Back to Journals » Cancer Management and Research » Volume 14
Plasma Matrix Metalloproteinase-1 as a Prognostic Biomarker in Oral Cavity Squamous Cell Carcinoma
Authors Tsai TY, Kao HK, Huang Y , Chang YT, Young CK, Hung SY, Chang YS, Yu JS, Chang KP
Received 13 September 2022
Accepted for publication 8 December 2022
Published 15 December 2022 Volume 2022:14 Pages 3459—3468
DOI https://doi.org/10.2147/CMAR.S389742
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Ahmet Emre Eşkazan
Tsung-You Tsai,1,* Huang-Kai Kao,2,3,* Yenlin Huang,4 Ya-Ting Chang,5 Chi-Kuang Young,1 Shao-Yu Hung,3 Yu-Sun Chang,5 Jau-Song Yu,1,5,6 Kai-Ping Chang1,2,5
1Department of Otolaryngology-Head and Neck Surgery, Chang Gung Memorial Hospital, Kweishan, Taoyuan, Taiwan; 2College of Medicine, Chang Gung University, Taoyuan, Taiwan; 3Department of Plastic and Reconstructive Surgery, Chang Gung Memorial Hospital, Kweishan, Taoyuan, Taiwan; 4Department of Pathology, Chang Gung Memorial Hospital, Kweishan, Taoyuan, Taiwan; 5Molecular Medicine Research Center, Chang Gung University, Taoyuan, 33302, Taiwan; 6Department of Cell and Molecular Biology, College of Medicine, Chang Gung University, Taoyuan, 33302, Taiwan
*These authors contributed equally to this work
Correspondence: Kai-Ping Chang, Department of Otolaryngology-Head and Neck Surgery, Chang Gung Memorial Hospital & College of Medicine, Chang Gung University, No. 5, Fu-Hsing St, Kweishan, Taoyuan, 33305, Taiwan, Tel +886-3-3281200 ext.3967, Fax +886-3-3979361, Email [email protected]
Purpose: Plasma matrix metalloproteinase-1 (MMP-1) is a collagenase encoded by the MMP-1 gene. However, the prognostic value of plasma MMP-1 levels in oral cavity squamous cell carcinoma (OSCC) has yet to be elucidated. The study is the first to use a cohort of OSCC patients to assess the association of plasma MMP-1 levels with clinicopathological factors/survival outcomes in OSCC patients.
Patients and Methods: A total of 677 patients were retrospectively enrolled, including 276 oral potentially malignant disease (OPMD) and 401 OSCC patients from 2013 to 2021. Pretreatment plasma MMP-1 levels were measured with an enzyme-linked immunosorbent assay, and the values were compared between OPMD and OSCC patients. Furthermore, the association of plasma MMP-1 levels and clinicopathological characteristics/survival outcomes in OSCC patients was investigated.
Results: Plasma MMP-1 levels were significantly higher in OSCC patients than in OPMD patients (p = 0.04). In the OSCC group, plasma MMP-1 levels were significantly higher in females, tumor depth ≥ 10 mm, advanced pT classification and advanced overall stage (p = 0.04, < 0.001, < 0.001, 0.002, respectively). Higher plasma MMP-1 levels were significantly associated with poorer overall, disease-specific, disease-free, locoregional recurrence-free and distant metastasis-free survival (p = 0.003, 0.02, 0.005, 0.01, 0.001, respectively). Multivariate analysis revealed that plasma MMP-1 levels were a significant predictor for overall, disease-free, and distant metastasis-free survival (p = 0.03, 0.02, and 0.010, respectively).
Conclusion: Plasma MMP-1 levels are associated with more severe clinicopathological manifestations and can also be regarded as a significant prognostic factor for OSCC posttreatment outcomes.
