Back to Journals » Vascular Health and Risk Management » Volume 18
Plasma α-Glutathione S-Transferase in Patients with Chronic Mesenteric Ischemia and Median Arcuate Ligament Syndrome
Authors Kazmi SSH , Safi N , Berge ST, Kazmi M, Sundhagen JO , Julien K, Thorsby PM, Ånonsen KV , Medhus AW , Hisdal J
Received 9 March 2022
Accepted for publication 14 July 2022
Published 21 July 2022 Volume 2022:18 Pages 567—574
DOI https://doi.org/10.2147/VHRM.S365625
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Takashi Kajiya
Syed Sajid Hussain Kazmi,1,2 Nathkai Safi,1,2 Simen Tveten Berge,1,2 Marryam Kazmi,1,3 Jon Otto Sundhagen,1 Kari Julien,4 Per Medbøe Thorsby,2,4 Kim Vidar Ånonsen,5 Asle Wilhelm Medhus,2,5 Jonny Hisdal1,2
1Department of Vascular Surgery, Division of Cardiovascular and Pulmonary Diseases, Oslo University Hospital, Ullevål, Oslo, Norway; 2Institute of Clinical Medicine, Faculty of Medicine, University of Oslo, Oslo, Norway; 3Faculty 2, Poznan University of Medical Sciences, Poznan, Poland; 4The Hormone Laboratory, Department of Medical Biochemistry, Oslo University Hospital, Aker, Oslo, Norway; 5Department of Gastroenterology, Oslo University Hospital, Ullevål, Oslo, Norway
Correspondence: Syed Sajid Hussain Kazmi, Tel +47 92468309, Email [email protected]
Background: Chronic mesenteric ischemia (CMI) due to either atherosclerosis of the mesenteric arteries or median arcuate ligament syndrome (MALS) is an underdiagnosed entity. The etiology of MALS and its existence have been debated and questioned. We aimed to identify plasma biomarkers indicating mesenteric ischemia in patients with CMI and MALS.
Methods: Plasma α-glutathione S-transferase (α-GST), intestinal fatty acid-binding protein (I-FABP), citrulline, and ischemia modified albumin (IMA) were analyzed in fifty-eight patients with CMI (Group A, n=44) and MALS (Group B, n=14) before and after revascularization. The plasma levels of these potential biomarkers were compared with those of healthy individuals (Group C, n=16). Group comparison was performed with the Mann–Whitney U-test. Cross-tabulation and its derivatives were obtained. Receiver operating characteristic (ROC) curves and area under the curve (AUC) were calculated.
Results: Plasma levels of α-GST were significantly raised in the patients with CMI (7.8 ng/mL, p< 0.001) and MALS (8.4 ng/mL, p< 0.001), as compared with the control Group C (3.3 ng/mL). The threshold for normal median plasma α-GST levels of 4 ng/mL yielded a sensitivity of 93% and 86%, specificity of 86% and 88%, respectively, for the diagnosis of CMI due to atherosclerosis and MALS. AUC of ROC curves was 0.96 (p< 0.0001) for CMI and 0.85 (p< 0.002) for MALS. The patient groups did not differ from the healthy controls in any other biomarkers.
Conclusion: Plasma α-GST levels are elevated in CMI and MALS patients. Elevated plasma levels of α-GST suggest ischemia as the etiology of MALS.
