Back to Journals » International Journal of Nanomedicine » Volume 18
Plant-Derived Exosome-Like Nanovesicles: Current Progress and Prospects
Authors Mu N , Li J , Zeng L , You J , Li R , Qin A , Liu X, Yan F , Zhou Z
Received 13 June 2023
Accepted for publication 28 August 2023
Published 5 September 2023 Volume 2023:18 Pages 4987—5009
DOI https://doi.org/10.2147/IJN.S420748
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Mian Wang
Nai Mu,1,2,* Jie Li,3,* Li Zeng,4 Juan You,4 Rong Li,4 Anquan Qin,4 Xueping Liu,1 Fang Yan,3,5 Zheng Zhou1,2
1Department of Clinical Medicine, North Sichuan Medical College, Nanchong, Sichuan Province, People’s Republic of China; 2Geriatric Diseases Institute of Chengdu, Department of Orthopedics, Chengdu Fifth People’s Hospital, Chengdu, Sichuan Province, People’s Republic of China; 3Center for Medicine Research and Translation, Chengdu Fifth People’s Hospital, Chengdu, Sichuan Province, People’s Republic of China; 4Department of Pharmacy, Chengdu Fifth People’s Hospital, Chengdu, Sichuan Province, People’s Republic of China; 5Geriatric Diseases Institute of Chengdu, Department of Geriatrics, Chengdu Fifth People’s Hospital, Chengdu, Sichuan Province, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Fang Yan; Zheng Zhou, Tel +86 134 0849 8904 ; +86 182 0810 1005, Email [email protected]; [email protected]
Abstract: Exosomes are small extracellular vesicles, ranging in size from 30– 150nm, which can be derived from various types of cells. In recent years, mammalian-derived exosomes have been extensively studied and found to play a crucial role in regulating intercellular communication, thereby influencing the development and progression of numerous diseases. Traditional Chinese medicine has employed plant-based remedies for thousands of years, and an increasing body of evidence suggests that plant-derived exosome-like nanovesicles (PELNs) share similarities with mammalian-derived exosomes in terms of their structure and function. In this review, we provide an overview of recent advances in the study of PELNs and their potential implications for human health. Specifically, we summarize the roles of PELNs in respiratory, digestive, circulatory, and other diseases. Furthermore, we have extensively investigated the potential shortcomings and challenges in current research regarding the mechanism of action, safety, administration routes, isolation and extraction methods, characterization and identification techniques, as well as drug-loading capabilities. Based on these considerations, we propose recommendations for future research directions. Overall, our review highlights the potential of PELNs as a promising area of research, with broad implications for the treatment of human diseases. We anticipate continued interest in this area and hope that our summary of recent findings will stimulate further exploration into the implications of PELNs for human health.
Keywords: exosomes, nanocarriers, nanotherapeutics, plants, vesicles
Introduction
Exosomes have been a subject of research since 1983, when Johnstone and Pan et al discovered their release from ovine reticulocytes during in vitro culture.1 These tiny extracellular vesicles, ranging in size between 30–150nm,2 are formed by extracellular vesicles via processes such as endocytosis - fusion - exocytosis, and contain proteins, lipids, DNA, and non-coding RNA, among other substances. Subsequent studies have revealed that exosomes can be obtained from cells, tissues, or body fluids of almost all mammalian species. These sources include stem cells,3–5 cancer cells,6 immune cells,7 lung tissue, tumor tissue,8 blood, urine,9,10 and others. They have demonstrated excellent molecular transport properties and biocompatibility, making them promising for intercellular and interspecies communication.11–15 For example, exosomes can directly activate receptors on target cells’ surfaces, alter characteristics of targeted cells, be used as carriers to transport proteins, miRNAs, DNA, and various drugs that increase therapeutic efficacy or detection of certain diseases, like the large number of exosomes secreted by tumor cells, which can be used as a more specific indicator of early tumor screening and recurrence monitoring.
However, exosomes have limitations, including containing both immune activators and immunosuppressive factors that may reduce host resistance to pathogens, and high extraction difficulty and low purity, which significantly increase exosome production cost. Overcoming these limitations by improving the extraction process and expanding available sources is necessary, and this has led to the discovery of plant-derived exosome-like nanovesicles (PELNs). According to current research, there are three possible pathways for the biogenesis of plant extracellular vesicles: the exocyst-positive organelle (EXPO) pathway, the multivesicular bodies (MVBs) pathway, and the vacuolar pathway.16,17 Among these, the MVBs pathway is considered the main pathway for the formation of PELNs.18,19 The basic process involves inward budding of the plasma membrane to form early endosomes, which mature and communicate with the trans-Golgi network, forming MVBs. The intraluminal vesicles (ILVs) within MVBs can incorporate various substances such as RNA, DNA, lipids, etc. Upon fusion of MVBs with the plasma membrane, ILVs can be released into the extracellular space, forming PELNs.20,21 This pathway shares a high resemblance to the known biogenesis pathway of mammalian-derived exosomes (MDEs).22–25 The EXPO pathway involves the formation of double-membraned structures similar to autophagosomes, which can fuse with the plasma membrane to release a single-membraned vesicle towards the cell wall.26 The vacuolar pathway primarily occurs during plant defense against fungal pathogens. Upon infection, vacuoles fuse with the plasma membrane and release their content of hydrolytic enzymes and defense proteins to counteract the invasion of pathogens.27 It has been found that ELNs extracted from plants, such as ginger, ginseng, wheat, rhodiola, licorice, etc., not only have similar anti-fibrosis, anti-virus, anti-tumor effects as MDEs but also compensate for their deficiencies, like partial immunogenicity and limited source. Thus, PELNs hold great research value.
This review collates and summarizes current research on PELNs, analyzing the isolation, purification, identification, and functions of PELNs. The primary emphasis lies in their therapeutic potential for clinical diseases, categorized based on the human anatomical system. The objective is to furnish a comprehensive resource for future utilization of PELNs in the treatment of clinical diseases, while also proposing novel concepts and research methodologies to facilitate further investigation into PELNs.
