutathione (GSH) and hydrogen peroxide (H2O2). These components reduce the susceptibility of tumor cells to oxidative stress and position GSH/H2O2 as potential catalysts in the advancement of nanoparticles. The existing design strategy for GSH relies primarily on exploiting the GSH present in tumor cells to cleave disulfide bonds and facilitate drug release from nanoparticles. For example, Xu et al modified albumin by incorporating terminal sulfhydryl groups (-SH) and subsequently s-nitrosylated the albumin to generate nitrosylated human serum albumin (AlbSNO). They employed AlbSNO as a shell to construct nanoparticles that encapsulated paclitaxel (Ptx), resulting in the formation of Ptx@AlbSNO complexes. Specifically, AlbSNO can induce targeted release of nitric oxide (NO) upon reductive stimulation by the high GSH concentration found in the 4T1 and CT26 tumors. NO effectively inhibits platelet activation and the secretion of TGF-β, which is crucial in alleviating the immunosuppressive tumor microenvironment. In turn, disruption of the AlbSNO shell leads to the release of paclitaxel, which in turn develops anti-tumor effects.75 Hydrogen peroxide (H2O2), another vital reducing agent in the TME, plays a significant role in the elimination of reactive oxygen species (ROS) and platelet activation processes. Wang et al developed an H2O2-responsive NIR probe self-assembly-triggered nanosystem for targeted platelet nanoformulations CyBA/PFM. The CyBA/PFM NPs actively bind thrombi by associating with highly expressed P-selectin, and the rocket algae etiolated glycan segment within the vector also facilitates specific delivery of therapeutic polymers and probes to platelet-rich arterial thrombi. In a mouse model of FeCl3-induced arterial thrombosis, the phenyl borate group captures the electron-donating oxygen atom. Subsequently, H2O2 prompts the release of the phenyl boride group, resulting in nanoparticle dissociation and the generation of fluorescent CyOH. During this process, H2O2 is significantly consumed to reduce platelet activation and achieve inhibition of platelet function.74
The MMPs enzyme family plays a crucial role in the tumor progression during the invasion metastasis cascade of malignancies. MMP2 is overexpressed selectively on the plasma membranes of tumor endothelial cells and tumor stromal cells. Therefore, Li’s team utilized this property to develop a nanocomposite consisting of a core made of a block copolymer, poly(ether imide)-poly(lactic-co-glycolic acid)2 (PEI-(PLGA)2), loaded with the antiplatelet antibody R300 and the chemotherapy drug doxorubicin (Dox). The core was coated with an MMP2 cleavable peptide, lecithin, and polyethylene glycol phospholipid to form the shell. R300, a monoclonal antibody targeting the GPIbα subunit of the platelet vascular hematopoietic factor receptor, effectively depletes platelets by inducing microaggregation (3–5 platelets). The resulting macroaggregates are rapidly cleared through phagocytosis without activating the coagulation system. Upon intravenous injection into MCF7 tumor-bearing nude mice, the polyethylene glycolated phospholipids in the shell layer of the nanoparticles inhibit nonspecific aggregation, prolonging their half-life. Once they reach the tumor microenvironment, tumor-associated MMP2 triggers a burst release of the drug by cleaving the MMP2-cleavable peptide on the shell layer.71 Enzyme-responsive drugs pose greater challenges to the nanoshell than the two response modalities mentioned earlier. The selection of enzymes and the modification of the nanoshell require more stringent criteria. Further research is necessary to establish their potential as clinical candidates.
In addition to internal pathways, external stimuli play a crucial role in stimulating the desired effects. As one of the routine treatment modalities in cancer therapy, conventional radiation therapy is less effective on deep-seated tumors due to the diminishing energy of light in the body, and the abundance of GSH and H2O2 in tumor tissue can also weaken the harm of ROS produced by radiation. To address these shortcomings of radiotherapy, researchers have combined it with the development of nanoparticles, which use specially designed nanoparticles to deliver metal particles or photothermal agents with excellent photothermal conversion near the tumor to enhance the effect of radiotherapy in the presence of radiation for anti-tumor purposes. For instance, Chang’s group successfully developed a nanocomposite called Fu-Uk/ICG@SiAu NRs. This nanocomposite demonstrated high efficiency and safety in targeting tumor thrombi, exhibiting photothermal/enzymatic thrombolytic effects.69 Ma et al developed a BSA-IRLA@RVs complex that targets platelets through the encapsulation of protein nanoparticles within red blood cell membranes. Using the constructed cRGD immune membrane structure, the nanoparticles could actively target platelets and cancer cells, respectively, through overexpression of integrins αIIbβ3 and αvβ3, among others. The drug is loaded with biological nitric oxide synthase, l-arginine (LA), and the photosensitizer IR783, with the LA molecule acting as a nitric oxide precursor. In the U14 tumor-bearing mice model, IR783 utilizes oxygen under NIR stimulation to generate reactive oxygen species (ROS). This process leads to the oxidation of the guanidine group of LA, releasing nitric oxide (NO). Consequently, NO specifically inhibits tumor-associated platelet activation, resulting in weakened endothelial junctions and erythrocyte intravasation, along with a significant increase in drug concentration within the tumor tissue.72
Platelets Delivery Anti-Tumor Drugs
Researchers have designed a variety of drug delivery systems with platelets. At present, the main ideas are as follows: 1. platelet membrane-coated nanoparticles. 2. living platelets-based nanoparticles. 3. platelets with drug or antibody coupled on its surface. 4. platelet EV based nanoparticles (Table 2).