Keywords: oral cancer, MMP, squamous cell carcinoma, ELISA, OSCC
Introduction
Oral cavity squamous cell carcinoma (OSCC) is a common cancer in head and neck surgical oncology worldwide.1 Despite advancements in surgical techniques and the development of chemotherapy, radiotherapy and immunotherapy, the treatment outcomes of OSCC patients, especially in advanced stages, remain unsatisfactory, implying that the accurate prediction of prognosis are crucial issues.2 Until now, most reliable OSCC prognosticators used preoperatively are based on the findings of physical examinations, image studies and histological features.3 Although some circulating markers have been reported for outcome prediction before treatment, many researchers are still working diligently on discovering a more reliable blood marker for clinical utility.4–7
Matrix metalloproteinase-1 (MMP-1), also known as collagenase-1, breaks down the extracellular matrix by the cleavage of interstitial collagens (eg, type I, II, III) and certain matrix proteins (eg, gelatin).8 MMP-1 is believed to be associated with cancer cell behavior such as metastasis, angiogenesis, and inflammation.9 Enhanced expression of MMP-1 was observed in several types of cancers, including OSCC.10–13 In a recent study, to expand its clinical application in OSCC patients, Chang et al further investigated the association of salivary MMP-1 levels and clinical presentation, suggesting that salivary levels of MMP-1 may be a feasible biomarker for OSCC diagnosis by enzyme-linked immunosorbent assay (ELISA).14 Although the association of circulating MMP-1 levels with prognosis in several types of cancers, including lung, colon and breast cancers, has also been reported in previous studies,15–17 the prognostic roles of circulating MMP-1 levels in OSCC patients, to the best of our knowledge, have yet to be determined. The current study aimed to compare the difference in plasma MMP-1 levels of OSCC and OPMD patients and to investigate the associations between plasma MMP-1 levels and their relationship to clinicopathological factors/survival outcomes in OSCC patients.
Materials and Methods
Patients
In this study, the clinicopathological data of a cohort of OSCC and OPMD patients from 2013 to 2021 were retrospectively reviewed. Patients who visited the otolaryngology clinic for oral screening or the treatment of OPMD/OSCC were consecutively recruited. Based on World Health Organization recommendations, OPMD lesions (leukoplakia, erythroplakia, lichen planus and submucous fibrosis) were diagnosed either by clinical features or pathology, while OSCC was diagnosed with histopathological examinations. The following conditions were excluded: previous history of any malignancy, known distant metastasis or second primary cancer diagnosed before treatment; history of neoadjuvant radiation or chemotherapy therapy. Informed consent, approved by the Institutional Review Board of Chang Gung Memorial Hospital (Approval number: 201305685A3 and date: November 7th, 2013, to December 31st, 2022), was obtained from all subjects and the study complies with the Declaration of Helsinki. All OSCC patients were previously untreated and received surgery as the primary treatment modality. A thorough review of medical history, physical examination, laboratory tests, chest radiographs, imaging studies (including computed tomography or magnetic resonance imaging), liver ultrasonography, positron emission tomography or bone scans was conducted before the treatment was initiated. Primary tumors were excised with adequate margins using intraoperative frozen section controls transorally or via lip splitting, and neck dissections were simultaneously performed in level I–III (for clinical N0 neck disease) or level I–V (for clinical N+ disease). According to the defect size, primary closure or flap reconstruction was performed immediately after tumor ablation. The adequacy of the resection margin was assessed with intraoperative frozen sections. Adjuvant radiotherapy or chemoradiotherapy was administered according to the National Comprehensive Cancer Network guidelines. The 8th edition of the American Joint Committee on Cancer staging criteria was applied for pathological staging. The patients were followed up in the outpatient clinic every 2 months in the first year, every 3 months during the second and third years, and every 6 months thereafter.
Plasma Collection
Blood samples (10 mL) were collected from the patients and drawn into tubes containing ethylenediaminetetraacetic acid as an anticoagulant. The samples were centrifuged at 2000×g for 10 min for plasma separation and then aliquoted and stored at −70 ℃ refrigerators for further analysis.
Measurement of Plasma MMP-1
The pretreatment plasma MMP-1 level was measured, blinded to the identification data, by employing the human MMP-1 ELISA kit (S&T BIOMED Co, Ltd. Hsinchu, Taiwan). To generate the standard curve, 2-fold serial dilutions were performed from 8000 pg/mL MMP-1 protein down to 125 pg/mL (0 pg/mL as a blank). First, 96-well microplates precoated with the capture antibodies were plated with blanks, standards, controls and/or samples and incubated. After the detection of antibodies were added, 3,3′,5,5′-tetramethylbenzidine solution and stop solution were added sequentially with adequate washing and incubation. The optical density value at 450 nm was detected in each plate. Finally, the plasma levels of MMP-1 in the samples were calculated using a standard curve as a reference.
Statistical Analysis
All statistical analyses were performed using SAS software (version 9.4; SAS Institute, Cary, NC). To examine the differences in clinicopathological features between the patient groups, Chi-square tests were used. The Wilcoxon Rank Sum test was used to compare the differences in plasma MMP-1 levels between groups. The area under the curve (AUC) was calculated using receiver operating characteristic (ROC) analysis. Survival rates were demonstrated using Kaplan–Meier plots and were examined with the Log rank test. Overall survival was defined by the time to death by any cause from the date of surgical removal of the primary tumor. Disease-specific survival was defined by the time to death caused by OSCC from the date of surgical removal of the primary tumor. Disease-free survival was defined by the time to death by any cause or any tumor relapse from the date of surgical removal of the primary tumor. Locoregional recurrence-free survival was defined by the time to death by any cause or locoregional recurrence from the date of surgical removal of the primary tumor. Distant metastasis-free survival was defined by the time to death by any cause or the occurrence of distant metastasis from the date of surgical removal of the primary tumor. Furthermore, the association of the variables and survival was further analyzed with a multivariate Cox regression model. Seven patients were excluded from survival analyses due to incomplete adjuvant treatment. All patients were followed-up until June 2021 or until death. All p values were two-sided, and a p value less than 0.05 indicated statistical significance.
Results
Patient Characteristics and Clinicopathological Data
In the current study, 677 patients were enrolled from 2013 to 2021, including 276 OPMD and 401 OSCC patients. In the OSCC group, the mean age was 55.4 years, and the patients consisted of 367 (91.5%) males; 84.0% were smokers, 64.6% were alcohol consumers, and 52.5% were betel nut chewers. Alternatively, in OPMD patients, the mean age was 52.5, and the patients comprised 252 (91.3%) males; 88% were smokers, 65.2% were alcohol consumers, and 73.2% were betel nut chewers. Other clinicopathological characteristics of OSCC and OPMD patients are shown in Table 1.
 |
Table 1 Clinical Characteristics of Patients with Oral Cavity Squamous Cell Carcinoma (OSCC) and Oral Premalignant Disease (OPMD) |
Different MMP-1 Levels in OSCC and OPMD Patients
Levels of plasma MMP-1 in OPMD and OSCC groups were demonstrated by box plots, in which the middle line represented the median and lower/upper edge of the box edges corresponded to the 1st/3rd quartile, respectively; the lower/upper whisker caps denote the 5th/95th percentiles; lower and upper symbols outside the caps represent the plasma MMP-1 level lower than 5th and higher than 95th percentiles. Plasma MMP-1 levels were significantly higher in OSCC patients than in OPMD patients (mean ± standard deviation: 3404 ± 5931 vs 3208 ± 4207 pg/mL, p = 0.04; Figure 1A). However, ROC curve analyses showed limited efficacy of plasma MMP-1 levels in discriminating OSCC from OPMD (Figure 1B), with AUCs of only 0.547 (95% CI: 0.502–0.592). In general, an AUC of 0.5 suggests no discrimination, 0.7 to 0.8 is considered acceptable, 0.8 to 0.9 is considered excellent, and more than 0.9 is considered outstanding.18 Based on these results, plasma MMP-1 levels may not be an optimal marker for discriminating OSCC and OPMD patients.
Association Between Plasma MMP-1 and Patient Characteristics
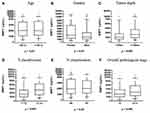
Using the median value of plasma MMP-1 (2018 pg/mL of the OSCC patient cohort) as a cutoff value, OSCC patients were stratified into two groups, and the clinicopathological factors are compared in Table 2. Significant differences in pT status, overall pathological status, extranodal extension, and lymphovascular invasion were observed between higher (≥2018 pg/mL) and lower (<2018 pg/mL) plasma MMP-1 patients (p = <0.001, 0.003, 0.04, 0.04, respectively). The box plots in Figure 2 illustrate the disparity of plasma MMP-1 levels in different clinicopathological factors. Plasma MMP-1 levels were significantly higher in female, tumor depth ≥10 mm, advanced pT classification (T3 and T4) and advanced overall stage (stage III and stage IV) patients (p = 0.04, <0.001, <0.001, 0.002, respectively).
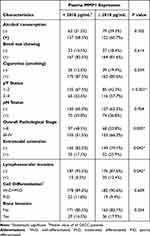 |
Table 2 Associations of MMP1 Protein Concentrations in Plasma with Clinicopathological Characteristics |
Association Between Plasma MMP-1 Levels and Survival Status in OSCC Patients
The Kaplan–Meier plots in Figure 3 illustrate the comparison of survival outcomes between higher and lower plasma MMP-1 levels. Using 2018 pg/mL (median value) as a cutoff value, higher plasma MMP-1 levels were significantly associated with poorer overall survival, disease-specific survival, disease-free survival, locoregional recurrence-free survival and distant metastasis-free survival (p = 0.003, 0.02, 0.005, 0.01, and 0.001, respectively). Furthermore, multivariate analysis revealed that higher plasma MMP-1 levels were significantly associated with worse overall survival, disease-free survival and distant metastasis-free survival (adjusted hazard ratio, 95% confidential interval, 1.583 [1.062–2.2358], 1.505 [1.051–2.154], 1.688 [1.139–2.502]; p = 0.024, 0.025, 0.009, respectively) after adjusting for age, sex, overall pathological stage (stage III~IV vs I~II), pathological N status, extranodal extension (positive vs negative), perineural invasion (positive vs negative), lymphovascular invasion (positive vs negative), surgical margin (<5mm vs ≥5mm), histological differentiation (poor differentiation vs well and moderate differentiation), and bone invasion (positive vs negative) (Table 3).
 |
Table 3 Multivariate Analysis of Survival Analyses Stratified by the Median MMP1 Concentration (≥2018 Pg/mL Vs <2018 Pg/Ml) in 394 Patients with Oral Cavity Squamous Cell Carcinoma |
Discussion
Matrix metalloproteinases, a family of zinc-dependent proteolytic enzymes, play an important role in the turnover of the extracellular matrix. If metalloproteinases and their inhibitors, tissue inhibitors of metalloproteinases, are out of balance, pathological changes may occur, including an inflammatory response, unrestricted remodeling, cell growth and migration.19,20 For decades, studies have revealed the association between MMPs and cancer development.21 MMPs promote cancer progression through genomic instability and cellular proliferation, angiogenesis, invasion and metastasis.9,22 Therefore, they were considered ideal pharmacological targets for cancer therapy and showed promising results in preclinical studies. Despite some previous clinical trials in various types of cancers, including breast cancer, lung cancer, and colorectal cancer, failed in the non-specific effect of broad spectrum MMP inhibitors, the therapeutic potential of highly selective MMP inhibitors, such as MMP-9 monoclonal antibodies, may still be evaluated in the future.23,24 Upregulated expression of MMPs has been observed in many studies among various types of cancers, and a recent systematic review and meta-analysis synthesized the current evidence and demonstrated that serum MMP-7 and MMP-9 levels as well as salivary MMP-1 and MMP-9 levels were significantly higher in OSCC patients.25 However, the role of preoperative MMP-1 levels in circulation on the association of OSCC treatment and its clinicopathological manifestations/survival outcomes remains unknown.
