Back to Journals » Infection and Drug Resistance » Volume 12
Plastic binding feature of polymyxins: the effect on MIC susceptibility measurements
Authors Sharafi T, Ardebili A
Received 11 June 2019
Accepted for publication 23 July 2019
Published 27 August 2019 Volume 2019:12 Pages 2649—2653
DOI https://doi.org/10.2147/IDR.S219130
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Toktam Sharafi,1,2 Abdollah Ardebili1,2
1Infectious Disease Research Center, Faculty of Medicine, Golestan University of Medical Sciences, Gorgan, Iran; 2Department of Microbiology, Faculty of Medicine, Golestan University of Medical Sciences, Gorgan, Iran
Correspondence: Abdollah Ardebili
Golestan University of Medical Sciences, P.O. Box: 4934174515, Gorgan, Iran
Tel +98 173 242 1651
Fax +98 173 244 0225
Email [email protected]
Abstract: The loss of polycationic antimicrobial peptides, polymyxins, due to adhesion to plastics is an important subject matter that influences in vitro susceptibility testing, including minimum inhibitory concentration (MIC) assays. This review reminds us that this issue can serve as a significant source of variation in the MIC values for polymyxins against Gram-negative bacteria.
Keywords: broth microdilution, colistin, Gram-negative bacteria, MIC, polymyxin B
Introduction
Given the rise of multidrug-resistant Gram-negative bacilli, such as Pseudomonas aeroginosa, Acinetobacter baumannii, and Klebsiella penomoniae, as well as the limited number of effective antibiotics, polymyxin class of antibiotics (polymyxin B and polymyxin E [colistin]) are currently the only remaining treatment options.1,2 This enforces a significant effort to maintain the antibacterial properties of these antibiotics and delay the emergence of resistance. Development of clear guidance regarding the indications, dosing, duration, and combination of polymyxins appears to be needed to improve clinical outcomes.1 In addition, standardization of routine susceptibility testing of clinical isolates for polymyxins may be a major contributor to achieve this purpose.3
Both the Clinical and Laboratory Standards Institute and the European Committee on Antimicrobial Susceptibility Testing have considered broth microdilution (BMD) as a gold standard reference method for MIC determination of all antibiotics.4,5 It has been recommended that the assay should be conducted with cation-adjusted Mueller–Hinton broth, a range of two-fold dilutions of an antibiotic, and a final inoculum size of 5×105 CFU/mL in round or conical bottom wells of a sterile plastic microdilution plate.6 Some technical problems have been reported with polymyxin MIC testing, including brand-to-brand heterogeneity in polymyxin formulations, drug binding to plastic materials, the presence of “skipped well” phenomenon, brand-to-brand variation in cation composition of MH media, and the potential antimicrobial activity of additives (eg, polysorbate-80, serum, and proteins) to reduce drug adhesion to microplates.3 Here, we specifically discuss on the type and nature of the container in which the assay is performed and enlighten their effects on MIC susceptibility to these antimicrobial compounds; the issues which can serve as a source of variability in MIC results and were generally neglected in the most published studies.