Keywords: biomarker, chronic mesenteric ischemia, intestinal ischemia, median arcuate ligament syndrome, α-GST
Introduction
The clinical presentation of chronic mesenteric ischemia (CMI), described as abdominal pain with postprandial worsening, results from intestinal hypoperfusion due to insufficient blood supply during times of increased intestinal metabolic demand.1,2 The resulting food aversion may lead to weight loss in these patients. However, these symptoms are not specific to CMI.3–5 Particularly, in patients with median arcuate ligament syndrome (MALS), which is a condition with stenosis of a single mesenteric artery caused by an external compression from the median arcuate ligament, it has long been debated whether the symptoms are due to intestinal ischemia or merely a neurogenic disorder.6–8
The diagnosis of CMI and MALS is usually consensus-based. It relies on the exclusion of alternative diagnoses and the verification of stenosis or occlusion of one or more mesenteric arteries on computed tomography angiography (CTA) and duplex ultrasound (DUS).9
A vast array of biomarkers for diagnosing mesenteric ischemia have previously been evaluated. These biomarkers are released from enterocytes injured by ischemia, ie, α-glutathione S-transferase (α-GST), intestinal fatty acid-binding protein (I-FABP), and citrulline.10–14 α GST is a light molecular weight iso-enzyme, particularly in high concentrations in hepatocytes. It has a half-life of less than 60 minutes.15–17 IFABP is a cytosolic enterocyte protein released by the dying mature enterocytes. It is a biomarker of high sensitivity and specificity for acute mesenteric ischemia.18 Furthermore, increased plasma levels of IFABP have also been found in patients with chronic mesenteric ischemia.19 Citrulline is a non-protein amino acid synthesized abundantly by the small intestine’s enterocytes. It is thought to be a suitable biomarker of enterocyte function, and its plasma levels are expected to decrease in conditions like mucositis and intestinal ischemia.12,13 In addition, ischemia modified albumin (IMA) is elevated in plasma from patients with acute mesenteric ischemia.20 Currently, clinical and laboratory investigations of these mesenteric ischemia biomarkers are exclusively performed in patients with acute mesenteric ischemia. Still, none of these has shown adequate sensitivity to be used as a screening test for acute mesenteric ischemia, and consequently, they are not suitable for routine clinical practice.21–23 Besides, these biomarkers have pathological plasma levels in the case of liver and renal diseases.12,24,25 On the other hand, studies investigating CMI patients for ischemia biomarkers are lacking.22
We aimed to investigate ischemia biomarkers (plasma α-GST, I-FABP, Citrulline, and IMA) in patients with CMI due to atherosclerotic changes or MALS. We hypothesized that patients with CMI have increased plasma levels of mesenteric ischemia biomarkers compared to healthy individuals.
Methods
Between 2016 and 2020, patients with a consensus diagnosis of CMI were included in this study at the Department of Vascular Surgery, Oslo University Hospital. The included patients had postprandial abdominal pain, changes in food intake patterns, and weight loss. All patients with CMI had an extensive gastrointestinal workup before inclusion in the study to exclude other more common causes of abdominal pain, food aversion, and weight loss. Based on the CTA and DUS findings, the patients were divided into Group A (atherosclerotic CMI) and Group B (MALS). A group of healthy individuals (Group C) served as the control group (Figure 1). The healthy Group C individuals did not use any medicines and had no known systemic disease.
 |
Figure 1 Flow chart of study individuals. |
An experienced physiologist in all patients performed Transabdominal DUS. The patients were investigated in at least 6 hours of fasting state. A peak systolic velocity (PSV) of ≥200 cm/s in the celiac artery or ≥275 cm/s in the superior mesenteric artery was considered clinically significant.26,27 DUS was repeated at follow-up at 3, 6, 12 months, and yearly after that.
The patients were investigated with 1 mm thickness, multidetector CTA (64 row-multidetector, Siemens Medical Systems: Florsheim, Germany). The scans were examined in multiple plains and confirmed ≥50% stenosis of the celiac and/or superior mesenteric artery. In the patients with MALS, a CTA acquired in the deep expiration phase confirmed ≥50% stenosis of the celiac artery lumen caused by an external compression from the median arcuate ligament. The patients with CTA and/or DUS verified ≥50% of the celiac artery and/ or superior mesenteric artery but without symptoms of chronic mesenteric ischemia were excluded. Also, patients with abdominal pain but with ≤50% stenosis of the celiac artery and/or superior mesenteric artery were not included in the study.
Blood Samples
Blood samples were drawn from the median cubital vein, with the patients in at least 6 hours of fasting. Ethylenediaminetetraacetic acid (EDTA) tubes were placed on ice before sampling and centrifuged for 15 minutes at four °C, 3000 rpm, within 30 minutes after sampling. Plasma samples were frozen in Nunc polypropylene vials and stored at −80 °C until analysis. In Group A and B patients, venous blood samples were taken before and three months after surgical or radiological revascularization. Blood samples were drawn only once from the healthy controls in Group C.