 |
Table 2 Platelet-Based Nanoparticles |
Platelet Membrane-Coated Nanoparticles
Platelet membrane-coated nanoparticles overcome the limitations of conventional coated non-scaling polymers like polyethylene glycol (PEG) and dextran, enhancing targeting capabilities and extending the half-life. Conventional anti-tumor therapy faces challenges in accessing the tumor cytoplasm, whereas platelet membrane-coated drugs utilize signaling molecules on the membrane surface to target tumors and facilitate platelet phagocytosis, significantly improving drug delivery. Platelet membrane-coated drugs are more intricate than platelet-targeted drugs, with the preparation and coating of platelet membranes being crucial. Platelets are initially isolated from the blood through differential or gradient centrifugation. Subsequently, they undergo repeated freeze-thaw or platelet lysis using hypotonic media. Afterward, centrifugation is performed to obtain platelet membrane fragments. These isolated platelet membranes are then mixed with inner core nanoparticles and coated onto the nanoparticles using ultrasound, co-extrusion, or microfluidic electroporation. The resulting nanocomposites are characterized using various techniques, including transmission electron microscopy, dynamic light scattering, sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and Western blotting analysis.84,85,97
Researchers have developed Platelet membrane-coated anti-tumor nanoparticles that target specific tumor features and activate multiple pathways, thereby enhancing safety. Zhuang et al encapsulated siRNA within acid-responsive porous metal-organic framework (MOF) nanoparticles. These nanoparticles were then coated with platelet membranes to create P-MOF-siRNA complexes. The P-MOF-siRNA complex specifically targeted breast cancer cells through the presence of platelet membranes on its surface. Upon endocytosis by the target cells, the acidic environment triggered the dissociation of the MOF scaffold, resulting in drug release and subsequent anti-tumor effects. In conclusion, this method presents a promising approach for developing clinical anti-tumor drugs through the preparation of acid-responsive platelet membrane-coated nanoparticles. Moreover, the highly reproducible P-MOF-siRNA complex offers versatility for exploring alternative therapeutic options by modifying the loaded drug.97
To target the role of GSH in tumor tissue, Ning’s team loaded the type I AIE photosensitizer TBP-2 into mesoporous organo-silica nanoparticles. Subsequently, these nanoparticles were coated with platelet membranes, resulting in the formation of PMBN complexes. The P-selectin present on the platelet membrane specifically targets CD44 in cancer stem cells (CSCs), leading to the phagocytosis of CSCs. Subsequently, intracellular GSH triggers the degradation of the disulfide bonds in the organo-mesoporous silica nanoparticles, resulting in the release of TBP-2. Ultimately, the release of TBP-2, when combined with external light, exerts an anti-tumor effect. In a murine breast cancer tumor model, the results demonstrated the successful targeting of CSCs by PMBN, leading to a decrease in the number of CSCs at the tumor site and reduced recurrence following treatment.94
Huang et al employed mesoporous silica nanoparticles (MSN) as carriers, loaded with the oxidative stress amplifier cinnamaldehyde (CA) and the acoustically sensitive agent IR780. These nanoparticles were further coated with platelet membranes. Upon ultrasound exposure, CA was released from the MSN, resulting in GSH depletion and subsequent ROS increase. This disruption of the cell membrane led to drug release, ultimately exerting anti-tumor effects in the 4T1 cancer-bearing mouse model. Experiments have demonstrated the effective inhibition of tumor growth by this drug. CA-induced GSH depletion hinders the ability of tumors to counteract ultrasound-induced oxidative stress. In summary, the combined approach of ultrasound treatment with anti-tumor drugs offers improved biosafety and efficacy, with significant potential for clinical application in combination with traditional anticancer therapies.82
The results of radiotherapy in targeting platelet nanoparticles are equally favorable in platelet-coated nanoparticles. Qi’s team developed a mesoporous Fe-SA enzyme (PMS) coated with platelet membranes, utilizing photothermal therapy (PTT) to induce mitochondrial damage in breast cancer. This approach enables secondary near-infrared (NIR-II) driven PTT at mild temperatures. The developed PMS exhibited favorable NIR-II photothermal properties, high peroxidase (POD) activity, and excellent tumor-targeting capabilities. Additionally, it demonstrated the ability to carry protein drugs. Upon targeting and subsequent phagocytosis by the tumor, the SAzyme, possessing high POD activity, catalyzes the generation of -OH from endogenous H2O2 within the tumor, resulting in mitochondrial damage. In turn, mitochondria encode genes that contribute to the development of heat resistance in eukaryotic cells. This suggests that damaging mitochondria can reduce the heat resistance of tumor cells, enabling their eradication under mild PTT conditions. These findings are supported by data from in vivo experiments.88
Living Platelets-Based Nanoparticles
In addition to using platelet membranes to cover nanoparticles, researchers also use living platelets as a drug delivery system to treat diseases. At present, drugs such as doxorubicin (DOX) are used for platelet loading, which is mainly loaded into platelets through passive osmosis of drugs.107 In the study of Xia,108 they prepared Au-Hb@PLT complex. In tumor-bearing mice, the engineered platelets will activate and release gold nanoparticles under radiation, greatly enhancing the effect of radiotherapy. This laser-responsive living platelet complex shows a good effect in tumor therapy, and the BNPD-Ce6@Plt constructed by Xu et al has also proved this in the glioblastoma mouse model.109
In addition to external stimulation to activate engineering platelets, live platelets are also activated by tumor cells, which greatly reduces the difficulty of design. In the study of Li et al,110 living platelets loaded with DOX were activated by Lewis lung cancer cells and unloaded drugs, the results showed that the therapeutic effect was significantly higher than that of DOX alone, and there was no serious toxicity related to DOX in mice. In addition, some similar engineered platelets have been tested in many different tumors, and the results have shown positive significance (Table 2). However, it is worth noting that these engineered platelets can be activated by tumor cells, thrombosis, etc., which means that they may also be activated by other substances, especially in the complex background of the human blood system. Moreover, the preparation and storage of platelets have great restrictions on this kind of engineered platelets. Platelets stored at room temperature often have problems such as bacterial contamination, activation and degranulation, while platelets stored at low temperature have the problem of functional change. These defects limit the participation of living platelets in the construction of these engineered platelets, which need to be overcome in order to be widely used.111
Platelets with Drug or Antibody Coupled on Its Surface
As mentioned earlier, there are a large number of surface markers on the surface of platelets, which means a new research idea. By coupling antibodies or drugs with platelet membranes, the researchers improve the anti-tumor ability of drugs through the natural targeting between platelets and tumors. Wang et al112 coupled programmed death ligand 1 to the platelet surface. This engineered platelet performed well in the mouse model of primary melanoma or triple negative breast cancer, which significantly increased the overall productivity of mice. This idea was also verified in Li’s experiment, which used hydrogel to locally consume tumor-related macrophages, which enhanced the coupling of anti-programmed cell death protein 1 antibody to platelets and had a good inhibitory effect on tumor recurrence.113 However, it is worth noting that the coupling of platelets with these substances may lead to excessive concealment of platelet surface markers, thus weakening the targeting ability of platelets to tumor cells and affecting the anti-tumor effect.