Overview of PELNs
Extraction of PELNs
In the field of exosome research, there are five major categories of approaches that have been applied for the isolation and purification of these nano-sized vesicles. These methodologies comprise ultracentrifugation methods, separation techniques based on exosome dimensions, precipitation techniques, immunoaffinity capture techniques, in addition to microfluidic techniques. Here we present a brief summary of the principles, advantages, and disadvantages of these techniques, as outlined in Table 1.
 |
Table 1 Principles, Advantages and Disadvantages of Different PELNs Extraction Methods |
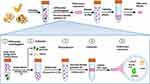
PELNs possess advantages such as high yield and easy accessibility. However, research on PELN extraction and purification currently lags considerably behind that of MDEs. The available methods for isolating and purifying PELNs are relatively limited and primarily based on the established techniques used for MDEs. While MDEs are typically isolated from biological fluids, PELNs are separated from apoplastic wash fluid, and differential centrifugation remains the fundamental extraction method for both.28,29 However, due to physiological differences between plants and animals, PELNs obtained through this method are often mixed with proteins, nucleic acid aggregates, and other vesicles. Therefore, further purification using density gradient ultracentrifugation is necessary to separate contaminants.30 The presence of high-molecular-weight components, such as cellulose and starch, in plant sap often leads to difficulties in centrifugation. To improve separation efficiency and address certain limitations of conventional centrifugation techniques, researchers have proposed various combinations of centrifugation methods. One widely used approach combines the use of differential centrifugation with sucrose density gradient centrifugation.31,32 This method has gained popularity due to its ease of use, affordability, and ability to achieve high extraction purity. Following this method, extracellular vesicles typically reside in the intermediate layer of a 30% - 45% sucrose solution, as illustrated in Figure 1.33
Furthermore, several other separation techniques, such as immunoaffinity capture,34,35 ultrafiltration29,36 or size-exclusion chromatography (SEC),37–40 co-precipitation methods,41–43 and microfluidic technologies,44 have been successfully applied to MDEs but exhibit divergent progress in PELNs research. For instance, immunoaffinity capture based on the formation of immune complexes targeting surface antigens of extracellular vesicles offers advantages such as rapid separation, simplicity, and high specificity, making it an ideal method for purifying extracellular vesicles. However, the limited knowledge regarding labeled proteins and characterized antibodies for PELNs restricts the further development of this technique.45 Woith et al successfully purified and visualized PELNs by applying freshly isolated and dried PELNs onto an agarose gel. This achievement has made it possible to combine high-speed centrifugation with ultrafiltration or SEC to collect purer PELNs.46 Polymer precipitation methods can separate extracellular vesicles from biological fluids by reducing their solubility. Combining ultracentrifugation with the ExoQuick system based on polymer precipitation significantly improves the colloidal stability and separation purity of PELNs, including ginseng-derived vesicles.47 Additionally, several emerging techniques have facilitated the successful separation of PELNs in experiments, providing convenience for future research and applications. Jackson et al employed a capillary channel polymer (C-CP) tip separation strategy based on hydrophobic interaction chromatography (HIC), achieving efficient separation of PELNs with reasonable time scales (<15 minutes) and low cost (<$1), while maintaining purity and integrity.48 Yang et al combined electrophoresis with a 300 kDa cut-off dialysis bag to successfully separate lemon-derived ELNs, which exhibited similar size and quantity to those obtained through standard ultracentrifugation, leading to significant time and cost savings.49 Kırbaş et al successfully isolated various biogenic ELNs, including pomegranate-derived ELNs, using a biphasic system that proved to be economically efficient and gentle, effectively removing non-protein impurities.50 It is noteworthy that the degree of variation between plants is much greater than that between animals, leading to significant differences in particle size, structure, yield, purity, and dispersibility of extracellular vesicles produced by different plant species. To date, there is no standardized procedure for PELNs isolation, which remains one of the obstacles for clinical applications of PELNs.
Identification of PELNs
The recognition of PELNs relies on several key parameters, including their morphology, size, and surface charge. The morphology of PELNs is typically observed using electron microscopy techniques, including scanning electron microscopy (SEM), transmission electron microscopy (TEM), cryo-electron microscopy, and atomic force microscopy (AFM). TEM, due to its superior resolution, provides a clearer view compared to SEM.51 However, both techniques require sample dehydration and fixation, which can cause deformation of PELNs, resulting in a cup-shaped appearance under the microscope.52 Cryo-electron microscopy allows for skipping the steps of fixation and staining, thus obtaining more authentic graphical representation of PELNs, approaching a spherical shape.53 AFM is a high-resolution microscope used for observing and measuring surface morphology and properties of materials. It can generate high-resolution three-dimensional topographic images,54 measure the mechanical properties of the object’s surface, such as hardness, elastic modulus, viscosity, etc.55 Additionally, it can quantitatively assess the biomarker levels of extracellular vesicles by detecting the single-molecule interaction forces between surface proteins of the vesicles and corresponding antibodies.56–58 The most commonly used methods for measuring the size and surface charge of PELNs are dynamic light scattering (DLS) and nanoparticle tracking analysis (NTA). DLS, due to its limitations such as low resolution and inability to measure particle concentration, has gradually been replaced by NTA.59,60 NTA captures the Brownian motion of extracellular vesicles within the size range of 10–2000 nm and calculates their concentration and hydrodynamic diameter using the Einstein equation.61,62 It provides higher resolution than DLS and is less susceptible to interference from intense scattering by larger particles, resulting in more stable results.11 Therefore, it has become the preferred technique for characterizing the size of PELNs. The known size range of PELNs currently falls between 50–500 nm, with surface charges ranging from neutral to −50 mV.63,64 However, when applied to PELNs detection, improper extraction of PELNs can introduce contamination from cell debris, lipid vesicles, colloidal particles, protein aggregates, and other impurities in the sample, inevitably affecting the results of NTA analysis. In addition, in recent years, scholars have discovered that the Localized Surface Plasmon Resonance (LSPR) technique exhibits high sensitivity when used for exosome detection.65 Furthermore, the application of extracellular vesicle characterization techniques based on AFM and LSPR in liquid biopsies is being widely investigated. These techniques have shown excellent prospects in the diagnosis of various tumors and cancers, such as malignant glioblastoma.56,66 We look forward to witnessing similarly remarkable achievements in PELNs research.