The blood samples were analyzed at the Hormone Laboratory at Oslo University Hospital, Aker, Norway. The ELISA kits for I-FABP, citrulline, and IMA had test detection ranges of 0.16–10 ng/mL, 5.0–100 nmol/mL, and 7.8–500 ng/mL, respectively. At the same time, the kit for α-GST had a detection range of 0.156 ng/mL-10 ng/mL. The ELISA kits for citrulline (MBS723693), I-FABP (MBS2507811), and IMA (MBS760561) were provided by MyBioSource, San Diego, CA, USA, and the ELISA kit for α-GST(CSB-E08906h), by Cusabio Technology, Houston, TX, USA. The intra-assay and inter-assay coefficient of variation (CV) for ELISA kits were <10% and <12%, <6.3% and 6%, <8% and <10%, <8% and <10%, respectively, for Citrulline, IFABP, IMA and α-GST. The assay procedures accompanying the ELISA kits were followed. The results of optic density measurements were corrected for the dilution factor to obtain the exact plasma concentration of the biomarker. In addition, plasma levels of p-amylase, aspartate aminotransferase (AST), alanine aminotransferase (ALT), C-reactive protein (CRP), and creatinine were determined. AMY-P, ASTLP, ALTLP, ALP2, CREP2, and CRPL3 kits were utilized, and samples were analyzed on Cobas 6000. The laboratory technician was blinded for the study groups.
Statistical Analysis
Continuous data are presented as median and interquartile ranges and analyzed with the Mann–Whitney U-test. Categorical data are presented as proportions and percentages. A p-value of <0.05 was considered statistically significant. Contingency tables were used to analyze categorical data and calculate sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and overall accuracy (OA). The diagnostic performance of the biomarkers was analyzed with a Receiver operating characteristic (ROC) curve, and the area under the curve (AUC) was calculated to estimate the diagnostic accuracy.28–30 SigmaPlot version 14.0 (Systat Software, San Jose, CA, USA) was used for data analysis.
The study complies with the Declaration of Helsinki. The Regional Committees for Medical and Health Research Ethics in the South-Eastern region of Norway approved the study (REK Sør-Øst B 2016/682-1). It was also registered on www.clinicaltrials.gov (NCT02914912). All study participants provided informed written consent.
Results
The study included fifty-eight patients consecutively and divided into Group A (CMI, n=44) and Group B (MALS, n=14). Thirty-six (62%) were females. Sixteen healthy individuals constituted the controls in Group C. Thirty patients in Group A and all in Group B underwent revascularization. Patients’ demographics are presented in Table 1. Fourteen patients in Group A did not receive revascularization. Nine of these patients had extensive atherosclerotic changes in the affected artery, CA (n=4) or SMA (n=3), or both arteries (n=2). Three patients refused to have any surgical or interventional treatment, and two patients were still waiting for treatment.
 |
Table 1 Patient Demographics |
Plasma Levels of α-GST
Median plasma α-GST levels were significantly raised in both Group A (7.8 ng/mL, p<0.001) and Group B (8.4 ng/mL, p<0.001) compared with Group C (3.3 ng/mL). After revascularization in thirty patients in Group A, a statistically significant reduction in the plasma α-GST levels was found (p=0.023). However, the median plasma levels of α-GST in the patients in Group A were still significantly higher than in the control group (5.5 ng/mL vs 3.3 ng/mL, p=0.001). In the patients with MALS (Group B), postoperative median plasma α-GST levels were no longer statistically different from the control group. Figure 2 illustrates the plasma α-GST levels in all three study groups.
By using >4 ng/mL as an upper threshold for normal plasma α-GST levels, the cross-tabulation analysis showed a sensitivity and specificity of 93% (95% CI 0.78 1.0) and 88% (95% CI 0.69 1.1), respectively, for the diagnosis of CMI. The test had a type I error of 12.5% and a type II error of 6.8%. A PPV of 95% and an NPV of 82% for a 73% prevalence of CMI in the study population were calculated. The overall accuracy of the test was 92%. Chi-Square test showed 2-sided p value <0.001. (Table 2)
 |
Table 2 Analysis of Plasma α-GST >4 ng/mL as the Cut-off for the Diagnosis of Patients with CMI of Atherosclerotic Origin and MALS |
In the patients with MALS, a sensitivity of 86% (95% CI 0.65 1.1) and a specificity of 88% (95% CI 0.69 1.1) were calculated. Overall accuracy was 87%, and the Chi-Square test showed a 2-sided p value <0.001. (Table 2)
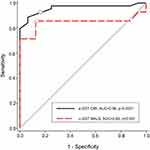
The patients with CMI in Group A had an AUC of 0.96 (p<0.000, 95% CI 0.91 1.0), whereas the patients with MALS in Group B had an AUC of 0.85 (p=0.001, 95% CI 0.66 1.0) (Figure 3).