Platelet EV Based Nanoparticles
Extracellular vesicles (EV), as a kind of cell information granules, are substances involved in a variety of functions such as coagulation, immunity and tumor progression in the human body.114 As the most abundant source of EV, platelet microparticle (PMP) is an important element in the process of tumor invasion and metastasis. Because PMP comes from platelets, it retains the characteristics of platelets to some extent, including surface markers such as CD41, CD42, P-selectin and functional substances such as platelet activating factor, angiogenic factor and chemokines.115,116 The tumor-promoting function of PMP is similar to that of platelets has been confirmed. Therefore, PMP is a potential substance for the development of antineoplastic drugs.117
Some studies have proved that EV, as a new nano-scale platform, can be used to carry drugs and treat related diseases. In jing’s experiment, they built a nanomedicine RGD-NPVs@MNPs/DOX developed by encapsulating melanin nanoparticles (MNPs) and doxorubicin (DOX) inside RGD peptide (c (RGDyC))-modified nanoscale platelet vesicles (RGD-NPVs). With the help of platelet vesicles, nanoparticles evaded immune clearance and targeted tumor cells, and finally activated and unloaded drugs under laser irradiation. The results showed that the nanoparticles enhanced the effect of radiotherapy and effectively killed tumor cells.106
Unfortunately, the research on anti-tumor drugs carried by PMP is still lack of focus, and the research of PMP in the development of anti-tumor drugs needs to be further explored.
Nanoparticles with Multiple Excitation Modes
In addition, nanoparticles combining multiple excitation modalities have also shown better results in experiments. These nanoparticles often provide two or more excitation modes, which can significantly increase the payload of the drug and unload the drug through external stimulation and the internal physical or chemical characteristics of the tumor. So as to improve the drug release efficiency and anti-tumor effect. However, due to the complexity of the tumor environment, a variety of excitation modes may antagonize each other and affect the drug release. For example, the anoxic environment caused by laser excitation will significantly reduce the drug release.118 Therefore, the nanoparticles bound by a variety of stimuli must be combined in an appropriate way.
For platelet-targeting nanoparticles, Yue et al conducted a study in which they utilized C3N4 nanosheets (CN) doped with the two-dimensional nanomaterial FeIII. These nanosheets were loaded with both chlorogenic acid (CA) and DOX to synthesize a pH/H2O2-responsive nanocomplex. As a result, the drugs exhibit more potent anti-tumor effects in the U14 tumor-bearing mice model.70 Wang’s team also prepared an ‘ion/gas’ bioactive nanogenerator (called IGBN) comprising a copper-based MOF and a loaded cisplatin-arginine (Pt-Arg) prodrug, which is a pH/GSH-responsive complex. The acidic pH in the tumor microenvironment breaks down the MOF, releasing Pt-Arg. Subsequently, GSH dissociates Pt-Arg to release active cisplatin and arginine (Arg). Arginine significantly enhances H2O2 production within the tumor and undergoes oxidation facilitated by H2O2 self-supply, resulting in the production of a self-enhancing cascade of NO gas. Ultimately, NO effectively inhibits platelet activation within the tumor in a 4T1 mammary adenocarcinoma model.73
For platelet-membrane-coated nanoparticles, Zhang’s team developed a photothermal/acid-responsive PDA@Dox NPs complex, which exhibited outstanding therapeutic effects and effectively prevented tumor recurrence in a breast cancer mouse model.102 Zhou developed an ultrasound and GSH-responsive MM-PEG@PLTs complex. The results demonstrated that ultrasound and GSH effectively triggered the controlled release of the contents, leading to tumor amino acid starvation, mTOR inhibition, and iron death. These effects significantly enhanced the anti-tumor response in breast cancer.103 Figure 2 illustrates the response modes of the mentioned above drugs.
Limitation and Perspective
These nanoparticles use the targeted effect of platelets on the tumor to aggregate at the tumor site and achieve site-specific drug release through specific stimulation. This strategy enhances drug targeting while minimizing systemic toxicity. However, their shortcomings are also obvious.
First of all, there are still great challenges in large-scale experiment and promotion of human-derived platelets because of ethical and other problems. At present, the storage of platelets also poses a challenge to the experiment. Platelets stored at room temperature often face the risk of pollution, which leads to a short shelf life, and even the latest pathogen technology only delays the shelf life to about 7 days.119 At the same time, as a highly dynamic cell, platelets usually communicate with their environment (for example, tumor educated platelets), and the obtained platelets often have certain donor characteristics.120 Therefore, whether the engineered platelets prepared by non-autologous platelets have other effects on tumors is a question that needs to be verified.
Not only that, the current research on these nanoparticles is still based on mouse experiments, the lack of clinical trials means a lot of unknown possibilities because of differences between mice and human body. It requires more clinical trials to verify whether these specially designed nanoparticles can work smoothly in the human body. In the existing experiments, these kinds of nanoparticles need sufficient membrane coverage, which will lead to the recognition and removal of nanoparticles by the immune system, and excessive clearance may lead to systemic damage due to the premature release of drugs. This puts forward higher requirements for the preparation of nanoparticles. In addition, the design of these drugs is often complex and does not improve efficacy, posing challenges for drug production and storage. Further optimization is necessary to promote their clinical translation. Although the targeting ability of the drug has been improved, experimental evidence shows that it accumulates in organs such as liver and kidney, indicating possible hepatotoxicity. Last but not least, the strategies of radiotherapy and chemotherapy are different in different stages of cancer, so further experiments are needed to determine the appropriate doses of drugs at different stages of cancer to ensure the effectiveness and safety of these drugs.