Characteristics of PELNs
The compositional analysis of PELNs is considered crucial for quality control, as the components of PELNs can vary depending on the plant source.46,67 Common techniques employed for the characterization of chemical components, such as protein analysis using bicinchoninic acid, Western blotting, fluorometric assays, sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE), and enzyme-linked immunosorbent assay (ELISA), are also used for PELNs.68 Additionally, nucleic acid analysis techniques, including next-generation sequencing, digital droplet polymerase chain reaction (PCR), and microarray analysis, have been used for PELNs.69 Lipidomic analysis of PELNs is also performed using sulfophosphovanilin assays and total reflection Fourier-transform infrared spectroscopy.70,71
The morphological and structural features of PELNs are depicted in Figure 2. Similar to that of MDEs, the membrane of PELNs is composed of a phospholipid bilayer. However, PELNs exhibit distinct lipid characteristics, such as a high concentration of phosphatidic acid, phosphatidylcholines, digalactosyldiacylglycerol, and monogalactosyldiacylglycerol, which are different from those of MDEs.72 These unique lipid compositions confer inherent cell-regulating properties to PELNs. Protein concentrations in PELNs are typically low, and most of these proteins are cytosolic proteins, including actin, proteases, and membrane proteins that function as channels and transporters within the membrane.73 In addition, the presence of surface marker proteins on exosomes plays a crucial role in their identification, characterization, and specific antigen-antibody reactions. For instance, CD81, CD9, CD63, TSG101, and flotillin are widely recognized surface marker proteins of MDEs.25,74,75 However, the identification of surface marker proteins for PELNs remains elusive. Although studies have discovered several highly abundant proteins such as patellin-3, heat shock proteins (HSP), aquaporins (AQP), clathrin heavy chain (Chc), and glyceraldehyde-3-phosphate dehydrogenase(GAPDH) in various plants including grapefruit,76–78 their homologues have been reported in MDEs. Notably, recent research has suggested that the synthetic protein PEN1, ABC transporter protein PEN3, and Tetraspanin-8 could be candidate surface marker proteins for PELNs.77,79 PEN1 has been identified in Arabidopsis and its enrichment in PELNs has been confirmed through Western blot analysis.80 PEN3 has been detected in Arabidopsis but has not yet been confirmed by Western blot. TET3 is a homologue of CD63 and is highly enriched in PELNs. However, due to limited experimental data, this perspective has not been widely accepted and further research is awaited for validation. Nucleic acids in PELNs include DNA and various RNAs, among which microRNA (miRNA) is a small RNA molecule with 22 nucleotides in length that lacks coding properties. miRNAs generally regulate messenger RNA (mRNA) translation or cleave mRNAs to modulate gene expression,81 or induce specific target gene expressions.82
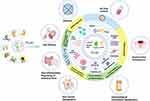
It is the existence of a variety of proteins, lipids, DNAs and a variety of complex RNAs that endow PELNs with important properties that can participate in information transmission and material exchange between cells.31,72 And there have been numerous studies that have applied these properties to the treatment of human diseases (Figure 3), which we will discuss in detail below.
Effects of PELNs in Human Diseases
The Role of PELNs in Digestive System Diseases
Over the past few years, there has been a growing awareness of the potential significance of PELNs in digestive system diseases. Importantly, studies have shown that PELNs are able to remain stable in digestive environments as they resist digestion by various enzymes such as pepsin and intestinal pancreatin,31,83–85 and further play a therapeutic role.
For instance, ginger-derived ELNs (GELNs) have been found to alter the composition of the microbiome and positively impact host physiology.31 In fact, oral administration of GELNs resulted in a reduction of acute colitis, an increase in intestinal repair, and prevention of chronic colitis.63 Another recent study revealed that ELNs derived from tea leaves were effective in both preventing and treating inflammatory bowel disease (IBD) in mice. The therapeutic effect was ascribed to downregulation of pro-inflammatory cytokines, reduction of oxidative stress, and maintenance of gut microbiota homeostasis.84 Similarly, grape-derived ELNs were shown to protect against DSS-induced colitis in mice via the Wnt/β-catenin signaling pathway.64 Nanoparticles derived from broccoli were also found to activate dendritic Cell AMP-Activated Protein Kinase and protect mice against colitis.86 Furthermore, a study by Zhuang et al found that GELNs activated nuclear factor erythroid 2-related factor 2 (Nrf2) through the regulation of the TLR4/TRIF pathway, protecting against liver injuries induced by alcohol.85
PELNs also have therapeutic effects on tumors of the digestive system. For example, ginger-derived ELNs have shown promising outcomes in the treatment of colitis-associated cancer (CAC) by reducing inflammatory cytokines and cyclin D1 mRNA levels, as well as suppressing intestinal epithelial cell proliferation in mice.63 ELNs derived from tea leaves also possess similar functions.87 Asparagus cochinchinensis derived ELNs have also been found to suppress hepatocellular carcinoma cell proliferation both in vitro and in vivo, without causing toxicity.32 Additionally, citrus lemon derived ELNs also exhibit anti-cancer effects by increasing the expression of pro-apoptotic genes and decreasing the expression of anti-apoptotic genes, stimulating cancer cell death by activating tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-mediated apoptosis, and reducing pro-angiogenic factors such as VEGF-A, IL-6, and IL-8, resulting in inhibited tumor growth.88 Proteomic analysis has further demonstrated that citrus lemon-derived ELNs exert their antitumor effects primarily through the induction of Acetyl-CoA carboxylase alpha (ACACA) downregulation.89
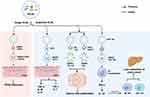
Extracellular vesicles have recently emerged as a promising drug delivery vehicle due to their high stability and safety, as well as their ability to cross biological barriers.90,91 PELNs are particularly advantageous as they are natural products with inherent safety, non-toxicity, and low immunogenicity (Figure 4). 83,92–94 For inflammatory bowel disease, ginger-derived nano-lipids loaded with siRNA were effective in delivering siRNA drugs to treat ulcerative colitis patients. siRNA-CD98 encapsulated in ginger-derived nano-lipid vectors for oral administration can effectively target colon tissue and inhibit CD98 expression, thereby reducing the side effects of conventional synthetic nanoparticles for colon diseases while improving specificity.92 Similarly, grapefruit-derived ELNs have been found to deliver the anti-inflammatory drug methotrexate (MTX) to the site of inflammation. This approach inhibited the production of pro-inflammatory factors TNF-α, IL-1β, and IL-6, while increasing the expression of anti-inflammatory factors such as heme oxygenase-1 and IL-10, resulting in improved therapeutic effects on DSS-induced colitis in mice.83 For tumors of the digestive system, Grapefruit-derived lipids into nanocarriers (GNVs) have been used for the treatment of lung, colon, and breast tumors.95 Combining GNVs with folic acid (GNV-FA) has significantly enhanced their targeting of tumors, while encapsulating the antitumor drug paclitaxel (PTX) on GNV-FA has allowed for more specific treatment of colon cancer. GNVs carrying miR-18a have also been found to inhibit liver metastasis of colon cancer by inducing IL-12 expression and activating NK cells and NKT cells.93
In addition to their protective and therapeutic effects on the gastrointestinal tract, GELNs have been found to bind specifically to proteins on Porphyromonas gingivalis, reducing the bacteria’s ability to invade the body. This application has potential value in the treatment of chronic periodontitis.96 Furthermore, ELNs extracted from lemon have been shown to improve the tolerance of Lactobacillus rhamnosus GG and Streptococcus thermophilus ST-21, effectively inhibiting the infection of Clostridioides difficile.97 Garlic-derived ELNs were able to bind to ligands on HepG2 cells and be internalized, resulting in the inhibition of inflammatory responses.98 Shiitake mushroom-derived ELNs have been found to inhibit NLRP3 activation and suppress IL-6 production while also inhibiting the activity of the IL1b gene by reducing its protein and mRNA content, thereby preventing GalN/LPS-induced acute liver damage.99
Taken together, the above studies demonstrate the potential of PELNs in treating not only digestive diseases, but also tumors. Although most research is still at the laboratory stage and further clinical trials are needed, these findings have significant potential for future development and clinical translational value.