Plasma Levels of the Other Biomarkers
Plasma citrulline levels were not different in the patient groups from the controls. Neither I-FABP nor human plasma IMA was elevated in the blood samples from either group at any time point compared with the control Group C (Table 3).
 |
Table 3 Median Plasma Levels of Candidate Mesenteric Ischemia Biomarkers in Patients with CMI Due to Atherosclerosis (Group A), and MALS(Group B), Compared with Healthy Individuals in Group C |
None of the study patients had a hepatic or pancreatic disease, and only five patients in Group A (CMI) (3 females and two males) had >90 µmol/mL serum creatinine. Also, the median plasma levels of amylase, AST, ALT, and CRP in the patient groups were within the normal range (Table 4).
 |
Table 4 Baseline Plasma Levels of Pancreas Amylase, AST, ALT, CRP and Creatinine in Patients with CMI (Group a, n=44), MALS (Group B, n=14), and Healthy Individuals (Group C, n=16) |
Discussion
This study demonstrates significantly raised plasma levels of α-GST in patients with CMI of atherosclerotic origin and with MALS. The threshold value of 4 ng/mL α-GST demonstrated very high sensitivity (90% and 86%) and PPV (95% and 86%), respectively, for the CMI patients with atherosclerotic lesion and MALS. The ROC curve for this cut-off value yields an excellent AUC of 0.96 and 0.85 in the patients with CMI and MALS, respectively.
To our knowledge, raised plasma α-GST levels have previously not been demonstrated in patients with CMI. In addition, this is the first time a mesenteric ischemia-specific marker from the mucosal enterocytes has been identified for patients with MALS. The etiology of MALS has long been disputed and debated in the medical literature, and even its existence has been questioned.6,31,32 Identifying raised plasma levels of an ischemia biomarker in patients with MALS before surgery, followed by a decrease in the postoperative plasma levels, strongly supports the ischemic etiology in MALS.7 These current findings also support previously reported findings of impaired microcirculation in the mucosal lining of the stomach and duodenum detected with laser Doppler flowmetry and visible light spectroscopy in patients with CMI and MALS.8,33
All study patients with CMI of atherosclerotic origin had multiple mesenteric artery lesions. However, the patients with MALS had only stenosis, caused by external compression of the celiac artery by the median arcuate ligament. The finding of raised plasma levels of α-GST in the patients with MALS in this study disagrees with the notion that there should be changes in at least two mesenteric arteries to develop chronic mesenteric ischemia.34
This study found the best combination of sensitivity and false-positive rate for the plasma α-GST threshold of 4 ng/mL, which corresponds with earlier findings by Delaney et al in patients with acute mesenteric ischemia.10
Although α-GST has been identified as an intestinal ischemia marker with good sensitivity and specificity in patients with acute mesenteric ischemia, later studies and meta-analyses have recommended IFABP as a biomarker with higher sensitivity and specificity than α-GST.21 Due to the small size of the study populations and thereby some uncertainty about the findings, the guidelines have not included routine use of these biomarkers in patients with acute mesenteric ischemia.34
In a previous study, elevated plasma I-FABP was found in 22 patients with CMI, at baseline and after gastric exercise tonometry. Furthermore, tonometry after a test meal provocation demonstrated significantly increased plasma I-FABP levels in 17 patients with CMI compared to the controls.19 The authors did not suggest any cut-off for abnormal plasma I-FABP levels. In our study population, plasma I-FABP levels were either below or within the detection range and did not differ significantly between the patients and the control group. The blood samples in our study were taken in a fasting state. Our study identifies plasma α-GST as a biomarker that seems easily detectable in patients with CMI and MALS without needing any provocation test before blood sampling.
After revascularization, CMI patients may develop reperfusion injury to the mucosal tissue, and, in severe cases, it may progress to reperfusion syndrome.35,36 The resulting inflammatory changes are self-limiting within several days after revascularization.36 The patients with CMI of atherosclerotic origin and MALS had a statistically significant reduction in plasma α-GST three months after revascularization. Although the second blood samples were drawn three months after the revascularization procedure, the plasma levels were still significantly higher in the patients in Group A than in the control group. In the patients with MALS (single artery stenosis), α-GST levels were no longer different from the controls. Still raised plasma levels of α-GST in Group A patients with atherosclerotic mesenteric artery disease could be due to revascularization performed on the only single artery in most of the patients in this Group A. The persistent stenosis or occlusion of the other mesenteric artery could have played a role in the persistently raised plasma α-GST levels irrespective of the time-point of the blood sampling.
Plasma levels of p-amylase, AST, ALT, CRP, and Creatinine were within the normal reference range, suggesting that the raised levels of plasma α-GST are due to intestinal ischemia in the patients with CMI and MALS I in this study.