At present, artificial synthetic platelets have been paid attention to, and great progress has been made in simulated platelet adhesion. These analogues can prolong storage life and minimize side effects, synthetic platelets are more controllable, and the problems of platelet supply are also solved. The use of synthetic platelet analogues to carry drugs may be a way out for future development.111
Conclusion
Platelets play an important role in tumor progression, and anti-tumor drugs inspired by platelets have become an important direction of cancer drug development in the future. In this review, researchers have worked hard to design a variety of platelet-based nanoparticles, and these strategies have proved their ability in experiments, and have good performance on the natural targeting, effectiveness and safety of tumor cells. With the development of intelligent delivery, personalized treatment for cancer will become possible, and these nanoparticles will greatly enhance the specificity of anti-tumor therapy. Accordingly, these nanoparticles can also be modified for the diagnosis of tumor cells. These nanoparticles show great potential in anti-tumor and will play a great role in clinical treatment in the future, but due to the lack of clinical trials to further prove their performance, more efforts are needed for their application in the future.
Data Sharing Statement
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This research was funded by the Sichuan Provincial Cadre Health Research Project (Chuan Gan Yan 2022-802); the Sichuan Science and Technology Program, China (2021JDRC0152, 2022YFS0006, 23NSFSC3334), Sichuan Province Medical Association Research Project (S21021).
Disclosure
The authors declare no competing interests in this work.
References
1. Linden MD. Platelet physiology. Methods Mol Biol. 2013; 992:13–30.
2. Haemmerle M, Stone RL, Menter DG, Afshar-Kharghan V, Sood AK. The platelet lifeline to cancer: challenges and opportunities. Cancer Cell. 2018;33:965–983. doi:10.1016/j.ccell.2018.03.002
3. Schlesinger M. Role of platelets and platelet receptors in cancer metastasis. J Hematol Oncol. 2018;11:125. doi:10.1186/s13045-018-0669-2
4. Cheng X, Zhang H, Hamad A, Huang H, Tsung A. Surgery-mediated tumor-promoting effects on the immune microenvironment. Semin Cancer Biol. 2022;86:408–419. doi:10.1016/j.semcancer.2022.01.006
5. Gay LJ, Felding-Habermann B. Contribution of platelets to tumour metastasis. Nat Rev Cancer. 2011;11:123–134. doi:10.1038/nrc3004
6. Suzuki-Inoue K. Platelets and cancer-associated thrombosis: focusing on the platelet activation receptor CLEC-2 and podoplanin. Blood. 2019;134:1912–1918. doi:10.1182/blood.2019001388
7. Bruno A, Dovizio M, Tacconelli S, Contursi A, Ballerini P, Patrignani P. Antithrombotic Agents and Cancer. Cancers. 2018;10:253. doi:10.3390/cancers10080253
8. Zhu YS, Tang K, Lv J. Peptide-drug conjugate-based novel molecular drug delivery system in cancer. Trends Pharmacol Sci. 2021;42:857–869. doi:10.1016/j.tips.2021.07.001
9. Zhang M, Hu W, Cai C, Wu Y, Li J, Dong S. Advanced application of stimuli-responsive drug delivery system for inflammatory arthritis treatment. Mater Today Bio. 2022;14:100223. doi:10.1016/j.mtbio.2022.100223
10. Alexander ET, Gilmour SK. Immunomodulatory role of thrombin in cancer progression. Mol Carcinog. 2022;61:527–536.
11. García-López MT, Gutiérrez-Rodríguez M, Herranz R. Thrombin-activated receptors: promising targets for cancer therapy? Curr Med Chem. 2010;17:109–128. doi:10.2174/092986710790112639
12. Jain S, Harris J, Ware J. Platelets: linking hemostasis and cancer. Arterioscler Thromb Vasc Biol. 2010;30:2362–2367. doi:10.1161/ATVBAHA.110.207514
13. Gnanenthiran SR, Pennings GJ, Reddel CJ, et al. Identification of a distinct platelet phenotype in the elderly: ADP hypersensitivity coexists with platelet PAR (Protease-Activated Receptor)-1 and PAR-4-mediated thrombin resistance. Arterioscler Thromb Vasc Biol. 2022;42:960–972. doi:10.1161/ATVBAHA.120.316772
14. Lee HJ, Wall B, Chen S. G-protein-coupled receptors and melanoma. Pigment Cell Melanoma Res. 2008;21:415–428. doi:10.1111/j.1755-148X.2008.00478.x
15. Fontana P, Zufferey A, Daali Y, Reny JL. Antiplatelet therapy: targeting the TxA2 pathway. J Cardiovasc Transl Res. 2014;7:29–38. doi:10.1007/s12265-013-9529-1
16. Tang J, Li MP, Zhou HH, Chen XP. Platelet inhibition agents: current and future P2Y12 receptor antagonists. Curr Vasc Pharmacol. 2015;13:566–577. doi:10.2174/1570161112666141127162209
17. Puri RN, Colman RW, Liberman MA. ADP-induced platelet activation. Crit Rev Biochem Mol Biol. 1997;32:437–502. doi:10.3109/10409239709082000
18. Wright JR, Chauhan M, Shah C, et al. The TICONC (Ticagrelor-Oncology) Study: implications of P2Y(12) inhibition for metastasis and cancer-associated thrombosis. JACC CardioOncol. 2020;2:236–250. doi:10.1016/j.jaccao.2020.04.009
19. Rovati G, Contursi A, Bruno A, Tacconelli S, Ballerini P, Patrignani P. Antiplatelet agents affecting GPCR signaling implicated in tumor metastasis. Cells. 2022;11:725. doi:10.3390/cells11040725
20. Mege D, Aubert M, Lacroix R, Dignat-George F, Panicot-Dubois L, Dubois C. Involvement of platelets in cancers. Semin Thromb Hemost. 2019;45:569–575. doi:10.1055/s-0039-1693475
21. Zhang R, Zhang G, Xiang B, et al. TRAF3 negatively regulates platelet activation and thrombosis. Sci Rep. 2017;7:17112. doi:10.1038/s41598-017-17189-1
22. Takemoto A, Miyata K, Fujita N. Platelet-activating factor podoplanin: from discovery to drug development. Cancer Metastasis Rev. 2017;36:225–234. doi:10.1007/s10555-017-9672-2
23. Fuentes E, Palomo I, Rojas A. Cross-talk between platelet and tumor microenvironment: role of multiligand/RAGE axis in platelet activation. Blood Rev. 2016;30:213–221. doi:10.1016/j.blre.2015.11.005
24. Fabricius H, Starzonek S, Lange T. The role of platelet cell surface P-selectin for the direct platelet-tumor cell contact during metastasis formation in human tumors. Front Oncol. 2021;11:642761. doi:10.3389/fonc.2021.642761
25. Ward Y, Lake R, Faraji F, et al. Platelets promote metastasis via binding tumor CD97 leading to bidirectional signaling that coordinates transendothelial migration. Cell Rep. 2018;23:808–822. doi:10.1016/j.celrep.2018.03.092
26. Robador JR, Feinauer MJ, Schneider SW, et al. Involvement of platelet-derived VWF in metastatic growth of melanoma in the brain. Neurooncol Adv. 2021;3:vdab 175.