The Role of PELNs in Respiratory System Diseases
Coronavirus disease-19 (COVID-19) is a global pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2),100 posing an unprecedented public health challenge. ELNs isolated from dietary plants, such as soybean, ginger, hamimelon, grapefruit, tomato, and pear, have been found to contain microRNAs (miRNAs) that can target human transcripts.101 These miRNAs have the potential to target multiple regions within SARS-CoV-2, making them a promising therapeutic option for the treatment of COVID-19.102 For instance, Zhou et al demonstrated that exosomes derived from honeysuckle contain miR-2911, which can bind to 28 binding sites in the genome of SARS-CoV-2 and significantly inhibit the replication of the virus.103 Similarly, Teng et al found that ginger-derived miRNA, miR396a-5p, effectively inhibited lung inflammation induced by Nsp12 and Nsp13, which are exosomes released from lung epithelial cells previously infected by SARS-CoV-2.104 The findings emphasize the potential of utilizing PELNs for COVID-19 treatment, particularly in light of the urgent need for effective therapies during the rapid spread of the disease, warranting further investigation.
Furthermore, PELNs also hold promise for the treatment of pulmonary fibrosis, a disease characterized by cell proliferation, extracellular matrix aggregation, and inflammatory damage that leads to progressive decline in lung function.105 Recent studies have shown that ELNs, also known as decoctosomes, derived from decoctions of Rhodiola crenulate and Taraxacum mongolicum exhibited anti-fibrosis and anti-inflammatory effects in both in vitro and in vivo disease models, leading to significant improvements in pulmonary fibrosis and lung inflammation in mice.106,107 However, the underlying mechanisms of these effects remain unclear and further research is warranted to elucidate the roles of PELNs in these diseases.
In conclusion, PELNs have exhibited substantial potential in treating respiratory system disorders, including COVID-19 and pulmonary fibrosis. These findings underscore the importance of further exploring the therapeutic applications of PELNs, particularly in the context of the ongoing global health challenges posed by respiratory diseases.
The Role of PELNs in Neurological System Diseases
The existence of blood-brain barrier (BBB) has always been an obstacle in the treatment of neurological diseases. Due to the existence of BBB, it is difficult for conventional chemotherapy drugs to achieve ideal intracranial blood concentration.108 Therefore, it is of great significance to find drugs that can enter the BBB and achieve effective intracranial blood concentration to improve the therapeutic effect of neurological diseases such as brain tumors.
PELNs have been shown to cross various physiological barriers, including the blood-brain barrier, through receptor-mediated cellular transport and membrane fusion.109 In one study, ELNs extracted from grapefruit were loaded with the anti-tumor drug doxorubicin onto heparin-based nanoparticles for glioma treatment. The results showed that these grapefruit-derived ELNs could effectively bypass the blood-brain barrier, accurately transport doxorubicin to the site of the tumor, and result in an improved anti-glioma effect.94 Grapefruit ELNs were also found to carry miR-17 and inhibit brain tumor progression through an intranasal route.110 In addition, exosomes extracted from ginseng root were reported to stimulate the neural differentiation of bone marrow mesenchymal stem cells through the PI3K signaling pathway. This finding suggests that PELNs may have great potential in regenerative medicine for the nervous system.111
Overall, PELNs represent a promising new avenue for the treatment of nervous system diseases such as glioma. To fully understand the underlying mechanisms and evaluate the clinical potential of these results, additional researches are required.
The Role of PELNs in Circulatory System Diseases
Circulatory system diseases, such as angina, hypertension, myocardial ischemia, and heart failure, are among the primary causes of morbidity and mortality worldwide.112 Medicinal plants offer numerous pharmacological and biological benefits, including cardioprotective, anti-atherogenic, anti-hypertensive, anti-inflammatory, and antioxidant properties, and their natural safety makes them a highly attractive therapeutic option.113
Research have demonstrated that PELNs exhibit cardioprotective effects. In one study, Liu et al found that ginseng root-derived exosomes can mitigate doxorubicin-induced H9C2 cardiomyocyte injury by safeguarding the mitochondrial apoptotic pathways.114 The antioxidant effects of PELNs may also be harnessed to protect the vascular system. For instance, blueberry-derived ELNs (B-ELNs) were found to prevent damage from various stressors to the vascular system by regulating the expression of genes induced by TNF-α and reducing reactive oxygen species (ROS) production and loss of cellular vitality. Furthermore, B-ELNs can act as innovative candidate therapeutic vectors of bioactive compounds.115 ELNs isolated and purified from Fragaria x Ananassa (cv. Romina) strawberry juice and lemon were also found to promote cardioprotective effects by demonstrating a significant antioxidant effect after being taken up by adipose mesenchymal stem cells (ADMSCs) in vitro. Strawberry-derived ELNs, which contain high amounts of vitamin C, are speculated to have protective effects against oxidative stress.116 Meanwhile, ELNs extracted from lemons carried sufficient amounts of citrate and vitamin C that also exhibited significant antioxidant effects after uptake by MSC in vitro.117
While these studies provide valuable insights into the potential of PELNs to treat circulatory diseases, clinical studies are still lacking. Further research is required before these findings can be applied in the clinic effectively.