MMP-1, the earliest identified member of the MMP family, is a collagenase expressed in endothelial and epithelial cells, macrophages, and various cancer cells.26 The expression of MMP-1 in tumor tissues is related to tumorigenesis or invasiveness in various cancers.27–32 Although it is considered a secreted protein, only a few studies have reported serum or plasma MMP-1 levels derived from cancer patients, including bladder, lung, colon and breast cancer patients.15–17,33,34 In the current study, the plasma MMP-1 levels in OSCC and OPMD patients were compared, and significantly higher plasma MMP-1 levels were observed in OSCC patients. However, the diagnostic performance was unfortunately not feasible as an optimal diagnostic marker, with an AUC of only 0.547 (95% CI: 0.502–0.592). Choudhry et al also reported higher expression of serum MMP-1 levels in OSCC patients, with an AUC of only 0.642. However, the study was conducted in a relatively small cohort (38 patients in both the OSCC and control groups), and the serum MMP-1 levels of OSCC patients were compared with those of healthy subjects rather than OPMD patients. Alternatively, the measurement of salivary MMP-1 levels was conducted in a study by Chang et al, in which salivary MMP-1 exhibited a more effective diagnostic performance (sensitivity 76.58%, specificity 86.76%) in discriminating OSCC from high-risk non-OSCC patients using ELISA.14
The association of plasma levels of MMP-1 and clinicopathological characteristics in OSCC patients has yet to be clarified in previous studies. One of the major findings in the current study is that the plasma MMP-1 levels were found to be significantly associated with tumor depth, pT classification and overall stage. These results implied that the levels of plasma MMP-1 might reflect the extent or severity of the disease and were compatible with previous studies. Li et al indicated that the plasma MMP-1 levels were significantly higher in advanced stage lung cancer patients (including non-small-cell lung carcinoma and small-cell lung carcinoma), and the levels were also significantly associated with the histological grade. Kulić et al reported that in early-stage breast cancer patients, serum MMP-1 levels were associated with tumor size and the number of involved lymph nodes.
Recent studies have reported that the expression of MMP-1 protein in tumor tissue might be an effective biomarker for predicting survival outcomes in various types of cancers, including esophageal, thyroid and gastric cancers.35–37 Luukkaa et al, by performing immunohistochemical stain on formalin-fixed and paraffin-embedded tissue samples, also reported that the expression of MMP-1 in tumor tissues was associated with the prognosis in salivary gland cancer.38 On the other hand, the association of serum or plasma MMP-1 levels and survival outcomes varies among previous studies on other malignancies. Jonsson et al claimed that in colon cancer patients, among MMP-1, −2, −7, −8, and −9, a higher plasma level of MMP-1 was significantly associated with poorer long-term cancer-specific survival.15 Li et al also indicated that the plasma MMP-1 level was a negative independent prognosticator for overall survival in lung cancer patients.16 In contrast, Kulić et al demonstrated that lower serum MMP-1 was associated with poorer overall survival in early breast cancer.17 However, the role of circulating MMP-1 levels in head and neck cancers, including OSCC patients, remains unknown.
In OSCC, Fan et al, using immunohistochemical analysis, indicated that the expression levels of MMP-1 and protease-activated receptor-1 may be a valuable tool to assess patient prognosis.39 Interestingly, Lassig et al found that MMP-1 levels in surgical drains were also associated with poorer clinical outcomes in oral cavity and oral pharyngeal cancer.40 Regarding serum or plasma MMP levels, although previous studies have reported the association of prognosis and serum or plasma levels of MMP-2 and MMP-9, the current study is the first to report the association of clinicopathological factors and prognostic value with plasma MMP-1 levels.41 Furthermore, the multivariate analyses in our study, also the first to the best of our knowledge, show that the plasma levels of MMP-1 are a significant prognosticator for overall survival, disease-free survival and distant metastasis-free survival in OSCC patients.