Nature, type, and surface treatment of microplates
Polypropylene and polystyrene are two types of commonly used polymers for plastic laboratory consumables. Polypropylene is a thermoplastic polymer used to construct plastic laboratory supplies, including beakers, bottles, flasks, specimen containers, test tubes, and microplates. Although they are widely used in laboratories and industries because of their high chemical resistance, as well as high level of temperature tolerance, application of polypropylene plastics in microbiology is unfortunately hampered by their low optical clarity, so it is problematic when growth is evaluated visually or by optical density, eg, in MIC measurements.7 Polystyrene as a clear, hard, and brittle polymer is used for bottles, flasks, test tubes, microplates, and petri dishes. Due to the low melting point of polystyrene between 64°C and 80°C, polystyrene products are not autoclavable; hence, they are the classic laboratory disposables.7
Microplates used in BMD assay to measure MIC values are generally made of polystyrene. As an important issue, because of passive interactions between samples, such as DNA, proteins, or cationic antibiotics, and well surface of microplate, adsorption of samples to the plate surface takes place.8,9 Due to their material properties, polypropylene products present less biomolecule adsorption than polystyrene. However, surface properties of polystyrene microplates are often modified by manufactures in many ways, whether by physical, chemical, or coating methods, to accommodate requirements for various applications in cell culture, immunosorbent assays, etc. There are different types of surface modification of microplates by applications (Figure 1). Polystyrene microplates without surface treatment (non-treated) are hydrophobic in nature and have low biomolecular binding feature. However, for very sensitive applications, even low amounts of biomolecular binding can interfere with the assay. Tissue culture microplates provide the standard surface for culture of anchorage-dependent cells. These products are subjected to special surface treatment, causing the incorporation of polar groups, such as hydroxyl (–OH), carboxyl (–COOH), and amino (–NH2) residues, to increase the surface hydrophility and introduce a negative or positive charge, consistent with cell attachment. High-binding surface polystyrene microplates feature a relatively high number of ionic groups and/or hydrophobic regions with the ability to bind to large biomolecules. Non-binding surface is another treatment technology for polystyrene microplates that creates a nonionic hydrophilic polyethylene oxide-like surface to minimize passive molecular interactions, leading to reduced non-specific immobilization of biomolecules.
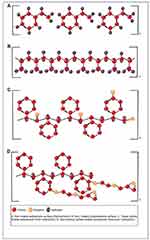 |
Figure 1 Different types of surface modification of plates used in broth microdilution assay. |
What are the effects of microplate type on polymyxin MIC testing?
Polymyxins, as lipopeptide antibiotics, are well-known biomolecules and their inherent polycationic property causes them to adhere to a wide range of materials, including plastics used in microbiology laboratories.3 This causes the antibiotic to be lost considerably during the in vitro experiments, such as MIC assays, resulting in unreliable data from standard laboratory procedures. While its implications are potentially serious, neither scientific communities nor literature have satisfactorily characterized the matter of loss of polymyxins during experiments, although a number of recent papers have discussed the problem.8–12
In this issue, several studies demonstrated that plate type can lead to significant variations in MIC assay results.10–12 Bock et al reported that MIC values for cationic antibiotics (polymyxins) and some cationic disinfectants (chlorhexidine digluconate and octenidine hydrochloride) are influenced significantly by plate type.12 By polypropylene plates, the median MIC values to both polymyxin B and colistin for E. coli NCTC 10,418 and P. aeruginosa NCTC 13,359 reference stains were >4-fold and ≥4-fold lower, respectively, when compared to polystyrene. Singhal et al found that the validity of BMD with polystyrene plates (BMD-Ps) was lower than that of a reference standard, where this assay was unable to identify three colistin- and polymyxin B-susceptible isolates of carbapenem-resistant A. baumannii.11 In addition, Albur et al evaluated MICs of colistin for the isolates, including 56 P. aeruginosa, 29 Acinetobacter spp., and 61 Enterobacteriaceae using two different types of polystyrene microtitre trays (MTTs), namely non-coated V-bottom MTTs (NMTTs) and tissue-culture-coated round-bottom MTTs (TCMTTs).10 The authors found an overall 5.3-fold increase in MIC value using TCMTTs, most likely due to decreased free antibiotic concentration within the wells resulted by the enhanced negative electric charge of microplate well surface.10 The different containers, including 10-mL soda-lime glass tubes, 15-mL polypropylene tubes, 10-mL polystyrene tubes, and 1.5 mL low-protein-binding polypropylene microtubes, have been compared in a comprehensive study with regard to the total colistin binding.8 At different initial concentrations of colistin ranging from 0.125 μg/mL to 8 μg/mL, low-protein-binding polypropylene microtube showed the least loss (with 59–90% of expected values left at 24 hrs), followed by polypropylene tubes (with 23–90% of expected values left at 24 hrs), glass tubes (with 25–80% of expected values left at 24 hrs), and polystyrene tubes (with 8.4–84% of expected values left at 24 hrs). Taken together, these findings indicate that the polymyxin concentration will appear to be lower than the actual concentration, so care should be taken during the all the steps of experiments to reduce the effect of adsorption. Low-protein binding or non-binding surface plastic materials appear to be suitable for cationic compound susceptibility testing. A study by Kavanagh et al demonstrated that non-binding surface plates provided significantly more potent MIC values for some cationic antimicrobial peptides, including lipoglycopeptides and polymyxins in comparison with other types of plates used.9 Regarding the polymyxins, non-binding plates resulted in MIC values of ≤0.03 μg/mL for both colistin and polymyxin B versus E. coli ATCC 25,922, while untreated and tissue culture-treated polystyrene plates gave less potent MICs.9 Likewise, MIC assays for cationic antimicrobial peptides can be performed with some additives, such as broth containing 50–95% human or mouse serum, 3–4% human or bovine serum albumin as a protein supplement, 2% lysed horse blood, and 0.002% polysorbate-80 to improve drug availability.13–16 In the case of serum or protein supplement, it seems that because of the adsorption of proteins to plate surfaces, binding sites of antibiotics are occupied, resulting in avoiding or minimizing the possibility of the cationic molecule adsorption to plates. In addition, polysorbate-80 is a dispersing agent that has been shown to inhibit the binding of drug to plastics at a final concentration of 0.002%.17,18 Nevertheless, it is important to note that high concentrations of serum as well as polysorbate-80 may show a synergistic effect in combination with antibiotics on bacterial growth.14,19 In addition, MIC assessments of cationic antibiotics that bind to both protein and plastic may be confused by contrary effects. Protein binding of the polymyxin lipopeptides is estimated to be 50–60%20 that reduces the concentration of free antibiotics available for antimicrobial activity, lessening their MIC potency; while the added protein also decreases non-specific binding to plastic, leading to more potent MIC values.
As for the type of test container, loss of polymyxins during the experiment has been shown to vary depending on the manufacturer. Two recent studies found that the brand of microplates effects on polymyxin activity.8,9 The Thermo Scientific Nunc, Corning Life Sciences, and Trek Diagnostics Systems plates were compared in BMD assay against the E. coli ATCC 25,922.9 For colistin, Treck non-treated polystyrene plate led to at least MIC value (≤0.03 μg/mL), followed by Corning (0.06 μg/mL) and Nunc (0.125–1 μg/mL) plates. For polymyxin B, Corning nontreated polystyrene plate gave more potent MIC value (0.06 μg/mL) than Treck (0.06–0.125 μg/mL) and Nunc (0.125–0.25 μg/mL) plates.9 The study by Karvanen et al found remarkable differences in colistin concentrations between different brands of noncoated round-bottom polystyrene microplates.8 The author observed that after 24-hrs incubation, only 2% and 70% of an expected 8 μg/mL concentration of colistin remained in the Greiner Bio-One and Nunc polystyrene microplates, respectively.
Conclusion
In summary, the loss of polymyxins during the time course of BMD susceptibility assays serves as a great condition-specific systematic error in microbiology laboratories, emphasizing the need to characterize relevant issues, such as plate types and suppliers and any additives used. Out of materials employed in different studies, low-protein binding or non-binding surface microplates appear to be beneficial to improve the problem of antibiotic adsorption and can exclude the need for adding serum or albumin protein. Future investigations should be conducted to determine which type of plate provides true polymyxin MIC that is most relevant to the clinical activity of drug.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Wertheim H, Van Nguyen K, Hara GL, et al. Global survey of polymyxin use: a call for international guidelines. J Glob Antimicrob Resist. 2013;1(3):131–134. doi:10.1016/j.jgar.2013.03.012
2. Klein EY, Van Boeckel TP, Martinez EM, et al. Global increase and geographic convergence in antibiotic consumption between 2000 and 2015. Proc Natl Acad Sci USA. 2018;115(15):E3463–E3470. doi:10.1073/pnas.1717295115
3. Ezadi F, Ardebili A, Mirnejad R. Antimicrobial susceptibility testing for polymyxins: challenges, issues, and recommendations. J Clin Microbiol. 2019;57:e01390–e01418. doi:10.1128/JCM.01390-18
4. Clinical and Laboratory Standards Institute. 2018. Performance standards for antimicrobial susceptibility testing. CLSI document M100, 28th ed.
5. European Committee on Antimicrobial Susceptibility Testing. Breakpoint tables for interpretation of MICs and zone diameters, version8.1; 2018. Available from: http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_8.1_Breakpoint_Tables.pdf.
6. Clinical and Laboratory Standards Institute. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically;approved Standard. 11th ed. CLSI standard M07. Wayne, PA: Clinical and Laboratory Standards Institute; 2018. Available from: https://www.techstreet.com/mss/products/preview/2003549.
7. Zaehrlinger H. The age of plastic labware. Lab Times. 2008;2:54–58.
8. Karvanen M, Malmberg C, Lagerbäck P, Friberg LE, Cars O. Colistin is extensively lost during standard in vitro experimental conditions. Antimicrob Agents Chemother. 2017;61(11). doi:10.1128/AAC.00857-17
9. Kavanagh A, Ramu S, Gong Y, Cooper MA, Blaskovich MA. Effects of microplate type and broth additives on microdilution MIC susceptibility assays. Antimicrob Agents Chemother. 2019;63(1):e01760–e01818. doi:10.1128/AAC.01760-18
10. Albur M, Noel A, Bowker K, MacGowan A. Colistin susceptibility testing: time for a review. J Antimicrob Chemother. 2014;69(5):1432–1434. doi:10.1093/jac/dkt503
11. Singhal L, Sharma M, Verma S, et al. Comparative evaluation of broth microdilution with polystyrene and glass-coated plates, agar dilution, E-test, Vitek, and disk diffusion for susceptibility testing of colistin and polymyxin B on carbapenem-resistant clinical isolates of Acinetobacter baumannii. Microb Drug Resist. 2018;24(8):1082–1088. doi:10.1089/mdr.2017.0251
12. Bock LJ, Hind CK, Sutton JM, Wand ME. Growth media and assay plate material can impact on the effectiveness of cationic biocides and antibiotics against different bacterial species. Lett Appl Microbiol. 2018;66(5):368–377. doi:10.1111/lam.12863
13. Wiegand I, Hilpert K, Hancock RE. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat Protoc. 2008;3(2):163–175. doi:10.1038/nprot.2007.521
14. Zeitlinger MA, Derendorf H, Mouton JW, et al. Protein binding: do we ever learn? Antimicrob Agents Chemother. 2011;55(7):3067–3074. doi:10.1128/AAC.01433-10
15. Tsuji BT, Leonard SN, Rhomberg PR, Jones RN, Rybak MJ. Evaluation of daptomycin, telavancin, teicoplanin, and vancomycin activity in the presence of albumin or serum. Diagn Microbiol Infect Dis. 2008;60(4):441–444. doi:10.1016/j.diagmicrobio.2007.11.011
16. Arhin FF, Sarmiento I, Belley A, et al. Effect of polysorbate 80 on oritavancin binding to plastic surfaces: implications for susceptibility testing. Antimicrob Agents Chemother. 2008;52(5):1597–1603. doi:10.1128/AAC.01513-07
17. Sader HS, Rhomberg PR, Flamm RK, Jones RN. Use of a surfactant (polysorbate 80) to improve MIC susceptibility testing results for polymyxin B and colistin. Diagn Microbiol Infect Dis. 2012;74(4):412–414. doi:10.1016/j.diagmicrobio.2012.08.025
18. Sutherland CA, Nicolau DP. To add or not to add polysorbate 80: impact on colistin MICs for clinical strains of Enterobacteriaceae and Pseudomonas aeruginosa and quality controls. J Clin Microbiol. 2014;52(10):3810. doi:10.1128/JCM.01454-14
19. Brown MR, Winsley BE. Effect of polysorbate 80 on cell leakage and viability of Pseudomonas aeruginosa exposed to rapid changes of pH, temperature and tonicity. J Gen Microbiol. 1969;56(1):99–107. doi:10.1099/00221287-56-1-99
20. Li J, Milne RW, Nation RL, Turnidge JD, Smeaton TC, Coulthard K. Use of high-performance liquid chromatography to study the pharmacokinetics of colistin sulfate in rats following intravenous administration. Antimicrob Agents Chemother. 2003;47(5):1766–1770. doi:10.1128/aac.47.5.1766-1770.2003
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