The Role of PELNs in Endocrine System Diseases
The modern diet, characterized by high levels of fat and sugar, has been associated with escalated susceptibilities to diabetes, cardiovascular disorders, and liver damage. Several studies have investigated the potential of plant extracts as a way of mitigating the effects of these diets. For example, previous research has found that an ethanol extract of ginger can significantly reduce lipid accumulation in cells and inhibit the expression of genes related to adipogenesis, thus exerting an anti-obesity effect.118
Recent studies by Kumar et al have shown that GELNs affect the expression of aryl hydrocarbon receptor (AhR), a ligand-activated transcription factor that regulates various transcriptional processes in the body,119 including glucose metabolism.120 Overexpression of AhR has previously been shown to lead to insulin resistance, while mice without AhR showed improved insulin sensitivity and glucose tolerance.121–123 The group found that GELNs induced miR-375 expression, which inhibited AhR expression. This led to a marked improvement in glucose tolerance and insulin resistance among mice.124 Similarly, hvu-MIR168-3p extracted from rice aleurone cells facilitates the upregulation of glucose transporter 1 (SLC2A1) expression in human cells, leading to decreased blood glucose levels. The mechanism for this effect may be related to the silencing of mitochondrial electron transport chain complex I genes by hvu-MIR168-3p.125 In addition, hvu-MIR168-3p can down-regulate low density lipoprotein receptor ligand protein 1 (LDLRAP1) and increase blood LDL levels in animals.126 Subsequent studies have also demonstrated that common variants of MIR168a exhibit different silencing effects on LDLRAP1.127,128 Studies investigating the therapeutic potential of nanovesicles extracted from orange juice (ONVs) have reported positive results. ONVs reversed intestinal changes in obese mice, increasing the size of intestinal villi while reducing triglyceride content. ONVs regulate the expression levels of several mRNAs, some of which are involved in immune response, fat absorption, and celiac release. Therefore, ONVs may be effective in preventing or inhibiting obesity-related gastrointestinal inflammation caused by high-fat and high-sugar diets.129 Other studies have explored the use of plant extracts encapsulated in nanoparticles for therapeutic purposes. For example, nanoparticle preparations from pterostilbene have been found to reduce blood glucose levels via an injection in a diabetic rat model.130 Likewise, curcumin nanoparticles prepared with a modified emulsion-diffusion-evaporation method successfully reduced fasting blood glucose and glycated hemoglobin levels in a diabetic rat model.131 Finally, curcumin nanoparticles and aged garlic extract suspension have shown promise in treating diabetic cardiomyopathy, reducing inflammation, myocardial fibrosis, and the risk of cardiovascular events.132 We anticipate more applications of PELNs in the treatment of endocrine system diseases in the future.
The Role of PELNs in Genitourinary System Diseases
As mentioned earlier, PELNs contain miRNAs that can regulate growth, metabolism, and development of cells across species. Recent studies have investigated the potential of miR159 from PELNs to treat breast cancer. Chin et al detected miR159 in human blood as well as in breast tumors and found that the content of miR159 in PELNs is negatively correlated with the progression of breast cancer. The team synthesized a miRNA mimic and administered it orally to a xenograft breast cancer mouse model. The findings revealed that sustained oral administration for 16 days resulted in the inhibition of tumor cell proliferation, and the test group mice exhibited a significant reduction in tumor weight. The mechanism of action for this miRNA mimic is thought to be that miR159 targets TCF7 and indirectly regulates the transcription of MYC gene, leading to a decline in MYC protein and the inhibition of tumor cell growth and metabolism. Therefore, miRNAs in PELNs may have therapeutic effects on other tumor cells.133 In another study, researchers isolated ELNs from Moringa seeds (MELNs) and examined their potential to treat human acute lymphoblastic leukemia cells and cervical adenocarcinoma cells. The results showed that MELNs could inhibit the expression of B-cell lymphoma-2 (BCL-2) and reduce the intracellular mitochondrial membrane potential, thereby inhibiting the proliferation of both types of tumor cells.134 This study presents a promising new approach to the treatment of cervical cancer and leukemia.
Overall, these studies highlight the potential of PELNs and their miRNA content as a therapeutic strategy for cancer treatment. Subsequent investigations are warranted to optimize the delivery modalities of these molecules, in order to achieve optimal therapeutic efficacy.
The Role of PELNs in Musculoskeletal System Diseases
There is currently no research that has specifically investigated the effect of PELNs on skeletal-related diseases. However, the therapeutic benefits of plant exosomes in wound healing have been demonstrated in multiple studies.135 For example, exosomes extracted from wheat were found to promote wound healing in vitro by enhancing cell viability and migration efficiency, as well as promoting the formation of new blood vessels.136 Similarly, ginger- and lemon-derived ELNs have been shown to mediate apoptosis, which is a key process in wound healing that involves the removal of inflammatory cells and the evolution of granulation tissue to scar tissue. As PELNs and MDEs have very similar morphological structures and components, there is reason to believe that PELNs may also have therapeutic effects on skeletal-related diseases such as fracture healing, osteoarthritis recovery, and intervertebral disc degeneration, similarly to MDEs. However, further research is needed to validate these inferences in relevant clinical trials.
Overall, PELNs offer an exciting avenue for exploring new therapeutic approaches for a variety of medical conditions. They are less risky and do not pose ethical concerns associated with stem cell transplantation, and can be delivered directly to the affected area or intravenously to achieve systemic effects. With continued research, we may discover even more extensive applications for these promising bioactive substances in the future.
PELNs-Based Therapy in Clinical Trials
As for clinical trials of PELN therapies, one Phase I clinical trial (NCT01294072) which investigated the ability of PELNs to deliver curcumin to normal and malignant colon tissue was registered, and it has already started to recruit patients. Another clinical trial (NCT03493984) that aimed to investigate the potential of exosomes derived from aloe as well as ginger in alleviating insulin resistance and chronic inflammation in individuals diagnosed with polycystic ovary syndrome was withdrawn due to the absence of enrolled patients. The PELNs related clinical trials are summarized in Table 2.
 |
Table 2 PELNs-Based Therapy in Clinical Trials |
Discussion and Outlook
Since its discovery in sheep reticulocytes in the 1880s, exosomes have been widely investigated for their potential applications in the diagnosis and treatment of various diseases. Although MDEs currently dominate the field of disease diagnosis, such as the detection of glioblastoma cell-derived EVs from blood and cerebrospinal fluid for glioblastoma diagnostics and prognostication,138 and the identification of miR-126 isolated from serum as a potential indicator of non-small cell lung cancer progression.139 Research on PELNs primarily focuses on their therapeutic potential in disease treatment. This includes their ability to exhibit anti-fibrotic, antiviral, anti-tumor effects, as well as their role in regulating the gut microbiota. Table 3 summarizes existing studies on the effects of PELNs in human diseases. However, given the exceptional qualities of PELNs, we believe that with further research, they could also be directly or indirectly applied to early disease diagnosis.