Conclusion
The current study is the first to use a cohort of OSCC patients to investigate the roles of circulating MMP-1 levels on clinicopathological factors and treatment outcome. Higher levels are associated with more severe clinicopathological manifestations including tumor depth, pT classification and overall stage. Moreover, multivariate analyses revealed that higher levels of plasma MMP-1 levels could be used as a significant prognostic factor for poorer posttreatment outcomes, including overall survival, disease-free survival, and distant metastasis-free survival. These findings of the current study might add some potential prognostic implications of plasma MMP-1 levels for the treatment of OSCC patients.
Data Sharing Statement
Datasets used for analysis for this study are available for review, without compromising patient confidentiality, upon reasonable request.
Ethics Approval and Consent to Participate
The study complied with the Declaration of Helsinki and its protocol was approved by the Institutional Review Board of Chang Gung Memorial Hospital, Taiwan.
Consent for Publication
Written informed consent was obtained from each patient for study participation and publication.
Acknowledgment
This study was supported by a grant (MOST 111-2314-B-182A-078 -MY3) from the Ministry of Science and Technology and by grants (CMRPG3J1253 and CMRPG3M0101) from Chang Gung Memorial Hospital, Taiwan. The authors thank all the members of the Cancer Center at Chang Gung Memorial Hospital for their invaluable help.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors have no disclosure to declare in this work
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.21492
2. Howard A, Agrawal N, Gooi Z. Lip and oral cavity squamous cell carcinoma. Hematol Oncol Clin North Am. 2021;35(5):895–911. doi:10.1016/j.hoc.2021.05.003
3. Chamoli A, Gosavi AS, Shirwadkar UP, et al. Overview of oral cavity squamous cell carcinoma: risk factors, mechanisms, and diagnostics. Oral Oncol. 2021;121:105451. doi:10.1016/j.oraloncology.2021.105451
4. Nagler RM, Barak M, Peled M, Ben-Aryeh H, Filatov M, Laufer D. Early diagnosis and treatment monitoring roles of tumor markers Cyfra 21-1 and TPS in oral squamous cell carcinoma. Cancer. 1999;85(5):1018–1025. doi:10.1002/(SICI)1097-0142(19990301)85:5<1018::AID-CNCR2>3.0.CO;2-R
5. Bhatavdekar JM, Patel DD, Vora HH, Balar DB. Circulating prolactin and TPS in monitoring the clinical course of male patients with metastatic tongue cancer: a preliminary study. Anticancer Res. 1993;13(1):237–240.
6. Yen TC, Lin WY, Kao CH, Cheng KY, Wang SJ. A study of a new tumour marker, CYFRA 21-1, in squamous cell carcinoma of the head and neck, and comparison with squamous cell carcinoma antigen. Clin Otolaryngol Allied Sci. 1998;23(1):82–86. doi:10.1046/j.1365-2273.1998.00101.x
7. Krimmel M, Hoffmann J, Krimmel C, Cornelius CP, Schwenzer N. Relevance of SCC-Ag, CEA, CA 19.9 and CA 125 for diagnosis and follow-up in oral cancer. J Craniomaxillofac Surg. 1998;26(4):243–248. doi:10.1016/s1010-5182(98)80020-6
8. Pardo A, Selman M. MMP-1: the elder of the family. Int J Biochem Cell Biol. 2005;37(2):283–288. doi:10.1016/j.biocel.2004.06.017
9. Maciejczyk M, Pietrzykowska A, Zalewska A, Knaś M, Daniszewska I. The significance of matrix metalloproteinases in oral diseases. Adv Clin Exp Med. 2016;25(2):383–390. doi:10.17219/acem/30428
10. Yen CY, Chen CH, Chang CH, et al. Matrix metalloproteinases (MMP) 1 and MMP10 but not MMP12 are potential oral cancer markers. Biomarkers. 2009;14(4):244–249. doi:10.1080/13547500902829375
11. An HJ, Lee YJ, Hong SA, et al. The prognostic role of tissue and serum MMP-1 and TIMP-1 expression in patients with non-small cell lung cancer. Pathol Res Pract. 2016;212(5):357–364. doi:10.1016/j.prp.2015.11.014
12. Boström P, Söderström M, Vahlberg T, et al. MMP-1 expression has an independent prognostic value in breast cancer. BMC Cancer. 2011;11(1):348. doi:10.1186/1471-2407-11-348
13. Yang R, Xu Y, Li P, et al. Combined upregulation of matrix metalloproteinase-1 and proteinase-activated receptor-1 predicts unfavorable prognosis in human nasopharyngeal carcinoma. Onco Targets Ther. 2013;6:1139–1146. doi:10.2147/ott.S50389
14. Chang YT, Chu LJ, Liu YC, et al. Verification of saliva matrix metalloproteinase-1 as a strong diagnostic marker of oral cavity cancer. Cancers. 2020;12(8). doi:10.3390/cancers12082273
15. Jonsson A, Falk P, Angenete E, Hjalmarsson C, Ivarsson ML. Plasma MMP-1 expression as a prognostic factor in colon cancer. J Surg Res. 2021;266:254–260. doi:10.1016/j.jss.2021.04.021
16. Li M, Xiao T, Zhang Y, et al. Prognostic significance of matrix metalloproteinase-1 levels in peripheral plasma and tumour tissues of lung cancer patients. Lung Cancer. 2010;69(3):341–347. doi:10.1016/j.lungcan.2009.12.007
17. Kulić A, Dedić Plavetić N, Vrbanec J, Sirotković-Skerlev M. Low serum MMP-1 in breast cancer: a negative prognostic factor? Biomarkers. 2012;17(5):416–421. doi:10.3109/1354750x.2012.678885
18. Mandrekar JN. Receiver operating characteristic curve in diagnostic test assessment. J Thorac Oncol. 2010;5(9):1315–1316. doi:10.1097/JTO.0b013e3181ec173d
19. Herszényi L, Hritz I, Lakatos G, Varga MZ, Tulassay Z. The behavior of matrix metalloproteinases and their inhibitors in colorectal cancer. Int J Mol Sci. 2012;13(10):13240–13263. doi:10.3390/ijms131013240
20. Brew K, Nagase H. The tissue inhibitors of metalloproteinases (TIMPs): an ancient family with structural and functional diversity. Biochim Biophys Acta. 2010;1803(1):55–71. doi:10.1016/j.bbamcr.2010.01.003
21. Bassiouni W, Ali MAM, Schulz R. Multifunctional intracellular matrix metalloproteinases: implications in disease. FEBS J. 2021;288(24):7162–7182. doi:10.1111/febs.15701
22. Airola K, Karonen T, Vaalamo M, et al. Expression of collagenases-1 and −3 and their inhibitors TIMP-1 and −3 correlates with the level of invasion in malignant melanomas. Br J Cancer. 1999;80(5–6):733–743. doi:10.1038/sj.bjc.6690417
23. Winer A, Adams S, Mignatti P. Matrix metalloproteinase inhibitors in cancer therapy: turning past failures into future successes. Mol Cancer Ther. 2018;17(6):1147–1155. doi:10.1158/1535-7163.Mct-17-0646
24. Cathcart JM, Cao J. Inhibitors: past, present and future. Front Biosci. 2015;20(7):1164–1178. doi:10.2741/4365
25. Rezaei F, Imani MM, Lopez-Jornet P, Sadeghi M. Estimation of serum and salivary matrix metalloproteinase levels in oral squamous cell carcinoma patients: a systematic review and meta-analysis. Postepy Dermatol Alergol. 2021;38(2):106–114. doi:10.5114/ada.2021.104285
26. Brinckerhoff CE, Rutter JL, Benbow U. Interstitial collagenases as markers of tumor progression. Clin Cancer Res. 2000;6(12):4823–4830.
27. Hilska M, Roberts PJ, Collan YU, et al. Prognostic significance of matrix metalloproteinases-1, −2, −7 and −13 and tissue inhibitors of metalloproteinases-1, −2, −3 and −4 in colorectal cancer. Int J Cancer. 2007;121(4):714–723. doi:10.1002/ijc.22747
28. Ito T, Ito M, Shiozawa J, Naito S, Kanematsu T, Sekine I. Expression of the MMP-1 in human pancreatic carcinoma: relationship with prognostic factor. Mod Pathol. 1999;12(7):669–674.
