Back to Journals » Infection and Drug Resistance » Volume 12
Plasma indoleamine 2,3-dioxygenase activity as a potential biomarker for early diagnosis of multidrug-resistant tuberculosis in tuberculosis patients
Authors Shi W , Wu J , Tan Q , Hu CM, Zhang X, Pan HQ , Yang Z, He MY, Yu M , Zhang B , Xie WP, Wang H
Received 24 January 2019
Accepted for publication 29 March 2019
Published 14 May 2019 Volume 2019:12 Pages 1265—1276
DOI https://doi.org/10.2147/IDR.S202369
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Joachim Wink
Wen Shi,1 Juan Wu,1 Qi Tan,1 Chun-Mei Hu,2 Xia Zhang,2 Hong-Qiu Pan,3 Zhen Yang,4 Meng-Yu He,1 Min Yu,1 Bo Zhang,5 Wei-Ping Xie,1 Hong Wang1
1Department of Respiratory and Critical Care Medicine, Jiangsu Province Hospital. The First Affiliated Hospital of Nanjing Medical University, Nanjing, Jiangsu Province, People’s Republic of China; 2Department of Tuberculosis, The Second Hospital of Nanjing, Nanjing, Jiangsu Province, People’s Republic of China; 3Department of Tuberculosis, The Third Hospital of Zhenjiang City, Zhenjiang, Jiangsu Province, People’s Republic of China; 4Department of Respiratory Medicine, Jiangbei Hospital, Nanjing, Jiangsu Province, People’s Republic of China; 5Department of Population and Quantitative Health Sciences, University of Massachusetts Medical School, Worcester, MA, USA
Purpose: Multidrug-resistant tuberculosis (MDR-TB) remains a challenge of global TB control, with difficulty in early detection of drug-sensitive tuberculosis (DS-TB). We investigate the diagnostic significance of IDO as a potential biomarker to discriminate MDR patients among the TB patients.
Patients and methods: Plasma indoleamine 2,3-dioxygenase (IDO) was measured by the ratio of kynurenine (Kyn) to tryptophan (Trp) concentrations, using high performance liquid chromatography-mass spectrometry (LC-MS/MS). Chest computed tomography (CT) imaging signs from TB patients were collected and analyzed in 18 DS-TB patients, 16 MDR-TB patients, 6 lung cancer (LC) patients, and 11 healthy individuals. Lung imaging signs from TB patients were collected and analyzed.
Results: We found that plasma IDO activity was significantly higher in the MDR-TB patients than in the DS-TB patients (p=0.012) and in the LC patients (p=0.003). We evaluated the diagnostic significance of plasma IDO activity in discriminating the MDR-TB group from the DS-TB group using a receiver operating characteristic (ROC) curve. With a cutoff level of 46.58 uM/mM, the diagnostic sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) for IDO activity were 87.50%, 72.22%, 73.68%, and 86.67%, respectively. Plasma IDO activity was higher in cavity cases than in non-cavity cases (p=0.042), proving a positive correlation between lung cavity number and cavity size (p<0.05, separately) among all the TB patients studied.
Conclusion: Our findings confirmed that plasma IDO activity might have an auxiliary diagnosis value for early discrimination of MDR-TB patients from DS-TB patients. Among the TB patients with cavitary lung lesions, higher plasma IDO activity can indicate a higher risk of MDR-TB.
Keywords: IDO, MDR-TB, LC-MS/MS, cavitary lung lesion
Introduction
Multidrug-resistant tuberculosis (MDR-TB) is a major threat to global tuberculosis control.1 In 2017, there were 10 million new and relapse tuberculosis (TB) cases worldwide; over 558,000 of these TB patients suffered from MDR-TB, and only 25% of MDR-TB patients were notified.2 Missing people with MDR-TB, due to a failure of the local healthcare system3 and lack of tools for diagnosis4 can lead to the systematic selection and spread of MDR-TB strains. This increases the risk that we may lose control of TB in the future.5 In comparison with drug-sensitive tuberculosis (DS-TB), MDR-TB treatments tend to use more toxic second-line anti-TB drugs in combination, and in a prolonged treatment regimen (the standard is 9–24 months for MDR-TB versus 6 months for DS-TB)6 with less treatment efficiency, potential patient incompliance and, unaffordably high treatment costs.7,8 Clinical confirmation of MDR-TB mostly depends on identification of Mycobacterium tuberculosis (MTB) by smear or culture and determination of the strain by drug-susceptibility testing (DST). However, this testing usually takes longer than four weeks.9,10 More efficient methods based on GeneXpert/RIF have been recommended by the World Health Organization (WHO) for screening drug resistant-TB.11 However, these methods are limited in early detection for MDR-TB due to inadequate sensitivity (46%), and giving a false-positive result when few bacilli are present in a clinical specimen (smear negative).12,13 Current determination of TB in immunology, pathology, and radiology cannot discriminate drug-resistant TB from drug-sensitive TB.14,15 The lack of adequate tools is contributing to delayed detection or even missed diagnoses of MDR-TB.16 As a result, these missed cases can transmit the pathogen, and extend the MDR-TB population in uncontrolled conditions.
Indoleamine 2,3-dioxygenase (IDO) is an intracellular, non-secreted enzyme, and is a key rate-limiting enzyme that catalyzes the catabolism of tryptophan (Trp) to kynurenine (Kyn).17,18 Induction of IDO in cells of the immune system by IFN-γ is a technique that was developed in the late 1980s.19,20 due to the anti-proliferative features of IDO on bacteria, protozoa, and tumor cells.21 Numerous studies in the past two decades have indicated that induced IDO acts as a suppressive regulator in host immunity to promote and prolong TB infection.12–26 In both animal models and patient specimens, IDO was found to be enriched in the inner layer of the granuloma22,23 and capable of promoting long-term survival of the bacilli by limiting the proliferation of CD4+T cells and macrophages.27 Recent studies have demonstrated that IDO activity in the serum, sputum, or pleura from pulmonary TB (PTB) patients is increased, and can be an independent predictor of mortality.24,26 Most of these studies were focused on TB patients; yet, these studies did not distinguish whether the TB patients infected with drug-sensitive or drug-resistant MTB strain. So, we still cannot determine the role that IDO plays in MDR-TB infection. Therefore, the differences in IDO activity in MDR-TB patients compared to DS-TB patients have not yet been adequately investigated.
In our previous work, MDR-TB patients—mostly with more severe clinical symptoms and radiological lung focuses—were recognized by an extreme drop of CD4+T helper subset cell responses before treatment which was markedly different from that in DS-TB cases, including a more sharply decreased Th1 frequency, lower IFN-γ concentration, and higher over-induced inflammatory cytokine expressions in peripheral blood mononuclear cells (PBMCs).28,29 Thus, we hypothesize that the increased Trp and decreasing Kyn levels within IDO activity play a crucial role. In this study, we investigate the diagnostic significance of IDO as a potential biomarker to discriminate MDR patients from other TB patients. We also investigated the IDO activity identification significance in MDR-TB patients compared with lung cancer (LC) patients, and the correlation of plasma IDO activity with the frequency and size of cavitary lung lesions of TB patients.
Our findings indicated plasma IDO activity was a novel biomarker for early diagnosis MDR-TB, and suggested a potential target of host direct treatment (HDT) for MDR-TB in future by indicating the crucial role of IDO in the immune response against MDR-TB. Our findings confirmed that plasma IDO activity can remarkably discriminate MDR-TB patients from DS-TB patients, as well as from lung cancer patients. Meanwhile we demonstrated a higher plasma IDO activity in cavitary TB patients than in non-cavitary TB patients. Plasma IDO activity level was illustrated a positive correlation with the number and size of lung cavitary lesions in patients with TB, according to imaging signs. This hinted that plasma IDO activity can also be a biomarker to boost imaging signs of the cavity, indicating the risk of MDR-TB infection and pathogen transmittance. In conclusion, our pilot investigation sheds light on plasma IDO activity performance in the early identification of MDR-TB patients, which may be an auxiliary tool for promptly conducting the correct patients into an isolation ward and thereby alleviating the spread of MDR-TB.
Material and methods
Ethics statement
This study protocol was approved by the ethics committee of the First Affiliated Hospital of Nanjing Medical University (Jiangsu Province Hospital) and was performed adhering to the ethical principles of the Declaration of Helsinki (APPROVAL NUMBER/ID 2017-SRFA-163). All patients provided written informed consent before enrollment.
Subjects and diagnostic criteria
From December 2017 to May 2018, 34 newly diagnosed pulmonary TB patients, including 18 cases of DS-TB, 16 cases of MDR-TB, and 6 cases of LC, along with 11 HCs, were enrolled in the study and admitted to The Public Health Medical Center of Nanjing City, Jiangbei Hospital, Third Hospital of Zhenjiang City, and The First Affiliated Hospital of Nanjing Medical University. All participants were over 18 years old. All of the active tuberculosis patients recruited were bacteriologically confirmed, and presented with a protracted cough, occasional fever, blood-streaked phlegm or hemoptysis, night sweats, fatigue, weight loss, and a positive chest computed tomography (CT) with lung lesions and/or cavities. TB patients with human immunodeficiency virus (HIV), cancer, autoimmune diseases, liver and kidney diseases, undergoing immunosuppressive treatment, blood system dysfunction, or who were pregnant or lactating women were excluded. DS-TB patients were confirmed by a positive bacterial culture with drug-sensitive stains and had no history of TB. MDR-TB patients were diagnosed with a positive sputum culture and baseline drug sensitivity tests using at least two drugs—isoniazid and rifampin. LC patients were diagnosed on the basis of detecting malignant cells in a pulmonary biopsy. Our study contemporaneously enrolled healthy controls (HCs) with normal chest X-rays, no cough, fever, or weight loss, and no recent exposure to TB.
Determination of radiological findings
Of the 34 pulmonary TB patients, chest CT scans showing the lung lesions of 18 patients with DS-TB and 16 patients with MDR-TB were collected before initial treatment regimen and reviewed in a random order by a medical group of senior physicians in the department of respiratory medicine. The determination of the imaging signs included the lung cavity prevalence, bilateral lungs, cavity number, maximum cavity diameter, and sum diameters of all cavities. Radiological findings on the chest CT scans of individual patients were confirmed by the physician’s decision.
Measurement of tryptophan and kynurenine
Chemicals
L-Trp (Sigma-Aldrich, USA) and L-Kyn (Sigma-Aldrich, USA) were dissolved in methanol and stored frozen at –20 ℃. Methanol and formic acid (high-performance liquid chromatography (HPLC) grade) were purchased from Tedia (USA). Other organic solvents and chemicals were all HPLC grade.
Analysis parameters of liquid chromatography-tandem mass spectrometry (LC-MS/MS)
The solvent and sample were separated by an auto sampler (Agilent, USA) and a chromatographic pump (Agilent, USA). The column was Reversal Phase-18 (RP-18) with C18 protection pre-column. The column oven was set at 25 ℃. The mobile phase was methanol/water (95:5 v/v) (water containing 0.1% formic acid), and the flow rate of the LC system was 200 µl/min. The analysis time for each serum sample was four minutes.
The sample profiling analysis was performed by a triple quadrupole mass spectrometer QTRAP®5000 (AbSciex, USA) with an ion source of electrospray ionization (ESI); a detection method of positive ion detection; a detection molecular weight of 209.1→145.9 (Kyn) and 205.3→149.0 (Trp); a scanning method of selection reaction monitoring; a scanning time of 0.1 s; a scanning range of 10–300 m/z (Kyn) and 10–300 m/z (Trp); and a collision energy of 12.7 V (Kyn) and 22.3 V (Trp).
Sample and plasma pretreatment
The stock solutions of L-Kyn and L-Trp were further diluted with methanol into a series of working solutions and stored at –20 ℃, and were thawed at room temperature before LC/MS analysis. The standard sample concentration ranges were 10–500 ng/ml (Kyn) and 12.5–400 ng/ml (Trp).
Ethylenediaminetetraacetic acid–anticoagulated plasma samples were obtained according to the standard operating procedure at the time of admission, and 100 µl of plasma and 300 µl of methanol were added to a 1.5 ml centrifuge tube. These were centrifuged at 12,000 rpm for 30 min at 4 ℃. Then, the supernatants were collected and stored at –80 ℃. After this, the mixture was acutely vortexed for 10 s and centrifuged at 12,000 rpm for 5 min at 4 ℃, then the supernatant was transferred to the auto sampler. The injection volume of every sample was 10 µl for LC-MS/MS.
The plasma IDO activity was estimated by the plasma ratio of the Kyn concentration to the Trp concentration.
Statistical analysis
Summary statistics were shown for the continuous measurements with their means and standard deviations (SDs), and for the categorical measurements with their frequencies and percentages. Logarithmic transformations were performed for the Kyn concentrations and IDO activities to avoid the influence of non-normality. Transformed Kyn concentrations and IDO activities were adopted in the statistical analysis. A Student t-test was used to compare each of the two groups. A one-way analysis of variance (ANOVA) test with the least significant differences (LDSs) post-hoc test was used for multiple-group comparison. Categorical data were analyzed using Fisher’s exact tests. The Mann-Whitney test was used for nonparametric unpaired groups. The diagnostic significance of the IDO activity in discriminating the MDR-TB patients from the DS-TB and LC patients was evaluated by an area under the receiver operating characteristic (ROC) curve (AUC), sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV). Spearman’s t-test was used to detect the correlation between the Try-Kyn pathway and radiological findings. Data analysis were performed using SPSS software (SPSS 25.0, Chicago, IL, USA), and a significance level of 0.05 was considered in the hypothesis testing.
Results
Demographics and clinical parameters
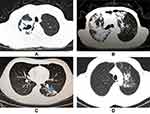
A total of 51 participants were included, with 11 HCs, 18 DS-TB cases, 16 MDR-TB cases, and 6 LC cases (3 lung adenocarcinoma cases, 2 squamous cell lung cancer cases, and 1 small cell lung cancer case). The baseline demographic characteristics of the study participants are summarized in Table 1. There was no difference among groups in the distribution of age, gender, body mass index (BMI), or smoking status. Meanwhile, radiological findings of the DS-TB and the MDR-TB patients are summarized in Table 2. A higher proportion of the MDR-TB patients demonstrated cavitary lung lesions compared to the DS-TB patients. No difference of bilateral lung lesion prevalence between the DS-TB patients and the MDR-TB patients was found. Figure 1 shows the imaging signs of the DS-TB (Figure 1A and B) and MDR-TB patients (Figure 1C and D).
 | Table 1 Baseline characteristics of the participants in the four groups |
 | Table 2 Radiological characteristics of the DS-TB and MDR-TB patients |
Plasma concentrations of Kyn, Trp, and IDO activity in the four study groups
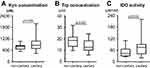
As shown in Figure 2A, the Kyn concentration in plasma was significantly higher in patients with MDR-TB than in the HC, DS-TB, and LC groups (988.26±332.75 nM vs 757.33±160.98 nM, p=0.013; 988.26±332.75 nM vs 743.50±145.42 nM, p=0.003; 988.26±332.75 nM vs 725.79±103.42 nM, p=0.022), whereas no significant difference was found between the HC and DS-TB groups (757.33±160.98 nM vs 743.50±145.42 nM, p=0.844) (Figure 2A). Compared with MDR-TB patients, the HC, DS-TB, and LC patients had a significantly higher Trp concentration (23.21±5.35 µM vs 12.19±3.79 µM, p<0.0001; 19.05±6.11 µM vs 12.19±3.79 µM, p<0.0001; 21.27±5.65 µM vs 12.19±3.79 µM, p=0.001) (Figure 2B). In addition, we also observed a decreasing trend in the plasma Trp concentration in the DS-TB group compared with the HC group, with the difference being significant (19.05±6.11 µM vs 23.21±5.35 µM, p=0.044). As a result, our data revealed significantly higher IDO activity (Kyn/Trp ratio) in the MDR-TB individuals than in the HC, DS-TB, and LC groups (90.61±49.09 µM/mM vs 35.18±13.92 µM/mM, p=0.003; 90.61±49.09 µM/mM vs 43.84±19.53 µM/mM, p=0.012; 90.61±49.09 µM/mM vs 35.91±9.56 µM/mM, p=0.003), whereas no significant difference was found between the HC and DS-TB groups (35.18±13.92 µM/mM vs 43.84±19.53 µM/mM, p=0.687) (Figure 2C).
Diagnostic significance of plasma concentrations of Kyn, Trp, and IDO activity for discriminating MDR-TB patients from TB patients
To evaluate the potential values of the plasma tryptophan-kynurenine pathway for the diagnosis of MDR-TB, three ROC curves were plotted (Figure 3). Using optimal cut-off points, the diagnostic ability of these parameters is shown in Table 3. At a cutoff of 46.58 µM/mM, IDO activity had a sensitivity of 87.50%, a specificity of 72.22%, a PPV of 73.68%, and an NPV of 86.67% (AUC =0.882, Figure 3C, Table 3). We also analyzed the diagnostic value of plasma Kyn concentration and Trp concentration, and the two key indicators of the plasma tryptophan-kynurenine pathway can distinguish MDR-TB groups as well (Kyn, AUC =0.764; Trp, AUC =0.833, Figure 3A and B, Table 3). In the analysis, plasma IDO activity had a higher AUC than Kyn or Trp concentration.
 | Table 3 Diagnostic significance of plasma concentrations of Kyn, Trp, and IDO activity for discriminating MDR-TB patients from TB patients |
Association of plasma concentrations of Kyn, Trp, and IDO activity with radiological findings
In addition, we explored the Spearman correlation analysis of the three plasma indexes with radiological findings in the TB (DS-TB, MDR-TB) patients. As presented in Table 4, Trp concentration was negatively correlated with cavity number, the maximum diameter of the cavity, and the sum of cavity diameters (r = –0.373, p=0.030; r = –0.386, p=0.024; r = –0.408, p=0.017). IDO activity showed a positive association with the maximum diameter of the cavity, and the sum of cavity diameters (r =0.441, p=0.009; r =0.383, p=0.026; r =0.410, p=0.016). Subsequently, subgroup analyses were carried out and no significant differences were found in the DS-TB and MDR-TB patients separately.
 | Table 4 Correlation coefficient between cavity number, maximum cavity diameter, sum of cavity diameters, and kynurenine, tryptophan, and IDO activity by patient group |
Plasma concentrations of Kyn, Trp, and IDO activity in cavitary and non-cavitary pulmonary TB patients
Next, we investigated plasma tryptophan-kynurenine pathway indexes in non-cavitary pulmonary TB patients (n=15) and cavitary pulmonary TB patients (n=19) on CT scanning. No significant difference was found in Kyn concentration between the two subgroups, but there was a higher trend in patients with cavities (911.09±336.75 nM vs 792.30±162.21 nM, p=0.296) (Figure 4A). Compared with non-cavitary TB patients, cavitary patients had significantly lower Trp concentrations (13.88±5.21 µM vs 18.28±6.55 µM, p=0.037) (Figure 4B) and higher IDO activity (79.03±50.71 µM/mM vs 49.15±23.00 µM/mM, p=0.042) (Figure 4C).
Diagnostic value of plasma concentrations of Kyn, Trp, and IDO activity for discriminating LC and MDR-TB patients
Finally, based on ROC curves, we evaluated the diagnostic significance of plasma tryptophan-kynurenine pathway indexes for distinguishing MDR-TB from other lung diseases (LC). With an optimal calculated IDO activity threshold of 49.23 µM/mM, the AUC was 0.958, with a sensitivity and specificity of 87.50% and 100.00%, respectively, and with a PPV and NPV of 100.00% and 75.00%, respectively (Figure 5C, Table 5). The AUCs of Kyn and Trp were 0.802 and 0.917, respectively (Figure 5A and B, Table 5).
 | Table 5 Diagnostic significance of plasma concentrations of Kyn, Trp, and IDO activity for discriminating MDR-TB patients from the LC patients |
Discussion
Despite increased research activity in tuberculosis, clinicians remain hindered by gaps in knowledge and understanding of both pathogenesis and the host factors that contribute to the susceptibility of MDR-TB disease.30,31 We are clearly lacking host directed biomarkers for indicating disease status, especially in the early stage of MDR-TB.32 To date, few studies have discussed IDO activity in MDR-TB patients, either to clearly determine its profile in host immunity against MDR-TB or in lung lesions from MDR-TB patients.
It has been recognized that tryptophan metabolism brings twofold consequences. First, starving the bacteria by depleting the local microenvironment of tryptophan, which is essential for most pathogen metabolism.33,34 Second, inducing the apoptosis of host immune cells,35,36 mostly lymphocytes activated by tryptophan downstream catabolites, including kynurenine and 3-hydroxyanthranilic acid (3-HAA). IDO activity is evolved in suppressing CD4+T cell proliferation.37 inducing antigen-presenting cells (APC).38 and promoting the differentiation of CD4+CD25+Foxp3+regulatory T cells (Treg) cells from naive CD4+ T cells.37,39,40 Therefore, IDO activity is believed to contribute to mycobacteria burden increase and persistence in chronic infection. The present study shows that IDO activity in the MDR-TB group was higher than in the DS-TB group, and IDO activity was also associated with lung cavitary lesions in the patients with TB. This is consistent with our previous finding that MDR-TB patients show lower CD4+IFN-γ+T cell response and over-induced Treg activation in PBMCs along with more severe clinical symptoms and lung lesions.28,29,41
Abundant evidence also indicates IDO activity not only participates in the pathogenesis of TB, but also can be a complementary tool for diagnosis and prognosis of TB.23–26,42 However, no studies have been conducted to evaluate its diagnostic significance in MDR-TB patients. In this regard, we clearly showed that serum IDO activity in MDR-TB discriminates this condition from DS-TB groups, with a cutoff level of 46.58 µM/mM, sensitivity 87.50%, specificity 72.22%, and positive predictive value (PPV) of 73.68%. We also measured plasma Kyn and Trp concentration as some of the IDO metabolic pathway products in MDR-TB patients and consistently found significant differentiations in MDR-TB patients from DS-TB patients. A study in Japan reported in 2011 enrolled 174 consecutive patients and showed that serum IDO activity was an independent predictor of mortality.24 A prospective clinical study for TB patients with HIV infection demonstrated that, compared to the higher IDO activity before diagnosis, IDO activity decreased after anti-TB treatment.42 A large-scale study will be conducted in future with a well-designed protocol to validate this finding.
In the present study, the IDO activity showed a significant positive correlation with lung cavity prevalence and lung cavity size. We also observed the association of the three plasma indexes with radiological findings in DS-TB and MDR-TB patients separately, and no significant differences were found. We further found IDO activity was relatively higher in 19 patients with pulmonary cavities, compared with 15 patients who presented with non-cavities on computed tomography (CT) scanning. Based on accumulating evidence from studies on animal and human specimens, M. tuberculosis infection is associated with high expression of IDO in the infected phagocytes in the center structure of the granuloma, preventing T cell protection and thereby further promoting bacterial survival, which leads to cavitary lesions and disease transmission.22,23 High bacillary titers in cavities increase the probability of establishing drug-resistant bacterial populations.43,44 Along with imaging findings, it is therefore understandable that thicker walls and larger cavities are more likely to be associated with MDR-TB and extensively drug-resistant tuberculosis (XDR-TB).45,46 While MDR-TBs tend to be more extensive in chest CT imaging than DS-TBs,47 imaging signs still cannot independently discriminate MDR-TB from DS-TB.45,48 Our findings imply that higher plasma IDO activity indicates more severe MTB destruction in the lung parenchymal of patients, related to more extensive cavity lung lesions (cavity prevalence and cavity size), leading to a considerably higher risk of MDR-TB developing and greater transmission of the pathogen.49,50 Although imaging data from more cases is needed to confirm our pilot evaluation in this study, IDO activity may be a feasible tool along with CT imaging for early identification of such patients. This is urgently needed to facilitate promptly conducting the correct TB patients into an isolation ward, thereby alleviating the spread of MDR-TB.
We found significantly higher plasma IDO activity in the MDR-TB group than in the lung cancer group, with a discriminating significance. Several studies have shown that an increase in IDO activity is associated with poor prognosis in cancer patients.51 In our study, due to the limitation of a small sample size of lung cancer cases with no small cell lung cancer (NSCLC) cases or small cell lung cancer (SCLC) cases, plasma IDO activity showed no statistical discrimination from HCs. Given the current absence of efficient biomarkers to distinguish MDR-TB from lung cancer (and vice versa),52 we call for more feasible and powerful tools for identification of these two diseases. There is overlap between pulmonary tuberculosis and malignant lesions. Using high radiodensities on enhanced CT, MRI or18 F-FDG PET/CT is a sensitive tool for identifying lung cancer53,54 however especially in MDR-TB patients parenchymal consolidations and scarring with parenchymal and cavitary nodal calcifications are more common and poorly defined, and patchy in reactivated disease,55–57 all of which could also be consistent with a lung cancer and thus leading to unnecessary invasive tissue collection and resections in these populations.58 An earlier study reported by Seo KJ et al upon analyzing clinical specimens from TB patients, demonstrated that is was possible to use IDO as an immunohistochemical marker to differentiate between tuberculous granuloma (TG) and non-tuberculousgranuloma (NG).23 However, the limitations of this method are the invasive procedures required to obtain the pathologic specimens, and the longer duration required for histopathology measurement. IDO’s expression in tuberculous granuloma of a nonhuman primate model was found to increase and to be associated with host–Mycobacterium tuberculosis interactions, which either result in acute infection or the control of infection in a latent state.23,40 However, we are still uncertain about increasing plasma IDO activity in human extrapulmonary tuberculosis or latent tuberculosis because of the difficulty in collecting human samples with variable confounding factors.40 We assumed that plasma IDO activity in human latent TB or extrapulmonary TB might be increasing but be lower than in human MDR-TB. This assumption will be validated in future study. Recently, another study on serum markers found than the evaluation of circulating cell-free DNA (ccfDNA) is more sensitive and specific than carcinoembryonic antigen (CEA), carbohydrate antigen 125 (CA125), and neuron‐specific enolase (NSE) in differentiating NSCLC from tuberculosis.59 Unfortunately, these approaches are currently costly, with a low throughput. Therefore, our data from a small group of NSCLC patients, while requiring confirmation in future studies with a larger cohort, shed light on the value of plasma IDO activity, which seems to be potentially feasible as a compensatory tool for imaging or histopathology identification of MDR-TB from among other lung malignant diseases. Our pilot study used IDO activity as an auxiliary biomarker to discriminate MDR-TB from DS-TB and lung cancer, which can contribute to developing a combined diagnostic procedure with other biomarkers. For instance, in Chao Liu et al.’s reporting, the panel containing Programmed cell death protein 1 (PD-1), interleukin-10 (IL-10), Interleukin-2 receptor alpha (IL-2Rα) and cancer antigen 15-3 (CA15-3) as biomarkers discriminated breast cancer from benign breast disease with high efficiency.60
Several limitations exist in this study. The sample cases were selected within the restrictions of distance, transport duration, and parallel period (2 h) for blood drawing to keep the temperature and biological metabolism conditions of the body consistent (the present cases were all from east China). Thus, future studies should be performed with more cases of MDR-TB from different regions, along with other pulmonary infections and more malignant cavitary lung disease cases as controls to determine if increasing of IDO activity is specific to MDR-TB. Second, we examined IDO activity, determined by the plasma Kyn/Trp ratio, but lacked molecular or pathological confirmation of IDO expression in these patients. Origin of increased IDO activity in the plasma of MDR-TB patients in this study is still not clear in terms of clarifying the mechanisms in MDR-TB. Previous studies have focused mostly on TB conditions, with uncertainty around MDR-TB. Future studies will be required to clarify these issues. Third, further studies evaluating MDR-TB patients from baseline to after a standard treatment regimen, at different time points, should be performed to confirm that IDO activity could be used to assist diagnosis of MDR-TB and to monitor anti-TB therapy.
Conclusion
IDO activity in MDR-TB patients was predominantly higher than that in DS-TB patients and HCs, with the diagnostic significance in discriminating from the DS-TB group and lung cancer group. We found that IDO activity was positively correlated with cavitary lung lesions in patients with TB, potentially indicating a more severe infectious condition and higher risk of developing MDR-TB. We conducted this study to propose and test plasma IDO activity as a novel biomarker candidate for the early detection of MDR-TB from DS-TB. Our finding suggested that plasma IDO activity can be used as a potential biomarker to assist chest CT scans in the early detection of MDR-TB. However, this study is only a pilot study, and a large-scale study is needed to confirm our findings. This pilot study allows a better understanding of IDO’s participation in the pathogenesis of MDR- TB and DS – TB, and can contribute to developing a combined diagnostic procedure with other biomarkers.
Acknowledgments
The authors would like to thank Fu-Qiang Wang and Analysis Center Nanjing Medical University for their comments and assistance in high performance liquid chromatography-mass spectrometry measurement and blood sample preparation.
This work was funded by The Key Project of National Science & Technology for Infectious Diseases of China [Grants #2015ZX10003001], [Grants #2018ZX10722301]; National Natural Science Foundation of China [Grants #81800011]; The Key Research and Development Project of Zhenjiang, China [Grants #SH2017022]
Disclosure
The authors report no conflicts of interest in this work.
References
1. Van der Heijden YF, Abdullah F, Andrade BB, et al. Building capacity for advances in tuberculosis research; proceedings of the third report international meeting. Tuberculosis (Edinb). 2018;113:153–162. doi:10.1016/j.tube.2018.09.009
2.
3.
4. Andrews JR, Noubary F, Walensky RP, Cerda R, Losina E, Horsburgh CR. Risk of progression to active tuberculosis following reinfection with Mycobacterium tuberculosis. Clin Infect Dis Mar. 2012;54(6):784–791.
5. Shah NS, Auld SC, Brust JC, et al. Transmission of extensively drug-resistant tuberculosis in South Africa. N Engl J Med. 2017;376(3):243–253.
6. Dowdy DW, Azman AS, Kendall EA, Mathema B. Transforming the fight against tuberculosis: targeting catalysts of transmission. Clin Infect Dis. 2014;59(8):1123–1129.
7.
8. Virenfeldt J, Rudolf F, Camara C, et al. Treatment delay affects clinical severity of tuberculosis: a longitudinal cohort study. BMJ Open. 2014;4(6):e004818.
9. Zumla A, George A, Sharma V, Herbert RH, Oxley A, Oliver M. The WHO 2014 global tuberculosis report–further to go. Lancet Glob Health. 2014;3(1):e10–e12.
10. Boehme CC, Nabeta P, Hillemann D, et al. Rapid molecular detection of tuberculosis and rifampin resistance. N Engl J Med. 2010;363(11):1005–1015. doi:10.1056/NEJMoa0907847
11. Uplekar M, Weil D, Lonnroth K, et al. WHO‘s new end TB strategy. Lancet. 2015;385(9979):1799–1801. doi:10.1016/S0140-6736(15)60570-0
12. Lawn SD, Mwaba P, Bates M, et al. Advances in tuberculosis diagnostics: the Xpert MTB/RIF assay and future prospects for a point-of-care test. Lancet Infect Dis. 2013;13(4):349–361. doi:10.1016/S1473-3099(13)70008-2
13. Dorman SE, Schumacher SG, Alland D, et al. Xpert MTB/RIF ultra for detection of Mycobacterium tuberculosis and rifampicin resistance: a prospective multicentre diagnostic accuracy study. Lancet Infect Dis. 2018;18(1):76–84. doi:10.1016/S1473-3099(17)30691-6
14. Gupta S, Kakkar V. Recent technological advancements in tuberculosis diagnostics - A review. Biosens Bioelectron. 2018;115:14–29. doi:10.1016/j.bios.2018.05.017
15.
16. Wallis RS, Kim P, Cole S, et al. Tuberculosis biomarkers discovery: developments, needs, and challenges. Lancet Infect Dis. 2013;13(4):362–372. doi:10.1016/S1473-3099(13)70034-3
17. Pfefferkorn ER. Interferon gamma blocks the growth of Toxoplasma gondii in human fibroblasts by inducing the host cells to degrade tryptophan. Proc Natl Acad Sci U S A. 1984;81(3):908–912.
18. Taylor MW, Feng GS. Relationship between interferon-gamma, indoleamine 2,3-dioxygenase, and tryptophan catabolism. FASEB J. 1991;5(11):2516–2522.
19. Werner ER, Bitterlich G, Fuchs D, et al. Human macrophages degrade tryptophan upon induction by interferon-gamma. Life Sci. 1987;41(3):273–280.
20. Carlin JM, Borden EC, Sondel PM, Byrne GI. Interferon-induced indoleamine 2,3-dioxygenase activity in human mononuclear phagocytes. J Leukoc Biol. 1989;45(1):29–34.
21. Curti A, Trabanelli S, Salvestrini V, Baccarani M, Lemoli RM. The role of indoleamine 2,3-dioxygenase in the induction of immune tolerance: focus on hematology. Blood. 2009;113(11):2394–2401. doi:10.1182/blood-2008-07-144485
22. Gautam US, Foreman TW, Bucsan AN, et al. In vivo inhibition of tryptophan catabolism reorganizes the tuberculoma and augments immune-mediated control of Mycobacterium tuberculosis. Proc Natl Acad Sci U S A. 2018;115(1):E62–E71. doi:10.1073/pnas.1711373114
23. Seo KJ, Yoo CY, Im SY, et al. A possible complementary tool for diagnosing tuberculosis: a feasibility test of immunohistochemical markers. Int J Clin Exp Pathol. 2015;8(11):13900–13910.
24. Suzuki Y, Suda T, Asada K, et al. Serum indoleamine 2,3-dioxygenase activity predicts prognosis of pulmonary tuberculosis. Clin Vaccine Immunol. 2012;19(3):436–442. doi:10.1128/CVI.05402-11
25. Suzuki Y, Miwa S, Akamatsu T, et al. Indoleamine 2,3-dioxygenase in the pathogenesis of tuberculous pleurisy. Int J Tuberc Lung Dis. 2013;17(11):1501–1506. doi:10.5588/ijtld.13.0082
26. Van Laarhoven A, Dian S, Aguirre-Gamboa R, et al. Cerebral tryptophan metabolism and outcome of tuberculous meningitis: an observational cohort study. Lancet Infect Dis. 2018;18(5):526–535. doi:10.1016/S1473-3099(18)30053-7
27. Barry S, Breen R, Lipman M, Johnson M, Janossy G. Impaired antigen-specific CD4(+) T lymphocyte responses in cavitary tuberculosis. Tuberculosis (Edinb). 2009;89(1):48–53. doi:10.1016/j.tube.2008.07.002
28. Tan Q, Xie WP, Min R, et al. Characterization of Th1- and Th2-type immune response in human multidrug-resistant tuberculosis. Eur J Clin Microbiol Infect Dis. 2012;31(6):1233–1242. doi:10.1007/s10096-011-1434-4
29. Tan Q, Min R, Wang H, et al. Modulation of IFN-gamma and IL-17 is critical in human immune responses to multidrug-resistant tuberculosis in Eastern Chinese Han population.
30. Russell DG, Barry CE
31.
32. Falzon D, Mirzayev F, Wares F, et al. Multidrug-resistant tuberculosis around the world: what progress has been made? Eur Respir J. 2015;45(1):150–160. doi:10.1183/09031936.00101814
33. Zhang YJ, Reddy MC, Ioerger TR, et al. Tryptophan biosynthesis protects mycobacteria from CD4 T-cell-mediated killing. Cell. 2013;155(6):1296–1308. doi:10.1016/j.cell.2013.10.045
34. Cervenka I, Agudelo LZ, Ruas JL. Kynurenines: tryptophan‘s metabolites in exercise, inflammation, and mental health. Science. 2017;357(6349):eaaf9794. doi:10.1126/science.aaf9794
35. Mellor AL, Keskin DB, Johnson T, Chandler P, Munn DH. Cells expressing indoleamine 2,3-dioxygenase inhibit T cell responses. J Immunol. 2002;168(8):3771–3776.
36. Fallarino F, Grohmann U, Vacca C, et al. T cell apoptosis by kynurenines. Adv Exp Med Biol. 2003;527:183–190.
37. Fallarino F, Grohmann U, You S, et al. The combined effects of tryptophan starvation and tryptophan catabolites down-regulate T cell receptor zeta-chain and induce a regulatory phenotype in naive T cells. J Immunol. 2006;176(11):6752–6761.
38. Munn DH, Sharma MD, Lee JR, et al. Potential regulatory function of human dendritic cells expressing indoleamine 2,3-dioxygenase. Science. 2002;297(5588):1867–1870.
39. Munn DH, Zhou M, Attwood JT, et al. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science. 1998;281(5380):1191–1193.
40. Mehra S, Alvarez X, Didier PJ, et al. Granuloma correlates of protection against tuberculosis and mechanisms of immune modulation by Mycobacterium tuberculosis. J Infect Dis. 2013;207(7):1115–1127.
41. Li N, Xie WP, Kong H, et al. Enrichment of regulatory T-cells in blood of patients with multidrug-resistant tuberculosis. Int J Tuberc Lung Dis. 2015;19(10):1230–1238.
42. Adu-Gyamfi CG, Snyman T, Hoffmann CJ, et al. Plasma indoleamine 2, 3-dioxygenase, a biomarker for tuberculosis in human immunodeficiency virus-infected Patients. Clin Infect Dis. 2017;65(8):1356–1358.
43. Mazzarella G, Bianco A, Perna F, et al. T lymphocyte phenotypic profile in lung segments affected by cavitary and non-cavitary tuberculosis. Clin Exp Immunol. 2003;132(2):283–288.
44. Ong CW, Elkington PT, Friedland JS. Tuberculosis, pulmonary cavitation, and matrix metalloproteinases. Am J Respir Crit Care Med. 2014;190(1):9–18.
45. Cha J, Lee HY, Lee KS, et al. Radiological findings of extensively drug-resistant pulmonary tuberculosis in non-AIDS adults: comparisons with findings of multidrug-resistant and drug-sensitive tuberculosis. Korean J Radiol. 2009;10(3):207–216.
46. Cheon H. Comparison of CT findings of between MDR-TB and XDR-TB: a propensity score matching study. Imaging Med. 2017;9:125–129.
47. Fishman JE, Sais GJ, Schwartz DS, Otten J. Radiographic findings and patterns in multidrug-resistant tuberculosis. J Thorac Imaging. 1998;13(1):65–71.
48. Kim SH, Min JH, Lee JY. Radiological findings of primary multidrug-resistant pulmonary tuberculosis in HIV-seronegative patients. Hong Kong J Radiol. 2014;17:4–8.
49. Van Dyck P, Vanhoenacker FM, Van Den Brande P, De Schepper AM. Imaging of pulmonary tuberculosis. Eur Radiol. 2003;13(8):1771–1785.
50. Zhang L, Pang Y, Yu X, et al. Risk factors for pulmonary cavitation in tuberculosis patients from China. Emerg Microbes Infect. 2016;5(10):e110.
51. Platten M, Wick W. Van den Eynde BJ. Tryptophan catabolism in cancer: beyond IDO and tryptophan depletion. Cancer Res. 2012;72(21):5435–5440.
52. Ho JC, Leung CC. Management of co-existent tuberculosis and lung cancer. Lung Cancer. 2018;122:83–87.
53. Qi LP, Chen KN, Zhou XJ, et al. Conventional MRI to detect the differences between mass-like tuberculosis and lung cancer. J Thorac Dis. 2018;10(10):5673–5684.
54. Niyonkuru A, Bakari KH, Lan X. (18)F-fluoro-2-deoxy-d-glucose PET/computed tomography evaluation of lung cancer in populations with high prevalence of tuberculosis and other granulomatous disease. PET Clin. 2018;13(1):19–31.
55. Lang S, Sun J, Wang X, et al. Asymptomatic pulmonary tuberculosis mimicking lung cancer on imaging: a retrospective study. Exp Ther Med. 2017;14(3):2180–2188.
56. Kim YI, Goo JM, Kim HY, Song JW, Im JG. Coexisting bronchogenic carcinoma and pulmonary tuberculosis in the same lobe: radiologic findings and clinical significance. Korean J Radiol. 2001;2(3):138–144.
57. Yu YY, Pinsky PF, Caporaso NE, et al. Lung cancer risk following detection of pulmonary scarring by chest radiography in the prostate, lung, colorectal, and ovarian cancer screening trial. Arch Intern Med. 2008;168(21):2326–2332. discussion 2332.
58. Davies PD, Pai M. The diagnosis and misdiagnosis of tuberculosis. Int J Tuberc Lung Dis. 2008;12(11):1226–1234.
59. Leng S, Zheng J, Jin Y, et al. Plasma cell-free DNA level and its integrity as biomarkers to distinguish non-small cell lung cancer from tuberculosis. Clin Chim Acta. 2018;477:160–165.
60. Liu C, Sun B, Xu B, et al. A panel containing PD-1, IL-2Ralpha, IL-10, and CA15-3 as a biomarker to discriminate breast cancer from benign breast disease. Cancer Manag Res. 2018;10:1749–1761.
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.