27. Cao L, Zhang Y, Mi J, et al. α-Hederin inhibits the platelet activating factor-induced metastasis of HCC cells through disruption of PAF/PTAFR axis cascaded STAT3/MMP-2 expression. Pharmacol Res. 2022;178:106180. doi:10.1016/j.phrs.2022.106180
28. Melnikova VO, Mourad-Zeidan AA, Lev DC, Bar-Eli M. Platelet-activating factor mediates MMP-2 expression and activation via phosphorylation of cAMP-response element-binding protein and contributes to melanoma metastasis. J Biol Chem. 2006;281:2911–2922. doi:10.1074/jbc.M508683200
29. Mammadova-Bach E, Gil-Pulido J, Sarukhanyan E, et al. Platelet glycoprotein VI promotes metastasis through interaction with cancer cell-derived galectin-3. Blood. 2020;135:1146–1160. doi:10.1182/blood.2019002649
30. Strasenburg W, Jóźwicki J, Durślewicz J, et al. Tumor cell-induced platelet aggregation as an emerging therapeutic target for cancer therapy. Front Oncol. 2022;12:909767. doi:10.3389/fonc.2022.909767
31. Lee DY, Im E, Yoon D, et al. Pivotal role of PD-1/PD-L1 immune checkpoints in immune escape and cancer progression: their interplay with platelets and FOXP3+Tregs related molecules, clinical implications and combinational potential with phytochemicals. Semin Cancer Biol. 2022;86:1033–1057. doi:10.1016/j.semcancer.2020.12.001
32. Schwarz S, Schlesinger M, Bendas G. Detection of tumor cell-induced platelet aggregation and granule secretion. Methods Mol Biol. 2021;2294:181–195.
33. Pereira-Veiga T, Schneegans S, Pantel K, Wikman H. Circulating tumor cell-blood cell crosstalk: biology and clinical relevance. Cell Rep. 2022;40:111298. doi:10.1016/j.celrep.2022.111298
34. Langiu M, Palacios-Acedo AL, Crescence L, Mege D, Dubois C, Panicot-Dubois L. Neutrophils, cancer and thrombosis: the new bermuda triangle in cancer research. Int J Mol Sci. 2022;23:1257. doi:10.3390/ijms23031257
35. Park J, Wysocki RW, Amoozgar Z, et al. Cancer cells induce metastasis-supporting neutrophil extracellular DNA traps. Sci Transl Med. 2016;8:361ra138. doi:10.1126/scitranslmed.aag1711
36. Kreidberg JA. Functions of alpha3beta1 integrin. Curr Opin Cell Biol. 2000;12:548–553. doi:10.1016/S0955-0674(00)00130-7
37. Cedervall J, Hamidi A, Olsson AK. Platelets, NETs and cancer. Thromb Res. 2018;164:S148–s152. doi:10.1016/j.thromres.2018.01.049
38. Snoderly HT, Boone BA, Bennewitz MF. Neutrophil extracellular traps in breast cancer and beyond: current perspectives on NET stimuli, thrombosis and metastasis, and clinical utility for diagnosis and treatment. Breast Cancer Res. 2019;21:145. doi:10.1186/s13058-019-1237-6
39. Rosell A, Martinod K, Mackman N, Thålin C. Neutrophil extracellular traps and cancer-associated thrombosis. Thromb Res. 2022;213(Suppl 1):S35–s41. doi:10.1016/j.thromres.2021.12.018
40. Wu Q, You L, Nepovimova E, et al. Hypoxia-inducible factors: master regulators of hypoxic tumor immune escape. J Hematol Oncol. 2022;15:77. doi:10.1186/s13045-022-01292-6
41. Liu X, Song J, Zhang H, et al. Immune checkpoint HLA-E: CD94-NKG2A mediates evasion of circulating tumor cells from NK cell surveillance. Cancer Cell. 2023;41:272–287.e279. doi:10.1016/j.ccell.2023.01.001
42. Maurer S, Kropp KN, Klein G, et al. Platelet-mediated shedding of NKG2D ligands impairs NK cell immune-surveillance of tumor cells. Oncoimmunology. 2018;7:
43. Dongre A, Weinberg RA. New insights into the mechanisms of epithelial-mesenchymal transition and implications for cancer. Nat Rev Mol Cell Biol. 2019;20:69–84. doi:10.1038/s41580-018-0080-4
44. Lüönd F, Sugiyama N, Bill R, et al. Distinct contributions of partial and full EMT to breast cancer malignancy. Dev Cell. 2021;56:3203–3221.e3211. doi:10.1016/j.devcel.2021.11.006
45. Huang Y, Hong W, Wei X. The molecular mechanisms and therapeutic strategies of EMT in tumor progression and metastasis. J Hematol Oncol. 2022;15:129. doi:10.1186/s13045-022-01347-8
46. Mittal V. Epithelial mesenchymal transition in tumor metastasis. Annu Rev Pathol. 2018;13:395–412. doi:10.1146/annurev-pathol-020117-043854
47. Labelle M, Begum S, Hynes RO. Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis. Cancer Cell. 2011;20:576–590. doi:10.1016/j.ccr.2011.09.009
48. Zhang Y, Unnithan RVM, Hamidi A, et al. TANK-binding kinase 1 is a mediator of platelet-induced EMT in mammary carcinoma cells. FASEB j. 2019;33:7822–7832. doi:10.1096/fj.201801936RRR
49. Pan S, Hu Y, Hu M, et al. Platelet-derived PDGF promotes the invasion and metastasis of cholangiocarcinoma by upregulating MMP2/MMP9 expression and inducing EMT via the p38/MAPK signalling pathway. Am J Transl Res. 2020;12:3577–3595.
50. Wang X, Zhao S, Wang Z, Gao T. Platelets involved tumor cell EMT during circulation: communications and interventions. Cell Commun Signal. 2022;20:82. doi:10.1186/s12964-022-00887-3
51. Paoli P, Giannoni E, Chiarugi P. Anoikis molecular pathways and its role in cancer progression. Biochim Biophys Acta. 2013;1833:3481–3498. doi:10.1016/j.bbamcr.2013.06.026
52. Liu Y, Zhang Y, Ding Y, Zhuang R. Platelet-mediated tumor metastasis mechanism and the role of cell adhesion molecules. Crit Rev Oncol Hematol. 2021;167:103502. doi:10.1016/j.critrevonc.2021.103502
53. Dhami SPS, Patmore S, Comerford C, et al. Breast cancer cells mediate endothelial cell activation, promoting von Willebrand factor release, tumor adhesion, and transendothelial migration. J Thromb Haemost. 2022;20:2350–2365. doi:10.1111/jth.15794
54. Reymond N, D’água BB, Ridley AJ. Crossing the endothelial barrier during metastasis. Nat Rev Cancer. 2013;13:858–870. doi:10.1038/nrc3628
55. Ie M S. Trophoblastic vasculogenic mimicry in gestational choriocarcinoma. Mod Pathol. 2011;24:646–652. doi:10.1038/modpathol.2010.231
56. Poto R, Cristinziano L, Modestino L, et al. Neutrophil extracellular traps, angiogenesis and cancer. Biomedicines. 2022;10. doi:10.3390/biomedicines10020431
57. Zhang P, Feng S, Liu G, et al. Mutant B-Raf(V600E) promotes melanoma paracellular transmigration by inducing thrombin-mediated endothelial junction breakdown. J Biol Chem. 2016;291:2087–2106. doi:10.1074/jbc.M115.696419
58. Mustafa S, Koran S, AlOmair L. Insights into the role of matrix metalloproteinases in cancer and its various therapeutic aspects: a review. Front Mol Biosci. 2022;9:896099. doi:10.3389/fmolb.2022.896099
59. Qi C, Wei B, Zhou W, et al. P-selectin-mediated platelet adhesion promotes tumor growth. Oncotarget. 2015;6:6584–6596. doi:10.18632/oncotarget.3164
60. Huang J, Li X, Shi X, et al. Platelet integrin αIIbβ3: signal transduction, regulation, and its therapeutic targeting. J Hematol Oncol. 2019;12:26. doi:10.1186/s13045-019-0709-6
61. Plantureux L, Mège D, Crescence L, Dignat-George F, Dubois C, Panicot-Dubois L. Impacts of cancer on platelet production, activation and education and mechanisms of cancer-associated thrombosis. Cancers (Basel). 2018;10. doi:10.3390/cancers10110441
62. Schmied L, Höglund P, Meinke S. Platelet-mediated protection of cancer cells from immune surveillance - possible implications for cancer immunotherapy. Front Immunol. 2021;12:640578. doi:10.3389/fimmu.2021.640578
63. Gupta GP, Nguyen DX, Chiang AC, et al. Mediators of vascular remodelling co-opted for sequential steps in lung metastasis. Nature. 2007;446:765–770. doi:10.1038/nature05760
64. Piao YS, Du YC, Oshima H, et al. Platelet-type 12-lipoxygenase accelerates tumor promotion of mouse epidermal cells through enhancement of cloning efficiency. Carcinogenesis. 2008;29:440–447. doi:10.1093/carcin/bgm274
65. Schumacher D, Strilic B, Sivaraj KK, Wettschureck N, Offermanns S. Platelet-derived nucleotides promote tumor-cell transendothelial migration and metastasis via P2Y2 receptor. Cancer Cell. 2013;24:130–137. doi:10.1016/j.ccr.2013.05.008
66. Cao Y, Chen E, Wang X, Song J, Zhang H, Chen X. An emerging master inducer and regulator for epithelial-mesenchymal transition and tumor metastasis: extracellular and intracellular ATP and its molecular functions and therapeutic potential. Cancer Cell Int. 2023;23:20. doi:10.1186/s12935-023-02859-0
67. Lambert AW, Pattabiraman DR, Weinberg RA. Emerging biological principles of metastasis. Cell. 2017;168:670–691. doi:10.1016/j.cell.2016.11.037
68. Zhou Z, Zhang B, Zai W, et al. Perfluorocarbon nanoparticle-mediated platelet inhibition promotes intratumoral infiltration of T cells and boosts immunotherapy. Proc Natl Acad Sci USA. 2019;116:11972–11977. doi:10.1073/pnas.1901987116
69. Chang LH, Chuang EY, Cheng TM, et al. Thrombus-specific theranostic nanocomposite for codelivery of thrombolytic drug, algae-derived anticoagulant and NIR fluorescent contrast agent. Acta Biomater. 2021;134:686–701. doi:10.1016/j.actbio.2021.07.072
70. Ke Y, Ma Z, Ye H, et al. Chlorogenic acid-conjugated nanoparticles suppression of platelet activation and disruption to tumor vascular barriers for enhancing drug penetration in tumor. Adv Healthc Mater. 2023;12:e2202205. doi:10.1002/adhm.202202205
71. Li S, Zhang Y, Wang J, et al. Nanoparticle-mediated local depletion of tumour-associated platelets disrupts vascular barriers and augments drug accumulation in tumours. Nat Biomed Eng. 2017;1:667–679. doi:10.1038/s41551-017-0115-8
72. Ma Z, Liu S, Ke Y, et al. Biomimetic nano-NOS mediated local NO release for inhibiting cancer-associated platelet activation and disrupting tumor vascular barriers. Biomaterials. 2020;255:120141. doi:10.1016/j.biomaterials.2020.120141
73. Wang S, Yin N, Li Y, et al. Copper-based metal-organic framework impedes triple-negative breast cancer metastasis via local estrogen deprivation and platelets blockade. J Nanobiotechnology. 2022;20:313. doi:10.1186/s12951-022-01520-8
74. Wang Y, Jian C, Long Y, Xu X, Song Y, Yin Z. H(2)O(2)-triggered “off/on signal” nanoparticles target P-selectin for the non-invasive and contrast-enhanced theranostics for arterial thrombosis. Acta Biomater. 2023;158:769–781. doi:10.1016/j.actbio.2022.12.026
75. Xu Y, Liu J, Liu Z, et al. Blockade of platelets using tumor-specific NO-releasing nanoparticles prevents tumor metastasis and reverses tumor immunosuppression. ACS Nano. 2020;14:9780–9795. doi:10.1021/acsnano.0c01687
76. Zhang X, Li X, Sun S, et al. Anti-tumor metastasis via platelet inhibitor combined with photothermal therapy under activatable fluorescence/magnetic resonance bimodal imaging guidance. ACS Appl Mater Interfaces. 2021;13:19679–19694. doi:10.1021/acsami.1c02302
77. Zhang Y, Wei J, Liu S, et al. Inhibition of platelet function using liposomal nanoparticles blocks tumor metastasis. Theranostics. 2017;7:1062–1071. doi:10.7150/thno.17908
78. Cao J, Yang P, Wang P, et al. ‘Adhesion and release’ nanoparticle-mediated efficient inhibition of platelet activation disrupts endothelial barriers for enhanced drug delivery in tumors. Biomaterials. 2021;269:120620. doi:10.1016/j.biomaterials.2020.120620
79. Xiao Y, Yu D. Tumor microenvironment as a therapeutic target in cancer. Pharmacol Ther. 2021;221:107753. doi:10.1016/j.pharmthera.2020.107753
80. Wang Y, Li W, Li Z, et al. Active recruitment of anti-PD-1-conjugated platelets through tumor-selective thrombosis for enhanced anticancer immunotherapy. Sci Adv. 2023;9:eadf6854. doi:10.1126/sciadv.adf6854
81. Li L, Fu J, Wang X, et al. Biomimetic “nanoplatelets” as a targeted drug delivery platform for breast cancer theranostics. ACS Appl Mater Interfaces. 2021;13:3605–3621. doi:10.1021/acsami.0c19259
82. Huang C, Ding S, Jiang W, Wang FB. Glutathione-depleting nanoplatelets for enhanced sonodynamic cancer therapy. Nanoscale. 2021;13:4512–4518. doi:10.1039/D0NR08440A
83. Chen Y, Shen X, Han S, et al. Irradiation pretreatment enhances the therapeutic efficacy of platelet-membrane-camouflaged antitumor nanoparticles. J Nanobiotechnology. 2020;18:101. doi:10.1186/s12951-020-00660-z
84. Ren D, Williams GR, Zhang Y, Ren R, Lou J, Zhu LM. Mesoporous doxorubicin-loaded polydopamine nanoparticles coated with a platelet membrane suppress tumor growth in a murine model of human breast cancer. ACS Appl Bio Mater. 2022;5:123–133. doi:10.1021/acsabm.1c00926
85. Zhou M, Lai W, Li G, et al. Platelet membrane-coated and VAR2CSA malaria protein-functionalized nanoparticles for targeted treatment of primary and metastatic cancer. ACS Appl Mater Interfaces. 2021;13:25635–25648. doi:10.1021/acsami.1c02581
86. Wang H, Wu J, Williams GR, et al. Platelet-membrane-biomimetic nanoparticles for targeted antitumor drug delivery. J Nanobiotechnology. 2019;17:60. doi:10.1186/s12951-019-0494-y
87. Chen Y, Zhao G, Wang S, et al. Platelet-membrane-camouflaged bismuth sulfide nanorods for synergistic radio-photothermal therapy against cancer. Biomater Sci. 2019;7:3450–3459. doi:10.1039/C9BM00599D
88. Qi P, Zhang J, Bao Z, Liao Y, Liu Z, Wang J. A platelet-mimicking single-atom nanozyme for mitochondrial damage-mediated mild-temperature photothermal therapy. ACS Appl Mater Interfaces. 2022;14:19081–19090. doi:10.1021/acsami.1c22346
89. Luo X, Cao J, Yu J, et al. Regulating acidosis and relieving hypoxia by platelet membrane-coated nanoparticle for enhancing tumor chemotherapy. Front Bioeng Biotechnol. 2022;10:885105. doi:10.3389/fbioe.2022.885105
90. Zou J, He J, Wang X, et al. Glycoprotein Ib-regulated micro platelet ghost for biosafe distribution and photothermal oncotherapy. J Control Release. 2022;351:341–360. doi:10.1016/j.jconrel.2022.09.036
91. Li W, Li F, Li T, et al. Self-actuated biomimetic nanocomposites for photothermal therapy and PD-L1 immunosuppression. Front Chem. 2023;11:1167586. doi:10.3389/fchem.2023.1167586
92. Wan S, Fan Q, Wu Y, et al. Curcumin-loaded platelet membrane bioinspired chitosan-modified liposome for effective cancer therapy. Pharmaceutics. 2023;15. doi:10.3390/pharmaceutics15020631
93. Du Y, Wang S, Luan J, Zhang M, Chen B, Shen Y. GOx-functionalized platelet membranes-camouflaging nanoreactors for enhanced multimodal tumor treatment. Int J Nanomedicine. 2022;17:2979–2993. doi:10.2147/IJN.S358138
94. Ning S, Zhang T, Lyu M, et al. A type I AIE photosensitiser-loaded biomimetic nanosystem allowing precise depletion of cancer stem cells and prevention of cancer recurrence after radiotherapy. Biomaterials. 2023;295:122034. doi:10.1016/j.biomaterials.2023.122034
95. Pei W, Huang B, Chen S, Wang L, Xu Y, Niu C. Platelet-mimicking drug delivery nanoparticles for enhanced chemo-photothermal therapy of breast cancer. Int J Nanomedicine. 2020;15:10151–10167. doi:10.2147/IJN.S285952
96. Yan H, Zhang Y, Zhang Y, et al. A ROS-responsive biomimetic nano-platform for enhanced chemo-photodynamic-immunotherapy efficacy. Biomater Sci. 2022;10:6583–6600. doi:10.1039/D2BM01291J