This study is a single-center study, and except for laboratory technicians, the rest of the study group was not blinded to the patients and the healthy controls. Another study limitation was that the optimal size for the study and adequate power was not determined at the start of the study. Future studies about the plasma biomarker for CMI and MALS may also include a control group of patients with abdominal pain and weight loss caused by other diseases. The studies should be designed with a study population that also considers the impact of reperfusion symptoms and the impact of single or multiple artery revascularizations on the plasma levels of α-GST in patients with CMI.
Conclusion
Patients with CMI of atherosclerotic origin and MALS have raised levels of plasma α-GST. Elevated plasma α-GST suggests MALS as an ischemic disorder.
Data Sharing Statement
De-identified data can be shared with investigators whose proposed use of the data has been approved by an independent review committee identified for this purpose. The proposal should be directed to the project leader, Syed Sajid Hussain Kazmi, [email protected]. Data requestors will need to sign a data access agreement to gain access.
Disclosure
Dr Asle Wilhelm Medhus reports grants from Takeda, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Terlouw LG, Moelker A, Abrahamsen J, et al. European guidelines on chronic mesenteric ischemia – joint United European Gastroenterology, European association for gastroenterology, endoscopy and nutrition, European society of gastrointestinal and abdominal radiology, Netherlands Association of hepatogastroenterologists, Hellenic society of gastroenterology, cardiovascular and interventional radiological society of Europe, and Dutch mesenteric ischemia study group clinical guidelines on the diagnosis and treatment of patients with chronic mesenteric ischaemia. United Eur gastroenterol j. 2020;8(4):371–395.
2. Haglund U, Bergkvist D. Intestinal ischemia – the basics. Langenbeck’s Arch Surg. 1999;384:233–238.
3. Ter Steege RW, Sloterdijk HS, Geelkerken RH, Huisman AB, van der Palen J, Kolkman JJ. Splanchnic artery stenosis and abdominal complaints: clinical history is of limited value in detection of gastrointestinal ischemia. World J Surg. 2012;36(4):793–799.
4. Mensink PBF, Van Petersen AS, Geelkerken RH, Otte JA, Huisman AB, Kolkman JJ. Clinical significance of splanchnic artery stenosis. BJS. 2006;93(11):1377–1382.
5. Alahdab F, Arwani R, Pasha AK, et al. A systematic review and meta-analysis of endovascular versus open surgical revascularization for chronic mesenteric ischemia. J Vasc Surg. 2018;67(5):1598–1605.
6. Weber JM, Boules M, Fong K, et al. Median arcuate ligament syndrome is not a vascular disease. Ann Vasc Surg. 2016;30:22–27.
7. Mensink PB, van Pettersen AS, Kolkman JJ, et al. Gastric exercise tonometry: the key investigation in patients with suspected celiac artery compression syndrome. J Vasc Surg. 2006;44:277–281.
8. Berge ST, Safi N, Sundhagen JO, Hisdal J, Kazmi SSH. Perioperative microcirculatory changes detected with gastroscopy assisted laser Doppler flowmetry and visible light spectroscopy in patients with median arcuate ligament syndrome. VHRM. 2020;16:331–341.
9. Terlouw LG, Verbeten M, van Noord D, et al. The incidence of chronic mesenteric ischemia in the well-defined region of a Dutch mesenteric ischemia expert center. Clin Transl Gastroenterol. 2020;11(8):e00200.
10. Delaney CP, O’Neill S, Manning F, Fitzpatrick JM, Gorey TF. Plasma concentrations of glutathione S-transferase are raised in patients with intestinal ischemia. BJS. 1999;86:1349–1353.
11. Kanda T, Fujii H, Fujita M, Sakai Y, Ono T, Hatakeyama K. Intestinal fatty acid binding protein is available for diagnosis of intestinal ischemia: immunochemical analysis of two patients with ischemic intestinal diseases. Gut. 1995;36:788–791.
12. Peters JHC, Nicolette JW, Beishuizen A, Teerlink T, Van Bodegraven AA. Intravenous citrulline generation test to assess intestinal function in intensive care unit patients. Clin Exp Gastroenterol. 2017;10:75–81.
13. Kuiken NSS, Rings EHHM, Blijlevens NMA, Tissing WJE. Biomarkers and non-invasive tests for gastrointestinal mucositis. Support Care Cancer. 2017;25:2933–2941.