 |
Table 3 Effects of PELNs on Human Systemic Diseases |
Furthermore, in recent years, there has been significant research interest in exploring the potential of PELNs as drug delivery tools. Apart from their ability to transport various drug molecules for the treatment of clinical diseases, such as previously mentioned MTX for colitis, PTX for colon cancer, and DOX for glioblastoma, studies have also indicated that PELNs can serve as carriers for treating plant diseases caused by fungal infections. For instance, nanoparticles extracted and processed from leaves of P. niruri, termed Green-Synthesized Silver Nanoparticles (GS-AgNP-LEPN), when heated after being mixed with AgNO3 solution, have shown efficacy in treating citrus canker caused by Xanthomonas axonopodis.140 These findings highlight the excellent research value and application potential of PELNs as drug carriers across multiple disciplines. However, the ability of PELNs to load and deliver drugs may be influenced by various factors, primarily encompassing the following aspects:
- Properties of PELNs: Different PELNs exhibit distinct characteristics, such as variations in size, charge, and stability. These properties inevitably have a significant impact on the loading of drugs into PELNs. Generally, smaller vesicles have a larger drug loading capacity due to their higher surface area-to-volume ratio. They also exhibit faster release rates and higher stability. However, both excessively large and small vesicle sizes can affect the efficiency of drug loading. Therefore, it is necessary to develop methods for producing uniformly sized extracellular vesicles.141 Fortunately, researchers have successfully employed the Bligh and Dyer technique (a liquid-liquid extraction method) to extract homogeneous-sized nano lipids from PELNs, which serve as carriers for more efficient drug delivery.142 PELNs typically carry a negative charge due to the presence of phosphates. Under the influence of electrostatic attraction, molecules with positive charges are absorbed and encapsulated by these negatively charged or neutral PELNs more efficiently.92 This opens up a new research direction for drug loading using PELNs, wherein the drug loading efficiency of PELNs can be enhanced by altering the charges carried by both the vesicles and the target molecules.
- Methods for drug loading: Currently, commonly used drug loading methods can be categorized into passive loading and active loading. Passive loading involves co-incubating PELNs with drug molecules at specific temperatures, relying on diffusion and lipophilic interactions between the drug molecules and the vesicles for binding.143 This method is relatively simple but may have a lower encapsulation efficiency.144 Active loading methods include ultrasound-assisted loading,145 electroporation,146 extrusion,147 and freeze-thaw cycles.148 It has been reported that active loading methods can increase the loading capacity of PELNs by more than 11-fold.149
- Exosome modification techniques: The ability of exosomes to deliver drugs can be enhanced by fixing specific bioactive molecules on their surface. For example, surface engineering techniques can be used to modify exosomes derived from ginger with an arrowhead pRNA-3WJ and FA, which enhances their ability to deliver genes to tumor sites.150 Similarly, hybridizing polyethyleneimine with grapefruit-derived exosomes can also increase their gene delivery capacity to brain tumors.110
- Administration routes: The administration routes of exosomes include oral administration, intravenous injection, intranasal delivery, local injection, etc.151 Currently, the most used route for PELNs administration is oral administration, which has the advantages of convenience, ease of acceptance by patients, and is particularly suitable for the treatment of gastrointestinal diseases.152 However, this route has drawbacks such as slow absorption and reduced drug loading after undergoing first-pass metabolism in the liver. Extraintestinal administration routes such as tracheal delivery and intravenous injection can avoid the above issues but come with risks of respiratory irritation or spasms and serious adverse drug reactions.153 Therefore, each administration route has its unique advantages and applicability, and the appropriate administration route should be chosen based on different diseases and drugs. In addition to these factors, the drug loading efficiency of PELNs is also influenced by factors such as the type of exosomes, drug properties, and interactions between PELNs and drugs. However, there is currently limited research in this area, and further studies are needed.
Although PELNs possess numerous advantageous biological and physical properties, attracting increasing attention from researchers, the current studies still have certain limitations and controversial issues. In light of this, we have summarized these challenges and controversies, and put forward our suggestions for future research directions, aiming to provide guidance for further investigation into PELNs.
Advantages of PELNs
- Excellent biocompatibility, tissue penetration, and physicochemical stability enable PELNs to cross various physiological barriers and exchange information between cells across species, acting as regulatory factors to exert anti-inflammatory and anti-tumor effects directly at the lesion site.
- Compared to synthetic nanoparticles, natural PELNs possess more significant advantages in biocompatibility, stability, in vivo distribution, prolongation of half-life, and cellular internalization. As they can load different types of substances upon demand, including small molecules, nucleic acids, and recombinant proteins, they are expected to become a very cost-effective drug delivery platform.
- PELNs can be produced on a large scale from an abundance of plant resources.154
Limitations of the Current Studies
- While current studies have demonstrated the numerous functions of PELNs, there is still much to be learned about their mechanisms. For example, it is unclear how plant exosomes interact with recipient cells and how they specifically modify those cells. Additionally, further investigations are required to elucidate the endocytic pathways involved in the uptake of exosomes derived from plants.
- Plant exosomes contain a variety of biomolecules that may cause unknown side effects. Therefore, it is crucial to conduct extensive clinical trials to investigate pharmacokinetics, pharmacodynamics, safety, stability, and efficacy prior to implementation.
- Most animal experiments involving plant exosomes have focused on oral administration, with only a few choosing intravenous injection. No experiments using local injection or coating administration have been found. Oral administration has advantages in simplicity, convenience, and relative safety, but first-pass elimination through the liver after oral administration significantly reduces the concentration and dose of the drug in the body circulation. Furthermore, slow absorption rates seriously affect treatment efficiency. Alternatively, intravenous administration provides faster and longer-lasting effects, but it comes with greater risks of adverse drug reactions. Thus, investigating different routes of administration and comparing their effectiveness can help find an optimal route of administration for exosomes from plants.
- To date, there is no unified plan for the separation and extraction of exosomes. Different methods can obtain varying content and purity of exosomes, leading to heterogeneity and affecting experimental results. Ultracentrifugation and sucrose density gradient centrifugation are commonly used due to their simplicity and ability to obtain a large number of exosomes. However, both methods require repeated centrifugation, which is time-consuming and may damage exosomes.33,155 Developing a complete exosome extraction method that shortens extraction time while ensuring quantity and purity and does not affect exosome activity is desired.
- Proteomic analysis can greatly improve the understanding of plant exosomes, but research on exosome-tagged proteins of plants is limited.156,157 This limitation hinders the characterization of PELNs.
- The utilization of PELNs as carriers for drug delivery carriers exhibits promising development prospects, but current research faces three main challenges: improving the load of active components of exosomes, improving their specificity for tissues to accurately deliver drugs to the desired sites, and improving the efficiency of exosome drug release. Studies using genetic or chemical methods to change the composition of exosomes derived from animal cells have led to engineered exosomes that exhibit improved biological functions. We expect similar approaches will yield positive results for exosomes from plants.
- Seasonal and geographical factors must be considered when studying plant exosomes because some plants only grow in specific seasons or regions. This factor complicates the study of plant exosomes, and researchers may face additional burdens if they consider artificial cultivation of some plants with long growth cycles. Tissue culture technology is a viable solution to these issues, but it comes with relatively high economic costs.
- A review of published literature on plant exosomes shows that most research focuses on the digestive system, tumor system, respiratory system, and circulatory system diseases. Research on hematological system, endocrine system, nervous system, and motor system diseases is lacking, indicating significant gaps in research on plant exosomes.
Recommendations for Future Research
- Further investigation into the mechanisms underlying the therapeutic effects of PELNs is necessary to gain a deeper understanding of PELN and promote its development.
- It is essential to conduct studies on the pharmacokinetics, pharmacodynamics, safety, and stability of plant extracellular vesicles as potential therapeutics to fully understand their clinical applications.
- Exploring different administration routes for PELN, such as local injection and topical application, can help identify the optimal delivery method for different diseases.
- A consistent focus on identifying the optimal method for extracting plant extracellular vesicles with maximum yield and purity while minimizing extraction time without compromising vesicle activity is critical.
- More research on specific protein markers for PELNs is needed to enhance the characterization of these important biological structures.
- Exploring strategies to enhance the cargo capacity of PELNs to enhance their tissue specificity for accurate drug delivery to desired sites, and to improve drug release efficiency is also crucial.
- Further research on the therapeutic effects of PELNs for diseases related to the blood system, endocrine system, nervous system, musculoskeletal system, and other systems will provide us more potential clinical applications.
Conclusion
After decades of development and research, PELNs have emerged as a recognized and highly promising natural bioactive ingredient. They possess excellent biological and physical properties, including low immunogenicity, high stability, and penetration through biological barriers. PELNs can directly act as therapeutics, exerting therapeutic effects in various animal disease models. They can also serve as carriers for accurate delivery and release of anti-inflammatory and anti-tumor drugs to target locations. Despite some limitations and unresolved issues in current research, such as the lack of a simple yet reliable method for extracting PELNs in high purity and quantity, the absence of studies on surface marker proteins of PELNs, and the unclear mechanisms underlying their therapeutic effects, the potential benefits of PELNs should not be ignored. Their numerous advantages make them a highly promising research field for current and future biomedical applications, deserving further investigation by scholars in relevant fields.
Acknowledgments
We would like to express our gratitude to Ms Qing Qing for her invaluable assistance with the English language editing of the manuscript.
Funding
This study was supported by the Major technology application demonstration project of Chengdu Science and Technology Bureau (2022-YF09-00014-SN), Chengdu High-Level Key Clinical Specialty Construction Project, and the Scientific Research Fund of Chengdu Fifth People’s Hospital.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Johnstone RM, Adam M, Hammond JR, Orr L, Turbide C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes).; Research Support, Non-U.S. Gov’t. J Biol Chem. 1987;262(19):9412–9420. doi:10.1016/S0021-9258(18)48095-7
2. Farooqi AA, Desai NN, Qureshi MZ, et al. Exosome biogenesis, bioactivities and functions as new delivery systems of natural compounds. Biotechnol Adv. 2018;36(1):328–334. doi:10.1016/j.biotechadv.2017.12.010
3. Zhang L, Jiao GJ, Ren SW, et al. Exosomes from bone marrow mesenchymal stem cells enhance fracture healing through the promotion of osteogenesis and angiogenesis in a rat model of nonunion. Stem Cell Res Ther. 2020;11(1):
4. Furuta T, Miyaki S, Ishitobi H, et al. Mesenchymal Stem Cell-Derived Exosomes Promote Fracture Healing in a Mouse Model. Stem Cells Transl Med. 2016:1620. doi:10.5966/sctm.2015-0285
5. Wang N, Li XJ, Zhong ZY, et al. 3D hESC exosomes enriched with miR-6766-3p ameliorates liver fibrosis by attenuating activated stellate cells through targeting the TGF beta RII-SMADS pathway. J Nanobiotechnol. 2021;19(1):
6. Zhang H, Yang M, Wu X, Li Q, Li M. The Distinct Roles of Exosomes in Tumor-Stroma Crosstalk within Gastric Tumor Microenvironment. Pharmacol Res. 2021;2(Pt 19):105785. doi:10.1016/j.phrs.2021.105785
7. Xu ZJ, Zeng SS, Gong ZC, Yan YL. Exosome-based immunotherapy: a promising approach for cancer treatment. Mol Cancer. 2020;19(1):
8. Huang CY, Liu ZM, Chen MZ, et al. Tumor-derived biomimetic nanozyme with immune evasion ability for synergistically enhanced low dose radiotherapy. J Nanobiotechnol. 2021;19(1):
9. Barreiro K, Lay AC, Leparc G, et al. An in vitro approach to understand contribution of kidney cells to human urinary extracellular vesicles. J Extracell Vesicles. 2023;12(2):
10. Xu ZJ, Xu YZ, Zhang K, et al. Plant-derived extracellular vesicles (PDEVs) in nanomedicine for human disease and therapeutic modalities. J Nanobiotechnol. 2023;21(1):
11. Dad HA, Gu TW, Zhu AQ, Huang LQ, Peng LH. Plant Exosome-like Nanovesicles: emerging Therapeutics and Drug Delivery Nanoplatforms. Mol Ther. 2021;29(1):13–31. doi:10.1016/j.ymthe.2020.11.030
12. van der Pol E, Boing AN, Harrison P, Sturk A, Nieuwland R. Classification, Functions, and Clinical Relevance of Extracellular Vesicles. Pharmacol Rev. 2012;64(3):676–705. doi:10.1124/pr.112.005983
13. Vader P, Breakefield XO, Wood MJA. Extracellular vesicles: emerging targets for cancer therapy. Trends Mol Med. 2014;20(7):385–393. doi:10.1016/j.molmed.2014.03.002
14. Emanueli C, Shearn AIU, Angelini GD, Sahoo S. Exosomes and exosomal miRNAs in cardiovascular protection and repair. Vascul Pharmacol. 2015;71:24–30. doi:10.1016/j.vph.2015.02.008
15. An T, Qin S, Xu Y, et al. Exosomes serve as tumour markers for personalized diagnostics owing to their important role in cancer metastasis. J Extracell Vesicles. 2015;4:27522. doi:10.3402/jev.v4.27522
16. Cong M, Tan S, Li S, et al. Technology insight: plant-derived vesicles-How far from the clinical biotherapeutics and therapeutic drug carriers? Adv Drug Deliv Rev. 2022;182:114108. doi:10.1016/j.addr.2021.114108
17. Kim J, Li SY, Zhang SY, Wang JX. Plant-derived exosome-like nanoparticles and their therapeutic activities. Asian J Pharm Sci. 2022;17(1):53–69. doi:10.1016/j.ajps.2021.05.006
18. An Q, van Bel AJE, Hueckelhoven R. Do Plant Cells Secrete Exosomes Derived from Multivesicular Bodies? Article. Plant Signal Behav. 2007;2(1):4–7. doi:10.4161/psb.2.1.3596
19. Movahed N, Cabanillas DG, Wan J, Vali H, Laliberte J-F, Zheng H. Turnip Mosaic Virus Components Are Released into the Extracellular Space by Vesicles in Infected Leaves. Plant Physiol. 2019;180(3):1375–1388. doi:10.1104/pp.19.00381
20. Karamanidou T, Tsouknidas A. Plant-Derived Extracellular Vesicles as Therapeutic Nanocarriers. Int J Mol Sci. 2022;23(1):
21. Li XF, Bao H, Wang Z, et al. Biogenesis and Function of Multivesicular Bodies in Plant Immunity. Front Plant Sci. 2018;9:
22. Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020;367(6478):640–+. eaau6977. doi:10.1126/science.aau6977
23. Willms E, Cabanas C, Mager I, Wood MJA, Vader P. Extracellular Vesicle Heterogeneity: subpopulations, Isolation Techniques, and Diverse Functions in Cancer Progression. Front Immunol. 2018;9:
24. Hessvik NP, Llorente A. Current knowledge on exosome biogenesis and release. Cell Mol Life Sci. 2018;75(2):193–208. doi:10.1007/s00018-017-2595-9
25. Thakur A, Ke XS, Chen YW, et al. The mini player with diverse functions: extracellular vesicles in cell biology, disease, and therapeutics. Protein Cell. 2022;13(9):631–654. doi:10.1007/s13238-021-00863-6
26. Wang JA, Ding Y, Wang JQ, et al. EXPO, an Exocyst-Positive Organelle Distinct from Multivesicular Endosomes and Autophagosomes, Mediates Cytosol to Cell Wall Exocytosis in Arabidopsis and Tobacco Cells. Plant Cell. 2010;22(12):4009–4030. doi:10.1105/tpc.110.080697
27. He BY, Hamby R, Jin HL. Plant extracellular vesicles: trojan horses of cross-kingdom warfare. FASEB Bioadv. 2021;3(9):657–664. doi:10.1096/fba.2021-00040
28. Zarovni N, Corrado A, Guazzi P, et al. Integrated isolation and quantitative analysis of exosome shuttled proteins and nucleic acids using immunocapture approaches. Methods. 2015;87:46–58. doi:10.1016/j.ymeth.2015.05.028
29. Konoshenko MY, Lekchnov EA, Vlassov AV, Laktionov PP. Isolation of Extracellular Vesicles: general Methodologies and Latest Trends. Biomed Res Int. 2018;2018:
30. Chen BY, Sung CWH, Chen CC, et al. Advances in exosomes technology. Clinica Chimica Acta. 2019;493:14–19. doi:10.1016/j.cca.2019.02.021
31. Mu JY, Zhuang XY, Wang QL, et al. Interspecies communication between plant and mouse gut host cells through edible plant derived exosome-like nanoparticles. Mol Nutr Food Res. 2014;58(7):1561–1573. doi:10.1002/mnfr.201300729
32. Zhang L, He FJ, Gao LN, et al. Engineering Exosome-Like Nanovesicles Derived from Asparagus cochinchinensis Can Inhibit the Proliferation of Hepatocellular Carcinoma Cells with Better Safety Profile. Int J Nanomedicine. 2021;16:1575–1586. doi:10.2147/ijn.S293067
33. Thery C, Amigorena S, Raposo G, Clayton A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protocols Cell Biol. 2006. doi:10.1002/0471143030.cb0322s30
34. Li P, Kaslan M, Lee SH, Yao J, Gao ZQ. Progress in Exosome Isolation Techniques. Theranostics. 2017;7(3):789–804. doi:10.7150/thno.18133
35. Guo SC, Tao SC, Dawn H. Microfluidics-based on-a-chip systems for isolating and analysing extracellular vesicles. J Extracell Vesicles. 2018;7(1):
36. Heinemann ML, Vykoukal J. Sequential Filtration: a Gentle Method for the Isolation of Functional Extracellular Vesicles. Methods Mol Biol. 2017;1660:33–41. doi:10.1007/978-1-4939-7253-1_4
37. Ruysschaert T, Marque A, Duteyrat JL, Lesieur S, Winterhalter M, Fournier D. Liposome retention in size exclusion chromatography. BMC Biotechnol. 2005;5:11. doi:10.1186/1472-6750-5-11
38. Rosana N, Fernando C, Alberto P. Serum Exosome Isolation by Size-Exclusion Chromatography for the Discovery and Validation of Preeclampsia-Associated Biomarkers. Methods Mol Biol. 2019. doi:10.1007/978-1-4939-9164-8_3
39. Sahil S, Taylor DD. Methods of isolating extracellular vesicles impact down-stream analyses of their cargoes. Methods. 2015;87:3–10.
40. Karimi N, Cvjetkovic A, Jang SC, et al. Detailed analysis of the plasma extracellular vesicle proteome after separation from lipoproteins. Cell Mol Life Sci. 2018;75(15):2873–2886. doi:10.1007/s00018-018-2773-4
41. Martins TS, Catita J, Rosa IM, Silva O, Henriques AG. Exosome isolation from distinct biofluids using precipitation and column-based approaches. PLoS One. 2018;13(6):
42. Patel GK, Khan MA, Zubair H, et al. Comparative analysis of exosome isolation methods using culture supernatant for optimum yield, purity and downstream applications. Sci Rep Mar. 2019;9:
43. Gyorgy B, Modos K, Pallinger E, et al. Detection and isolation of cell-derived microparticles are compromised by protein complexes resulting from shared biophysical parameters. Blood. 2011;117(4):E39–E48. doi:10.1182/blood-2010-09-307595
44. Gholizadeh S, Draz MS, Zarghooni M, et al. Microfluidic approaches for isolation, detection, and characterization of extracellular vesicles: current status and future directions. Biosens Bioelectron. 2017;91:588–605. doi:10.1016/j.bios.2016.12.062
45. Zeringer E, Barta T, Li M, Vlassov AV. Strategies for isolation of exosomes. Cold Spring Harb Protoc. 2015;2015(4):319–323. doi:10.1101/pdb.top074476
46. Woith E, Melzig MF. Extracellular Vesicles from Fresh and Dried PlantsSimultaneous Purification and Visualization Using Gel Electrophoresis. Int J Mol Sci. 2019;20(2):
47. Jang J, Jeong H, Jang E, et al. