Back to Journals » International Medical Case Reports Journal » Volume 17
Plasmodium Vivax as a Causative Agent for Cerebral Malaria in a Group of Adults at Mizan Tepi Teaching Hospital: Case Series
Authors Habtemariam Y , Asnake M , Alemu M, Shash EP , Tessema TW, Tesso ZG, Hawlet M
Received 17 October 2023
Accepted for publication 7 March 2024
Published 13 March 2024 Volume 2024:17 Pages 161—166
DOI https://doi.org/10.2147/IMCRJ.S440800
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Vinay Kumar
Yosef Habtemariam,1 Molla Asnake,2 Misikr Alemu,3 Erkyehun Pawlos Shash,3 Tsegaw Worku Tessema,2 Zerubabel Girma Tesso,1 Michael Hawlet4
1School of Medicine, College of Medicine and Health Sciences, Mizan-Tepi University, Mizan Aman, Ethiopia; 2School of Medicine, Adult ICU Unit, College of Medicine and Health Sciences, Mizan-Tepi University, Mizan Aman, Ethiopia; 3School of Medicine, Department of Internal Medicine, College of Medicine and Health Sciences, Mizan-Tepi University, Mizan Aman, Ethiopia; 4Department of Pediatrics and Child Health, School of Medicine, College of Medicine and Health Sciences, Wolkite University, Wolkite, Ethiopia
Correspondence: Molla Asnake, School of Medicine, Adult ICU Unit, College of Medicine and Health Sciences, Mizan-Tepi University, Mizan Aman, Ethiopia, Email [email protected]
Abstract: In 2022, there were 249 million cases of malaria globally, resulting in 608,000 deaths. The majority of cases and deaths occurred in the WHO (World Health Organization) African Region. A study in our region found that, out of 263,476 individuals, 148,734 had P. falciparum, 106,946 had P. vivax, and 7,796 had mixed infections. The prevalence of P. falciparum (Plasmodium falciparum) was 8.97% and P. vivax (Plasmodium Vivax) was 7.94%. Although there have been a few reported cases of cerebral malaria caused by P. vivax, there is currently no comprehensive analysis of such cases. All the cases that have been reported so far involved individuals living in malaria-endemic areas, who presented with symptoms characteristic of cerebral malaria. Cerebral malaria was diagnosed based on the clinical algorithm which WHO used except we used P. vivax instead of P. falciparum The diagnosis of these cases was confirmed through thin blood film examination and Rapid Diagnostic Tests (RDTs). Therefore, this report aims to provide additional data on the occurrence of P. vivax as a cause of cerebral malaria. It also recommends further studies to reassess the current clinical case definition of cerebral malaria mainly in endemic areas as it affects patient treatment outcome.
Keywords: cerebral malaria, P. Vivax and Blood Film
Introduction
P. falciparum and P. vivax are the most dangerous malaria parasites, with P. falciparum being the deadliest and most common in Africa. In 2022, there were 249 million cases of malaria globally, resulting in 608,000 deaths. The majority of cases and deaths occurred in the WHO African Region. A study in our region found that, out of 263,476 individuals, 148,734 had P. falciparum, 106,946 had P. vivax, and 7,796 had mixed infections. The prevalence of P. falciparum was 8.97% and P. vivax was 7.94%.1–3
Its burden decreased from 24.5 million cases in 2000 to 14.3 million in 2017; however, its rate increased in regions affected by political and economic instability.4 In sub-Saharan Africa most people do not express Duffy factor on the surface of red blood cells, as a result the prevalence of P. vivax infection in that region is low.5 In Ethiopia, Plasmodium vivax and mixed infections accounted for 35.5% and 6.3% of malaria infection, respectively. Dima, located 80 km from the hospital where the authors work, is ranked first in the country in terms of prevalence of slide-positive malaria (46.1%).6,7
Previously, P. vivax was considered a benign disease; however, now it is known that it can cause severe morbidity and mortality.8,9 Infection with P. falciparum should be considered if someone develops manifestations of severe disease except in the case of acute lung injury and acute respiratory distress syndrome (P. vivax is also associated).10 One form of severe malaria is cerebral malaria, which is defined as an encephalopathy that presents with impaired consciousness, delirium, and/or seizures.11 It is almost universally fatal If untreated; however, with treatment the mortality is between 15 to 20%.12,13 There are three criteria that should be fulfilled according to the standard clinical case definition of cerebral malaria, and being positive for P. falciparum parasitemia (not P. Vivax) is one.4,11 The WHO defines cerebral malaria as a clinical syndrome characterized by; 1) coma at least 1 hour after termination of a seizure or correction of hypoglycemia; 2) Confirmed asexual forms of Plasmodium falciparum parasites on peripheral blood smears; and 3) Absence of other known causes to explain the coma.14
To our knowledge, there are few cases which report cerebral malaria with P. vivax malaria; however, there is no case series in a single institution as a prospective study. Therefore, this report is written in order to provide more compressive additional data on P. vivax as a cause of cerebral malaria and to recommend further study in order to reconsider the clinical case definition of cerebral malaria.
Methods and Materials
In this case series, we include three cases that were presented to Mizan-Tepi University Teaching Hospital between September 2022 and July 2023. Ethical approval was obtained from Mizan-Tepi University College of Medicine and Health Science Research Ethics Review Committee (ref. no. CRERC/01/13, 01/02/2021). Their diagnosis was a case of cerebral malaria due to P. vivax using the WHO criteria except we substituted positive for P. vivax instead of positive for P. falciparum. All other causes of comas were ruled out as much as possible. The blood slides were thin film (advised for species identification) and seen by two senior laboratory technicians who worked for more than 15 years.14 After the blood film was done, we did an RDT test which has 100% specificity for P. vivax for confirmation.15
Laboratory Testes
- The RDT we used is a serology test that has been approved by the US Food and Drug Administration: the BinaxNOW®. The samples were taken by fingerpicking. It has P. falciparum sensitivity and specificity of 95% and 94%, respectively; the P. vivax sensitivity and specificity are 69% and 100%, respectively.
- For blood for smear preparation, the samples may be collected by fingerstick as it is more sensitive and slides were prepared using Giemsa staining. The samples were visualized for at least 20 minutes.
Inclusion Criteria
All patients who had signs and symptoms of cerebral malaria with a GCS (Glasgow Coma Scale) of < (9) and other causes of acute encephalopathy such as illicit drug use, hypoglycemia, hepatic and renal failure, alcohol intoxication, Wernicke encephalopathy, hypoxic-ischemic encephalopathy, heat stroke, shock, and electrolyte imbalance hads been ruled out. The presence of P. vivax on the thin slides was seen in two laboratories and confirmed with RDT tests.
Exclusion Criteria
Patients who had discordant test results, were treated with empiric antibiotic therapy, died during treatment, were comatose for only 1 hour after seizure, or had a focal neurologic deficit were excluded.
Case Series
Case 1
A 31-year-old male patient was diagnosed with cerebral malaria after being presented with Loc (loss of consciousness) for 8 hours duration. He also had fever, chills, and vomiting for 2 days. The vitals were normal except for a fever (39°C). Microscopy of thin blood smears revealed parasites morphologically similar to P. vivax with a parasitic load of +3 in thick blood film (Figure 1) and the rapid diagnostic test indicated the presence of P. vivax (Figure 2). He was treated with Artesunate over 4 days and was on a mechanical ventilator for 48 hours for indication of coma (Glasgow coma scale = 7/10). The patient had no history of chronic illness such as DM (diabetes mellitus), his CBC (complete blood count) was in the normal range, a HIV test was negative, his RBS (random blood sugar) at admission to ICU (Intensive care unit) was 187, and he had a normal electrolyte level, and LP (Lumbar Puncture) was not done due to the coma. He was discharged on his fifth admission day, and the blood film was negative on the second day.
 |
Figure 1 Thin blood film taken from finger prick showing P. vivax (Trophozoite stage). |
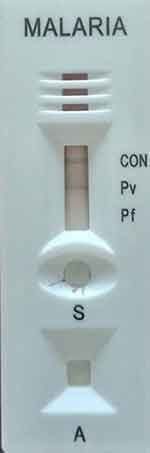 |
Figure 2 RDT test showing positive P. vivax result. |
Case 2
A 28-year-old female presented via ambulance following being referred from a nearby health center. The total illness duration was 2 days. She had a history of three episodes of seizure on the way to the hospital, LOC of 10 hours, and repeated vomiting. On examination, her GCS was 6/15, and her body temperature was 40°C. On thin blood film, there were P. vivax and on thick blood film, the parasite load was plus three. Immediately, the patient was admitted to the adult ICU and intubated. She was administered intravenous fluids and was prescribed artesunate and paracetamol. His seizures were controlled with diazepam and phenytoin. Her RBS was 191, and her HIV tests were negative. Her blood film changed to negative on the third day of admission. The patient was discharged after 4 days. Her CSF analysis was in the normal range.
Case 3
A 42-year-old male was presented to the emergency department by his family with a complaint of LOC of 4 hours duration. They also reported that he had a fever, chills, and vomiting. They did not witness abnormal body movement. His GCS was 12/15. He was tachycardic (106) and Febrile (19 oc}; however, his blood pressure and respiratory rate were normal. CBC was normal apart from mild normocytic, normochromic anemia. Abdominal ultrasound showed splenomegaly (4 cm below the costal margin). On thin blood film, there were P. vivax and on thick blood film, the parasite load was plus four. The patient was admitted to the ICU, resuscitated, and prescribed artesunate and paracetamol. Feeding was provided through an NG (Naso Gastric) tube. His blood film was negative on the second day. He became conscious on the second day and developed delirium which responded to non-pharmacological therapy. The patient was discharged on the fifth day with Quartum.
Summarized Results
The analysis included a total of three patients, among which two were males and one was a female patient. The mean age of the patients was 34 years (range = 28–42 years). All the patients presented with typical symptoms of malaria, such as fever (100%), headache (66%), and chills (100%). However, the clinical presentation of cerebral malaria was distinct, with symptoms including altered mental status (GCS <8), seizures (n = 1, 33%), and focal neurological deficits were not seen in all patients. Laboratory investigations revealed high levels of parasitemia (+3 and +4). All patients improved with the treatment of intravenous artesunate.
Discussion
Although cerebral malaria is commonly associated with P. falciparum malaria due to its epidemiology and pathogenesis, it should not be assumed that patients with severe malaria and positive P. vivax infections are solely the result of mixed infections.16,17 This case series highlights the growing recognition of P. vivax as a significant causative agent for cerebral malaria in adults at Mizan Tepi Teaching Hospital. Previously, P. vivax was considered as a benign malaria species; however, recent evidence suggests that it is able to lead to severe manifestations, including cerebral involvement. Among factors that may contribute to the pathogenicity of Plasmodium vivax is the presence of the Duffy antigen receptor on red blood cells. It is necessary for Plasmodium vivax invasion unlike P. falciferum. Even if non-conclusive, studies have demonstrated the ability of Plasmodium vivax to cause severe disease through mechanisms such as cytoadherence and sequestration, similar to Plasmodium falciparum.4,7,10
As provided by the WHO diagnosis algorithm of CM, the diagnosis was made after the exclusion of other causes of coma by different investigations except for meningitis because being comatose is a contraindication to do a lumbar puncture (a diagnostic test for meningitis) in a setup where CT (computed tomography) is not available to rule out increased ICP (intracranial pressure).18 However, the following evidence makes meningitis unlikely. One, the patient’s condition had improved after they were treated with only artesunate (antimalarial drug) but not with antibiotics (treatment for meningitis). Two, patient symptoms were explainable with cerebral malaria. Third, patients had a positive smear and RDT test, and they live in malaria-endemic areas. Fourth, absence of nuchal rigidity/pain (a typical symptom of meningitis) before losing consciousness and after regaining consciousness.19,20
All cases presented with symptoms of febrile illness and live in malaria-endemic areas, and it is stated that if a patient who lives in a malaria-endemic area presents with symptoms of febrile illness, physicians should consider malaria the most likely diagnosis.8,13 A patient with cerebral malaria presents with altered consciousness, seizures, and delirium; however, focal neurologic signs are unusual.21 Cases included in this case report presented with loss of consciousness, and only one patient manifested with seizures; however, none of them have a focal neurologic deficit. This evidence goes along with cerebral malaria. Being a child or elderly, being pregnant, having poor nutritional status, having HIV infection, and history of splenectomy are risk factors for cerebral malaria; however, none of the cases had these risk factors except the female patient who had a positive pregnancy test.22 The presence of cerebral malaria without having a specific risk factor, especially in the two male patients, may add a new finding to the science community. Although in cerebral malaria mortality is 15 to 20%, these patients have good outcomes, and it is due to the exclusion of patients who died during treatment. We excluded because we were not able to do EEG (Electroencephalography), CT, and LP due to the presence of contraindications and the absence of the diagnostic tool in our setup.12 As explained in the case presentation, all patients rapidly deteriorated to coma within 72 hours of their symptom commencement, and it indicates cerebral malaria or meningitis, however, due to the four reasons mentioned above the likelihood of meningitis is nil.23 Absence of pulmonary, GI (gastrointestinal), except vomiting, and GU (genitourinary) complaints, having normal liver and renal function tests, and patient improvement with only antimalarial treatment makes other infectious causes like tuberculosis which is also endemic in the area unlikely to be diagnosed.23,24
Using light microscopy by Giemsa-stained blood smears is the gold standard for the diagnosis of malaria.25 In a setting of low-density parasitemia diagnostic errors occur more but our cases have high parasitic load (+4). It also permits the determination of the infecting species and allows the exclusion of other infectious diseases such as filariasis, trypanosomiasis, Borrelia Recurrentis, and babesiosis that are common in the tropics as a cause of AFI (acute febrile illness).16 We used Giemsa stain and attached the figures. We confirmed the presence of P. vivax by RDT (100% specificity for P. vivax) and excluded the presence of P. falciparum (>90% specificity) in all cases.17
Conclusion
This case series illustrates the emerging role of P. vivax as a causative agent for cerebral malaria in adults at Mizan Tepi Teaching Hospital. Even if the diagnosis of cerebral malaria caused by Plasmodium vivax remains challenging due to overlapping clinical features with other forms of severe malaria, especially with P. Falceferum, we recommend prompt recognition of it as a cause of cerebral malaria and appropriate treatment which are crucial for favorable outcomes. Clinicians should be aware of the potential severe manifestations of P. vivax infection, especially in areas where this species is prevalent. Further research is warranted to better understand the pathogenesis, risk factors, and optimal management strategies for cerebral malaria caused by P. vivax.
Data Sharing Statement
The datasets collected and analyzed for the current study are available from the corresponding author and can be obtained upon reasonable request.
Ethics Approval
Ethical approval for this report was obtained from the College of Medicine and Health Science, Mizan-Tepi University [R.N. HSE/00429/2012].
Informed Consent
The nature of the case series was carefully explained to the patients after recovery. All patients agreed on these case series publications after the explaination and understood clearly the nature of these case reports, thereafter written informed consent was obtained. The consents were signed once assurances on the absence of medical problems, language or educational barriers which may have precluded understanding had been met.
Acknowledgments
The Mizan-Tepi University, specifically the ICU unit staff members and study participants, are all sincerely thanked by the authors for their technical assistance and guidance.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Disclosure
The authors declare that they have no conflicts of interest in this work.
References
1. World Health Organization. World Malaria Report 2022. Geneva: 2022.
2. Battle KE, Lucas TCD, Nguyen M, et al. Mapping the global endemicity and clinical burden of plasmodium vivax, 2000–17: a spatial and temporal modelling study. Lancet. 2019;394(10195):332–343. PMID: 31229233; PMCID: PMC6675736. doi:10.1016/S0140-6736(19)31096-7