14. Obulkasim M, Zhang L, Shen J. Serological biomarkers for acute mesenteric ischemia. Ann Transl Med. 2019;7(16):394.
15. Fedrico A, Tuccillo C, Crafa E, Loguercio C. The significance of alpha glutathione S-transferase determination in patients with chronic liver disease. Minerva Gastro-Enterologica e Dietologica. 1999;45(3):181–185.
16. Iwanaga Y, Komatsu H, Yokono S, Ogli K. Serum glutathione S-transferase alpha as a measure of hepatocellular function following prolonged anaesthesia with sevoflurane and halothane in paediatric patients. Pediatric Anesthesia. 2000;10(4):395–398.
17. Czuczejko J, Mila-Lierzenkowska C, Szewczyk-Golec K. Plasma α-Glutathione S-Transferase evaluation in patients with acute and chronic liver injury. Cand J Gastrenter Hep. 2019;1:1–6.
18. Evennett NJ, Petrov MS, Mittal A, Windsor JA. Systemic review and pooled estimates for the diagnostic accuracy of serological markers for intestinal ischemia. World J Surg. 2009;33:1374–1383.
19. Mensink PBF, Hol L, Borghuis-Koertshuis N, et al. Transient postprandial ischemia is associated with increased intestinal fatty acid binding protein in patients with chronic gastrointestinal ischemia. Eur J Gastroenterol Hepatol. 2009;21:278–282.
20. Gunduz A, Turedi S, Mentese A, et al. Ischemia-modified albumin in the diagnosis of acute mesenteric ischemia: a preliminary study. Am J Emerg Med. 2008;26:202–205.
21. Treskes N, Persoon AM, van Zanten ARH. Diagnostic accuracy of novel serological biomarkers to detect acute mesenteric ischemia: a systemic review and meta-analysis. Intern Emerg Med. 2017;12:821–836.
22. Clair DG, Beach JM. Mesenteric ischemia. N Engl J Med. 2016;374:959–968.
23. Montagnana M, Danese E, Lippi G. Biochemical markers of acute intestinal ischemia: possibilities and limitations. Ann Transl Med. 2018;6(17):341.
24. Acosta S, Nilsson T. Current status on plasma biomarkers for acute mesenteric ischemia. J Thromb Thrombolysis. 2012;33:355–361.
25. Heijkant TCVD, Aerts BAC, Teijink JA, Buurman WA, Luyer MDP. Challenges in diagnosing mesenteric ischemia. World J Gastroenterol. 2013;19(9):1338–1341.
26. Moneta GL, Yeager RA, Dalman R, Antonovic R, Hall LD, Porter JM. Duplex ultrasound criteria for diagnosis of splanchnic artery stenosis or occlusion. J Vasc Surg. 1991;14(4):511–520.
27. Safi N, Ånonsen KV, Berge ST, et al. Early identification of chronic mesenteric ischemia with endoscopic duplex ultrasound. Vasc Health Risk Manag. 2022;18:233–243.
28. Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristics (ROC) curve. Radiology. 1982;143:29–36.
29. Metz CE. Basic principles of ROC analysis. Semin Nucl Med. 1978;8(4):283–298.
30. Polo TCF, et al. Use of ROC curves in clinical and experimental studies. J Vasc Bras. 2020;19:1–4.
31. Gloviczki P, Ducan AA. Treatment of celiac artery compression syndrome: does it really exist? Perspect Vasc Surg Endovasc Ther. 2007;19(3):259–263.
32. Kim EN, Lamb K, Relles D, et al. Median arcuate ligament syndrome- review of this rare disease. JAMA. 2016;15(5):471–477.
33. Berge ST, Safi N, Medhus AW, et al. Gastroscopy assisted laser Doppler flowmetry and visible light spectroscopy in patients with chronic mesenteric ischemia. Scand J Clin Lab Invest. 2019;79(7):541–549.
34. Björck M, Koelemay M, Acosta S, et al. Editor’s choice – management of the diseases of mesenteric arteries and veins: clinical practice guidelines of the European Society of Vascular Surgery (ESVS). Eur J Vasc Endovasc Surg. 2017;53(4):460–510.
35. McCord JM. Oxygen-derived free radicals in postischemic tissue injury. N Engl J Med. 1985;312:159–163.
36. Grootjans J, Lenaerts K, Derikx JP, et al. Human intestinal ischemia-reperfusion induced inflammation characterized: experiences from a new translational model. Am J Pathol. 2010;176:2283–2291.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.