97. Zhuang J, Gong H, Zhou J, et al. Targeted gene silencing in vivo by platelet membrane-coated metal-organic framework nanoparticles. Sci Adv. 2020;6:eaaz6108. doi:10.1126/sciadv.aaz6108
98. Lu Q, Ye H, Wang K, et al. Bioengineered platelets combining chemotherapy and immunotherapy for postsurgical melanoma treatment: internal core-loaded doxorubicin and external surface-anchored Anti-PD-L1 antibody backpacks. Nano Lett. 2022;22:3141–3150. doi:10.1021/acs.nanolett.2c00907
99. Li T, Chen T, Chen H, et al. Engineered platelet-based micro/nanomotors for cancer therapy. Small. 2021;17:
100. Gao Y, Chen X, Wang B, et al. Engineering platelets with PDL1 antibodies and iron oxide nanoparticles for postsurgical cancer immunotherapy. ACS Appl Bio Mater. 2023;6:257–266. doi:10.1021/acsabm.2c00869
101. Li QR, Xu HZ, Xiao RC, et al. Laser-triggered intelligent drug delivery and anti-cancer photodynamic therapy using platelets as the vehicle. Platelets. 2023;34:2166677. doi:10.1080/09537104.2023.2166677
102. Zhang Y, Sun Y, Dong X, et al. A platelet intelligent vehicle with navigation for cancer photothermal-chemotherapy. ACS Nano. 2022;16:6359–6371. doi:10.1021/acsnano.2c00453
103. Zhou L, Feng W, Mao Y, Chen Y, Zhang X. Nanoengineered sonosensitive platelets for synergistically augmented sonodynamic tumor therapy by glutamine deprivation and cascading thrombosis. Bioact Mater. 2023;24:26–36. doi:10.1016/j.bioactmat.2022.11.020
104. Hu Q, Li H, Archibong E, et al. Inhibition of post-surgery tumour recurrence via a hydrogel releasing CAR-T cells and anti-PDL1-conjugated platelets. Nat Biomed Eng. 2021;5:1038–1047. doi:10.1038/s41551-021-00712-1
105. Fan X, Wang K, Lu Q, et al. Surface-anchored tumor microenvironment-responsive protein nanogel-platelet system for cytosolic delivery of therapeutic protein in the post-surgical cancer treatment. Acta Biomater. 2022;154:412–423. doi:10.1016/j.actbio.2022.10.031
106. Jing L, Qu H, Wu D, et al. Platelet-camouflaged nanococktail: simultaneous inhibition of drug-resistant tumor growth and metastasis via a cancer cells and tumor vasculature dual-targeting strategy. Theranostics. 2018;8:2683–2695. doi:10.7150/thno.23654
107. Cacic D, Hervig T, Reikvam H. Platelets for advanced drug delivery in cancer. Expert Opin Drug Deliv. 2023;20:673–688. doi:10.1080/17425247.2023.2217378
108. Xia D, Hang D, Li Y, et al. Au-hemoglobin loaded platelet alleviating tumor hypoxia and enhancing the radiotherapy effect with low-dose X-ray. ACS Nano. 2020;14:15654–15668. doi:10.1021/acsnano.0c06541
109. Xu HZ, Li TF, Ma Y, et al. Targeted photodynamic therapy of glioblastoma mediated by platelets with photo-controlled release property. Biomaterials. 2022;290:121833. doi:10.1016/j.biomaterials.2022.121833
110. Li QR, Xu HZ, Xiao RC, et al. Platelets are highly efficient and efficacious carriers for tumor-targeted nano-drug delivery. Drug Deliv. 2022;29:937–949. doi:10.1080/10717544.2022.2053762
111. Raghunathan S, Rayes J, Sen Gupta A. Platelet-inspired nanomedicine in hemostasis thrombosis and thromboinflammation. J Thromb Haemost. 2022;20:1535–1549. doi:10.1111/jth.15734
112. Wang C, Sun W, Ye Y, Hu Q, Bomba HN, Gu Z. In situ activation of platelets with checkpoint inhibitors for post-surgical cancer immunotherapy. Nature Bio Engine. 2017;1. doi:10.1038/s41551-016-0011
113. Li Z, Ding Y, Liu J, et al. Depletion of tumor associated macrophages enhances local and systemic platelet-mediated anti-PD-1 delivery for post-surgery tumor recurrence treatment. Nat Commun. 2022;13:1845. doi:10.1038/s41467-022-29388-0
114. Oggero S, de Gaetano M, Marcone S, et al. Extracellular vesicles from monocyte/platelet aggregates modulate human atherosclerotic plaque reactivity. J Extracell Vesicles. 2021;10(12084). doi:10.1002/jev2.12084
115. Żmigrodzka M, Guzera M, Miśkiewicz A, Jagielski D, Winnicka A. The biology of extracellular vesicles with focus on platelet microparticles and their role in cancer development and progression. Tumour Biol. 2016;37:14391–14401. doi:10.1007/s13277-016-5358-6
116. Johnson J, Wu YW, Blyth C, Lichtfuss G, Goubran H, Burnouf T. Prospective therapeutic applications of platelet extracellular vesicles. Trends Biotechnol. 2021;39:598–612. doi:10.1016/j.tibtech.2020.10.004
117. Yao C, Wang C. Platelet-derived extracellular vesicles for drug delivery. Biomater Sci. 2023;11:5758–5768. doi:10.1039/D3BM00893B
118. Opoku-Damoah Y, Wang R, Zhou J, Ding Y. Versatile nanosystem-based cancer theranostics: design inspiration and predetermined routing. Theranostics. 2016;6:986–1003. doi:10.7150/thno.14860
119. Modery-Pawlowski CL, Tian LL, Pan V, McCrae KR, Mitragotri S, Sen Gupta A. Approaches to synthetic platelet analogs. Biomaterials. 2013;34:526–541. doi:10.1016/j.biomaterials.2012.09.074
120. D’Ambrosi S, Nilsson RJ, Wurdinger T. Platelets and tumor-associated RNA transfer. Blood. 2021;137:3181–3191. doi:10.1182/blood.2019003978
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.