29. Inoue T, Yashiro M, Nishimura S, et al. Matrix metalloproteinase-1 expression is a prognostic factor for patients with advanced gastric cancer. Int J Mol Med. 1999;4(1):73–77. doi:10.3892/ijmm.4.1.73
30. Poola I, DeWitty RL, Marshalleck JJ, Bhatnagar R, Abraham J, Leffall LD. Identification of MMP-1 as a putative breast cancer predictive marker by global gene expression analysis. Nat Med. 2005;11(5):481–483. doi:10.1038/nm1243
31. Gouyer V, Conti M, Devos P, et al. Tissue inhibitor of metalloproteinase 1 is an independent predictor of prognosis in patients with nonsmall cell lung carcinoma who undergo resection with curative intent. Cancer. 2005;103(8):1676–1684. doi:10.1002/cncr.20965
32. Lin TS, Chiou SH, Wang LS, et al. Expression spectra of matrix metalloproteinases in metastatic non-small cell lung cancer. Oncol Rep. 2004;12(4):717–723.
33. Durkan GC, Nutt JE, Rajjayabun PH, Neal DE, Lunec J, Mellon JK. Prognostic significance of matrix metalloproteinase-1 and tissue inhibitor of metalloproteinase-1 in voided urine samples from patients with transitional cell carcinoma of the bladder. Clin Cancer Res. 2001;7(11):3450–3456.
34. Nikkola J, Vihinen P, Vuoristo MS, Kellokumpu-Lehtinen P, Kähäri VM, Pyrhönen S. High serum levels of matrix metalloproteinase-9 and matrix metalloproteinase-1 are associated with rapid progression in patients with metastatic melanoma. Clin Cancer Res. 2005;11(14):5158–5166. doi:10.1158/1078-0432.Ccr-04-2478
35. Tao YS, Ma XY, Chai DM, et al. Overexpression of MMP-1 and VEGF-C is associated with a less favorable prognosis in esophageal squamous cell carcinoma. Oncol Res Treat. 2012;35(11):651–656. doi:10.1159/000343637
36. Fujimoto D, Hirono Y, Goi T, Katayama K, Yamaguchi A. Prognostic value of protease-activated receptor-1 (PAR-1) and matrix metalloproteinase-1 (MMP-1) in gastric cancer. Anticancer Res. 2008;28(2a):847–854.
37. Buergy D, Weber T, Maurer GD, et al. Urokinase receptor, MMP-1 and MMP-9 are markers to differentiate prognosis, adenoma and carcinoma in thyroid malignancies. Int J Cancer. 2009;125(4):894–901. doi:10.1002/ijc.24462
38. Luukkaa H, Klemi P, Hirsimäki P, et al. Matrix metalloproteinase (MMP)-1, −9 and −13 as prognostic factors in salivary gland cancer. Acta Otolaryngol. 2008;128(4):482–490. doi:10.1080/00016480801922895
39. Fan HX, Chen Y, Ni BX, et al. Expression of MMP-1/PAR-1 and patterns of invasion in oral squamous cell carcinoma as potential prognostic markers. Onco Targets Ther. 2015;8:1619–1626. doi:10.2147/ott.S84561
40. Lassig AAD, Joseph AM, Lindgren BR, Yueh B. Association of oral cavity and oropharyngeal cancer biomarkers in surgical drain fluid with patient outcomes. JAMA Otolaryngol Head Neck Surg. 2017;143(7):670–678. doi:10.1001/jamaoto.2016.3595
41. Patel BP, Shah SV, Shukla SN, Shah PM, Patel PS. Clinical significance of MMP-2 and MMP-9 in patients with oral cancer. Head Neck. 2007;29(6):564–572. doi:10.1002/hed.20561
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.