Back to Journals » Journal of Blood Medicine » Volume 11
Plasma Folate Levels in Acutely Ill and Steady State Pediatric Sickle Cell Disease Patients in Ghana
Authors Adjei GO, Sulley AM , Goka BQ , Enweronu-Laryea C, Amponsah SK , Alifrangis M, Kurtzhals JAL
Received 5 August 2020
Accepted for publication 5 September 2020
Published 3 November 2020 Volume 2020:11 Pages 421—427
DOI https://doi.org/10.2147/JBM.S275150
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Martin H Bluth
George O Adjei,1 Abdul M Sulley,1 Bamenla Q Goka,2 Christabel Enweronu-Laryea,2 Seth K Amponsah,3 Michael Alifrangis,4 Jorgen AL Kurtzhals4,5
1Centre for Tropical Clinical Pharmacology and Therapeutics, University of Ghana Medical School, Accra, Ghana; 2Department of Child Health, University of Ghana Medical School, Accra, Ghana; 3Department of Medical Pharmacology, University of Ghana Medical School, Accra, Ghana; 4Centre for Medical Parasitology at Department of Immunology and Microbiology, University of Copenhagen, Copenhagen, Denmark; 5Centre for Medical Parasitology at Department of Clinical Microbiology, Copenhagen University Hospital (Rigshospitalet), Copenhagen, Denmark
Correspondence: George O Adjei
Centre for Tropical Clinical Pharmacology and Therapeutics, University of Ghana Medical School, Accra, Ghana
Email [email protected]
Background: Individuals with sickle cell disease (SCD) are susceptible to infective conditions that predispose them to hemolysis and anemia. Folic acid is recommended as a preventative measure against anemia in SCD patients; however, there is scarce literature on the implications of this practice.
Patients and Methods: Plasma concentrations of folate were measured in acutely ill pediatric SCD patients presenting with malaria or bacteremia and compared with those of SCD patients in steady state, or acutely ill non-SCD patients with confirmed malaria.
Results: The proportion of individuals with high (> 45.3 nmol/L) folate concentrations was 29.5% (13/44), 18.2% (8/44), 33.3% (6/18), and 0% in the SCD-malaria, SCD steady state, SCD bacteremia, and the non-SCD malaria groups, respectively. The proportion of SCD patients with high folate levels did not vary significantly at steady state and during confirmed malaria (p = 0.216), and during acute bacteremia (p = 0.20). The median (interquartile range) plasma folate levels were 34.50 (24.40– 52.00 nmol/L), 33.40 (15.83– 60.85 nmol/L), 30.85 (24.68– 39.65 nmol/L), and 13.30 (10.03– 17.18 nmol/L), respectively, in the SCD malaria, SCD bacteremia, SCD steady state, and the non-SCD malaria sub-groups. The median folate levels of SCD steady state, SCD malaria, and SCD bacteremia sub-groups differed significantly (p < 0.0001) when compared with non-SCD patients, but the levels in the SCD bacteremia and malaria groups were not significantly different from the SCD steady state group.
Conclusion: Elevated levels of plasma folate were found in a high proportion of pediatric SCD patients. The implications of such elevated folate levels in pediatric SCD patients are unknown but may suggest a need for review of current recommendations for prophylactic doses of folic acid in SCD patients.
Keywords: folate, sickle cell disease, malaria, bacteremia
Introduction
Individuals with sickle cell disease (SCD) have grossly reduced erythrocyte half-lives and are at increased risk of chronic hemolytic anemia1 and folate deficiency.2 Affected persons are expected to have higher needs for folate supplementation compared to non-SCD individuals. Accordingly, the World Health Organization (WHO) recommends folic acid supplementation to prevent anemia in persons with SCD. The rationale for routine supplementation with folic acid in the management of SCD patients includes maintenance of effective erythropoiesis, compensation for increased requirements,3 and reduction of endothelial cell damage.4 This notwithstanding, folate supplementation has been associated with adverse consequences such as masking of vitamin B12 deficiency,5 increased or reduced natural killer cytotoxicity among women with a diet low (< 233 µg/d) or high (>400 µg/d) in folate, respectively,6 and increased inflammation.7 Moreover, there is scarce literature on the potential effect of folic acid supplementation on important clinical outcomes in SCD patients.8
In endemic areas, malaria is the most common cause of acute crises in SCD patients and is associated with a worse outcome as compared to non-SCD patients. There is conflicting evidence on the role of folate supplementation in the context of malaria in SCD patients: high dietary folate has been shown to increase parasite replication in animals, and to aid in vitro growth for survival of malaria parasites,9 while other studies have demonstrated a protective effect of folate deficiency against malarial infection.10 In humans, pregnant women consuming high-folate diets have low malaria infection rates,11 while folate deficiency has also been implicated in susceptibility to malaria.12,13
There are no reports on whether currently recommended or prescribed doses of folic acid maintain folate levels within therapeutic levels, or whether variations in folate levels occur during acute infections in SCD patients. In this study therefore, we measured plasma folate concentrations in SCD patients in steady state and compared these with folate levels in acutely ill SCD patients with either parasitologically confirmed malaria or culture-positive bacteremia. We compared the folate levels in these SCD patient groups with those of acutely ill non-SCD patients with confirmed malaria.
Patients and Methods
Study Site
The study was conducted at the Pediatric Emergency Unit and Pediatric Sickle Cell Clinic of the Department of Child Health, Korle Bu Teaching Hospital (KBTH), Accra, Ghana. The Department of Child Health in KBTH provides service to children from birth to age 12 years, and over 35,000 children are seen in the general and sub-specialty clinics annually. The Pediatric Sickle Cell Clinic provides acute and follow-up care for children with SCD. It is held once a week with an average weekly attendance of 60–70. The Department has a “walk in” policy for children with SCD with an acute illness. These children are assessed in the outpatient clinic or emergency room 24 hours a day. All SCD patients attending the Pediatric Sickle Cell Clinic are routinely put on folic acid, 5 mg daily.
Study Population and Recruitment
Acutely ill children of known SCD status presenting to the Pediatric Emergency Department or Pediatric Sickle Cell Clinic of KBTH were referred to the study team, where they were examined and recruited if study criteria were fulfilled and if written informed consent was obtained from the accompanying parent or guardian. Inclusion criteria for the study were: child of confirmed SCD genotype aged between 6 months to 12 years with an acute febrile illness (history of fever within the past 72 hours, or an axillary temperature ≥ 37.5°C, at presentation). A detailed medical history and clinical examination was performed, and a preliminary working clinical diagnosis was made based on the presenting symptoms, signs, and evaluation assessment. SCD children in steady state visiting the clinic for scheduled evaluations and whose blood films were negative for malaria parasites on the day of visit were recruited as a comparison group. In addition, a group of acutely ill non-SCD children (Hb genotype, AA) with confirmed Plasmodium falciparum malaria were recruited from the adjoining primary care facility (Korle Bu Polyclinic) as a further comparison group. The inclusion and exclusion criteria for the non-SCD (HbAA) children were the same as for the SCD children apart from hemoglobin genotype.
After completion of initial study procedures, venous blood was obtained for routine hematological and microbiological investigations. Appropriate treatment was immediately initiated according to departmental guidelines.
Laboratory Investigations
Malaria Microscopy
Thick and thin blood smears were prepared from an EDTA blood sample and stained with Giemsa for identification and quantification of Plasmodia species. Asexual malaria parasitemia (expressed as parasites per microliter of whole blood) was determined by counting the number of asexual stage parasites relative to 200 white blood cells (WBC), and multiplied by the measured WBC count. A slide was declared negative if no parasites were seen in at least 100 oil immersion high-power fields on the thick smear. Each slide was read independently by two microscopists, and approximately 10% of all slides were read by a third expert microscopist who was independent of the study. In those with confirmed malaria, microscopy was repeated 14 days later.
Hematological Analyses
The hemoglobin level, total white blood cell (WBC), and differential counts were measured by means of an automated hematology analyzer (Sysmex KX-21N, Sysmex Inc, USA).
Blood Culture
Blood culture was done using a fully automated BACTEC 9240 blood culture system (Becton Dickinson Diagnostic Instrument Systems, Sparks, Md). A positive reading indicates the presumptive presence of viable microorganism. Positive cultures were flagged by an indicator light on the front of the BACTEC instrument. Vials stayed in the BACTEC for at least 5 days before declaring a negative result.
Folate Assay
Blood samples collected in standard gel tubes were immediately centrifuged and stored at 2–8°C, until analysis for folate levels. Folate levels were measured by use of Elecsys Folate III assay reagent kit on an Elecsys 2010 analyzer (Roche Diagnostic Limited, Switzerland), according to the manufacturer's protocol. This method is based on competitive test principle using folate-specific natural folate binding protein (FBP), in which folate present in a sample competes with the added biotin-labeled folate for binding sites on the FBP (labeled with ruthenium complex). The reference range was 4.5–45.3 nmol/L.14
Ethical Considerations
The study was approved by the Ethical and Protocol Review Committee, University of Ghana Medical School (MS-Et/1-P.5.4/2009–2010). Informed consent was obtained from parents or guardians of all recruited participants. The study was conducted in accordance with the Declaration of Helsinki.
Statistical Analysis
Median folate levels in various groups were compared using Kruskal–Wallis one-way analysis of variance (ANOVA) followed by Dunn’s multiple comparisons test. For repeated folate levels (within the same group), paired t-test was used to compare folate levels. Group proportions were compared with chi-square test. A p < 0.05 was an indication of statistically significant difference. Data were analysed using R version 3.6.3 (R Core Team, 2020) and GraphPad Prism Version 6.
Results
Recruited Participants
A total of 146 participants were included; these comprised 84 acutely ill patients with confirmed P. falciparum malaria, of which 44 were SCD patients and 40 were non-SCD (HbAA) patients; 44 were SCD children in steady state visiting the clinic for routine scheduled appointments (with a negative blood smear as well as negative malaria rapid diagnostic test [RDT] result on day of recruitment), and 18 SCD patients with an acute febrile illness, who were found to have a positive blood culture. Bacteria isolated were: Salmonella spp, n = 5; Staphylococcus aureus, n = 4; Enterococcus faecalis, n = 3; Enterobacter spp, n = 2; Streptococcus viridans, n = 2; Streptococcus pneumoniae, n = 1; and Klebsiella spp, n = 1.
Presenting Symptomatology and Baseline Clinical and Laboratory Parameters
In the SCD malaria sub-group, the predominant presenting symptoms were fever, pain syndrome, headache, chills/rigors, general malaise, jaundice, vomiting, and dark urine; while fever, pain syndrome, and cough were predominant in the SCD bacteremia group; and fever, vomiting, headache, abdominal pain, and loss of appetite occurred more frequently in the non-SCD malaria sub-group. The main presenting symptoms are shown (Table 1). A selection of baseline clinical and laboratory parameters of the recruited patients, showing lower hemoglobin and higher white blood cell and platelet counts in the SCD sub-groups, is shown (Table 2).
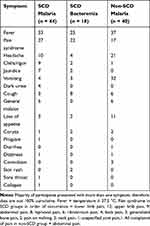 |
Table 1 Presenting Symptoms in the Acutely Ill Groups |
 |
Table 2 Baseline Demographic and Clinical Characteristics in Clinical Sub-Groups |
Median Folate Levels Among the Different Clinical Sub-Groups
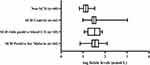
The data showed significantly overall comparatively high median serum folate levels in the SCD patients with malaria (median; interquartile range [IQR]), 34.50; 24.40–52.00 nmol/L versus 33.40; 15.83–60.85 nmol/L in SCD patients with acute bacteremia, and only slightly, non-significantly lower median (with IQR) levels in the SCD in steady state group (30.85; 24.68–39.65 nmol/L). The lowest median levels were observed in the non-SCD (HbAA) patients with acute malaria sub-group (13.30; 10.03–17.18 nmol/L), as shown in Figure 1.
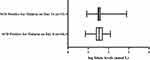
There was no significant difference in the median (IQR) folate levels between SCD in steady state and SCD patients with acute falciparum malaria, versus SCD patients with acute bacteremia (p > 0.05). The median (IQR) folate levels of the SCD participants sub-groups (SCD in steady state, SCD patients with acute falciparum malaria, and SCD patients with acute bacteremia) differed significantly (p < 0.0001) on comparison with non-SCD patients with malaria. The median (IQR) folate levels in the SCD patients with acute falciparum malaria on Day 14 (when malaria parasitemia had cleared) had reverted to levels comparable to those of the SCD at steady state. There was an appreciable difference between the median (IQR) folate levels in the SCD patients with acute falciparum malaria on Day 14 compared to Day 0 as shown in Figure 2; however, the difference did not attain statistical significance (p = 0.07). A summary of the median folate levels in the various sub-groups is shown in Figure 1.
Proportion of Individuals with High Folate Levels Among the Different Clinical Sub-Groups
The proportion of individuals with high (> 45.3 nmol/L) folate concentrations in the different clinical sub-groups was 29.5% (13/44), 18.2% (8/44), 33.3% (6/18), and 0%, respectively, in the SCD-malaria positive on Day 0, the SCD steady state, the SCD bacteremia, and the non-SCD (HbAA) groups. The proportion of SCD patients with folate levels > 45.3 nmol/L did not vary significantly at steady state and during confirmed malaria on Day 0 (p = 0.216), and during acute bacteremia (p = 0.20). None of the participants had folate levels below the normal reference range. The proportion of SCD malaria patients, 22.6% (7/31), with high folate levels (as well as median folate levels) 14 days later was comparable to that of the SCD steady state participants.
Hematological Indices in Sub-Groups with Elevated or Normal Folate Levels
A summary of selected hematological and other parameters in those with elevated versus normal folate in the groups are as shown in Table 3. The correlation coefficient (95% CI) between folate and hemoglobin was −0.09 (−0.24 to 0.07) (p = 0.288).
 |
Table 3 Selected Characteristics of Participants with Normal or Elevated Folate |
Discussion
In this study, elevated levels of plasma folate were observed in the SCD patient sub-groups. The observed high levels of folate in this study are consistent with findings from another study in SCD patients15 and are likely a result of “over-supplementation” (dietary intake plus daily intake of folic acid). Although the full implications of elevated folate levels are yet to be determined, high plasma folic acid levels have been shown to be correlated inversely with natural killer cell cytotoxicity,16 thus, further studies of prospective design may be needed to determine the potential contributory role of such elevated folate levels on infection status in SCD patients.
The possibility of a higher requirement for folate due to hemolysis, or an effect of oxidative stress imposed by the infecting pathogen, could account for the overall folate levels in the SCD malaria sub-group, especially as the median folate values in this sub-group on Day 14 (when parasitemia had cleared) was higher and comparable to the steady state sub-group.
Patients with SCD have decreased erythrocyte half-life and higher erythropoiesis rates,17 necessitating higher dietary intake of macro- and micronutrients. Folate metabolism is essential for the proper functioning of all living cells,18 and folate deficiency disproportionately inhibits development and propagation of rapidly proliferating cells.19 A high folate intake, on the other hand, may be associated with deleterious effects, including effects on embryonic development,20 organ injury,21 and, under certain circumstances, the risk of carcinogenesis.22
SCD patients with acute malaria have significantly elevated oxidative stress,23 which may derive from the immune activation by reactive oxygen species against the malarial parasite,24 and oxidative stress reduces intestinal absorption of dietary folic acid.25 It is considered, on the other hand, that, because reticulocytes are richer in folate than are matured erythrocytes, compensatory erythrocytosis and reticulocytosis associated with hemolysis would be expected to result in high intraerythrocytic folate levels,26 which would be contrary to the observations from this study. It has also been suggested that, since folate (and other B-group vitamins) facilitate DNA repair especially in the presence of oxidative stress,27 and folate depletion reduces proliferation of cells from hematopoietic lineages involved in the immunological response to infection,19,28 the overall effect of the observed lower folate levels in SCD patients with acute infection is uncertain, especially as folate supplementation may lead to downregulation of both intestinal and renal folate uptake processes. Other observations suggest that reference values of folate, which is established essentially for HbAA (non-SCD) individuals, may be inadequate to establish folate status of patients with diseases characterized by rapid erythrocyte turnover, including SCD patients.29 The full implications of the observed elevated folate levels may thus be difficult to determine, and further prospective studies of the effect of lower doses of oral folic acid supplementation, and the potential long-term effects of high folate levels in SCD patients receiving daily folic acid supplementation, are required.
In conclusion, the findings of this study showed elevated levels of plasma folate in this pediatric SCD population, suggesting that folate levels in SCD patients might be too high with current practice of folic acid supplementation. The findings also show that response of folate level to malaria or bacteremia might differ between SCD and non-SCD patients. These elevated folate levels may raise safety concerns in the light of scarcity of data on potential dose-related adverse effects related to folic acid intake. The long-term implications of these findings of elevated folate levels in pediatric SCD patients is difficult to determine but indicate a need to conduct studies in other regions, and may suggest a need for review of current recommendations regarding prophylactic doses of folic acid in SCD patients. Information on the correlation between reticulocyte counts and folate levels would have strengthened the data and is a limitation.
Funding
The study was funded by a grant from the Consultative Research Committee for Development Research (FFU), Grant number (09-080RH), and from the Building Stronger Universities project (BSU-3-UG).
Disclosure
The authors report no conflicts of interest in this work.
References
1. de Montalembert M, Guilloud-Bataille M, Feingold J, Girot R. Epidemiological and clinical study of sickle cell disease in France, French Guiana and Algeria. Eur J Hematol. 1993;51(3):136–140. doi:10.1111/j.1600-0609.1993.tb00613.x
2. Stuart MJ, Nagel RL. Sickle cell disease. Lancet. 2004;364(9442):1343–1360. doi:10.1016/S0140-6736(04)17192-4
3. Liu YK. Folic acid deficiency in sickle cell anemia. Scand J Hematol. 1975;14(1):71–79. doi:10.1111/j.1600-0609.1975.tb00295.x
4. Kennedy TS, Fung EB, Kawchak DA, Zemel BS, Ohene-Frempong K, Stallings VA. Red blood cell folate and serum vitamin B12 status in children with sickle cell disease. J Pediatr Hematol Oncol. 2001;23(3):165–169. doi:10.1097/00043426-200103000-00009
5. Dhar M, Bellevue R, Carmel R. Pernicious anemia with neuropsychiatric dysfunction in a patient with sickle cell anemia treated with folate supplementation. N Engl J Med. 2003;348(22):2204–2207. doi:10.1056/NEJMoa022639
6. Troen AM, Mitchell B, Sorensen B, et al. Unmetabolized folic acid in plasma is associated with reduced natural killer cell cytotoxicity among postmenopausal women. J Nutr. 2006;136(1):189–194. doi:10.1093/jn/136.1.189
7. Protiva P, Mason JB, Liu Z, et al. Altered folate availability modifies the molecular environment of the human colorectum: implications for colorectal carcinogenesis. Cancer Prev Res (Phila). 2011;4(4):530–543. doi:10.1158/1940-6207.CAPR-10-0143
8. Dixit R, Nettem S, Madan SS, et al. Folate supplementation in people with sickle cell disease. Cochrane Database Syst Rev. 2018;3:CD011130.
9. Meadows DN, Bahous RH, Best AF, Rozen R, Carvalho LJDM. High dietary folate in mice alters immune response and reduces survival after malarial infection. PLoS One. 2015;10(11):e0143738. doi:10.1371/journal.pone.0143738
10. Das KC, Virdi JS, Herbert V. Survival of the dietarily deprived: folate deficiency protects against malaria in primates. Blood. 1992;80(suppl 1):281a.
11. Hamilton PJ, Gebbie DA, Wilks NE, Lothe F. The role of malaria, folic acid deficiency and hemoglobin AS in pregnancy at Mulago hospital. Trans R Soc Trop Med Hyg. 1972;66(4):594–602. doi:10.1016/0035-9203(72)90305-7
12. Areekul S, Pinyawatana W, Charoenlarp P. Serum folate and folic acid absorption in patients with Plasmodium falciparum malaria. Southeast Asian J Trop Med Public Health. 1974;5:353–358.
13. Fleming AF. Iron deficiency in the tropics. Clin Hematol. 1982;11:365–388.
14. World Health Organization Serum and red blood cell folate concentrations for assessing folate status in populations. World Health Organization; 2015. Available from: https://apps.who.int/iris/handle/10665/162114.
15. Azzam M, Attalla S. Serum folate levels in patients with chronic hemolytic anemia on regular folic acid supplementation before and after dose modification. Indian Pediatr. 2019;56(10):845–848. doi:10.1007/s13312-019-1611-6
16. Rosenberg IH. Science-based micronutrient fortification: which nutrients, how much, and how to know? Am J Clin Nutr. 2005;82(2):279–280. doi:10.1093/ajcn/82.2.279
17. Martina WV, Martijn EG, van der Molen M, Schermer JG, Muskiet FA. Beta-N-terminal glycohemoglobins in subjects with common hemoglobinopathies: relation with fructosamine and mean erythrocyte age. Clin Chem. 1993;39(11):2259–2265. doi:10.1093/clinchem/39.11.2259
18. Fernandez-Villa D, Jimenez Gomez-Lavin M, Abradelo C, San Roman J, Rojo L. Tissue engineering therapies based on folic acid and other vitamin B derivatives. functional mechanisms and current applications in regenerative medicine. Int J Mol Sci. 2018;19:4068. doi:10.3390/ijms19124068
19. Koury MJ, Ponka P. New insights into erythropoiesis: the roles of folate, vitamin B12, and iron. Annu Rev Nutr. 2004;24:105–131. doi:10.1146/annurev.nutr.24.012003.132306
20. Mikael LG, Deng L, Paul L, Selhub J, Rozen R. Moderately high intake of folic acid has a negative impact on mouse embryonic development. Birth Defects Res a Clin Mol Teratol. 2013;97(1):47–52. doi:10.1002/bdra.23092
21. Christensen KE, Mikael LG, Leung KY, et al. High folic acid consumption leads to pseudo-MTHFR deficiency, altered lipid metabolism, and liver injury in mice. Am J Clin Nutr. 2015;101(3):646–658. doi:10.3945/ajcn.114.086603
22. Williams EA. Folate, colorectal cancer and the involvement of DNA methylation. Proc Nutr Soc. 2012;71(4):592–597. doi:10.1017/S0029665112000717
23. Atiku SM, Louise N, Kasozi DM. Severe oxidative stress in sickle cell disease patients with uncomplicated Plasmodium falciparum malaria in Kampala, Uganda. BMC Infect Dis. 2019;19:600.
24. Esan AJ. Evaluation of cortisol, malondialdehyde, blood glucose and lipid status on hemoglobin variants in malaria infected and non-malaria infected individual. Int J Hematol Disord. 2015;10:12691.
25. Couto MR, Gonçalves P, Catarino T, Araujo JR, Correia-Branco A, Martel F. The effect of oxidative stress upon the intestinal uptake of folic acid: in vitro studies with Caco-2 cells. Cell Biol Toxicol. 2012;28(6):369–381. doi:10.1007/s10565-012-9228-8
26. Antony AC. Evidence for potential underestimation of clinical folate deficiency in resource-limited countries using blood tests. Nutr Rev. 2017;75(8):600–615.
27. Basten GP, Duthie SJ, Pirie L, Vaughan N, Hill MH, Powers HJ. Sensitivity of markers of DNA stability and DNA repair activity to folate supplementation in healthy volunteers. Br J Cancer. 2006;94(12):1942–1947. doi:10.1038/sj.bjc.6603197
28. Dhur A, Galan P, Hercberg S. Folate status and the immune system. Prog Food Nutr Sci. 1991;15(1–2):43–60.
29. van der Dijs FP, Fokkema MR, Dijck-Brouwer DA, et al. Optimization of folic acid, vitamin B(12), and vitamin B(6) supplements in pediatric patients with sickle cell disease. Am J Hematol. 2002;69(4):239–246. doi:10.1002/ajh.10083
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.