3. Assemie A. Malaria prevalence and distribution of plasmodium species in Southern region of Ethiopia. J Parasitol Res. 2022:5665660. PMID: 35782658; PMCID: PMC9246638. doi:10.1155/2022/5665660
4. Mukhtar MM, Eisawi OA, Amanfo SA. Plasmodium vivax cerebral malaria in an adult patient in Sudan. Malar J. 2019;18(1):1–3. doi:10.1186/s12936-019-2961-1
5. Battle KE, Baird JK. The global burden of plasmodium vivax malaria is obscure and insidious. PLoS Med. 2021;18(10):e1003799. doi:10.1371/journal.pmed.1003799
6. Shiferaw M, Alemu M, Tedla K, Tadesse D, Bayissa S, Bugssa G. The prevalence of Malaria in Tselemti Wereda, North Ethiopia: a retrospective study. Ethiop J Health Sci. 2018;28(5):539–546. PMID: 30607068; PMCID: PMC6308779. doi:10.4314/ejhs.v28i5.4
7. Poespoprodjo JR, Fobia W, Kenangalem E. Vivax malaria: a major cause of morbidity in early infancy. Clin Infect Dis. 2009;48:1704–1712. doi:10.1086/599041
8. Wilson ME, Weld LH, Boggild A, et al. Fever in returned travelers: results from the geosentinel surveillance network. Clin Infect Dis. 2007;44:1560. doi:10.1086/518173
9. Svenson JE, MacLean JD, Gyorkos TW, Keystone J. Imported malaria. clinical presentation and examination of symptomatic travelers. Arch Intern Med. 1995;155:861. doi:10.1001/archinte.1995.00430080109013
10. Maguire JD, Fenton ME, Susanti AI, Walker JB. Plasmodium vivax-associated acute respiratory distress syndrome after extended travel in Afghanistan. Travel Med Infect Dis. 2007;5:301. doi:10.1016/j.tmaid.2007.04.001
11. World Health Organization. Severe and complicated malaria. Trans R Soc Trop Med Hyg. 2000;94:1. doi:10.1016/s0035-9203(00)90413-9
12. Stanley J. Malaria. Emerg Med Clin North Am. 1997;15:113. doi:10.1016/S0733-8627(05)70288-1
13. Mung’Ala-Odera V, Snow RW, Newton CR. The burden of the neurocognitive impairment associated with Plasmodium falciparum malaria in sub-saharan Africa. Am J Trop Med Hyg. 2004;71:64. doi:10.4269/ajtmh.2004.71.64
14. World Health Organization Severe falciparum malaria. World Health Organization, communicable diseases cluster. Trans R Soc Trop Med Hyg. 2000;94:S1–90.
15. Stauffer WM, Cartwright CP, Olson DA, et al. Diagnostic performance of rapid diagnostic tests versus blood smears for malaria in US clinical practice. Clin Infect Dis. 2009;49:908. doi:10.1086/605436
16. Kilian AH, Metzger WG, Mutschelknauss EJ, et al. Reliability of malaria microscopy in epidemiological studies: results of quality control. Trop Med Int Health. 2000;5:3. doi:10.1046/j.1365-3156.2000.00509.x
17. Okell LC, Ghani AC, Lyons E, Drakeley CJ. Submicroscopic infection in plasmodium falciparum-endemic populations: a systematic review and meta-analysis. J Infect Dis. 2009;200:1509. doi:10.1086/644781
18. Durand ML, Calderwood SB, Weber DJ, et al. Acute bacterial meningitis in adults. A review of 493 episodes. N Engl J Med. 1993;328:21. doi:10.1056/NEJM199301073280104
19. van de Beek D, de Gans J, Spanjaard L, et al. Clinical features and prognostic factors in adults with bacterial meningitis. N Engl J Med. 2004;351:1849. doi:10.1056/NEJMoa040845
20. Aronin SI, Peduzzi P, Quagliarello VJ. Community-acquired bacterial meningitis: risk stratification for adverse clinical outcome and effect of antibiotic timing. Ann Intern Med. 1998;129:862. doi:10.7326/0003-4819-129-11_Part_1-199812010-00004
21. Beare NA, Taylor TE, Harding SP, et al. Malarial retinopathy: a newly established diagnostic sign in severe malaria. Am J Trop Med Hyg. 2006;75:790. doi:10.4269/ajtmh.2006.75.790
22. Mohanty S, Mishra SK, Patnaik R, et al. Brain swelling and mannitol therapy in adult cerebral malaria: a randomized trial. Clin Infect Dis. 2011;53:349. doi:10.1093/cid/cir405
23. El-Amin EO, Elbashir MI, Elamin OE, et al. The underlying aetiologies of coma in febrile Sudanese children. Trans R Soc Trop Med Hyg. 2013;107:307–312. doi:10.1093/trstmh/trt013
24. Mahgoub H, Gasim GI, Musa IR, Adam I. Severe vivax malaria among Sudanese children at new halfa hospitals, Eastern Sudan. Parasit Vectors. 2012;5:154. doi:10.1186/1756-3305-5-154
25. Moody A. Rapid diagnostic tests for malaria parasites. Clin Microbiol Rev. 2002;15:66. doi:10.1128/CMR.15.1.66-78.2002
26. Ryan ET, Hill DR, Solomon T, et al. Hunter’s Tropical Medicine and Emerging Infectious Diseases Book.
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
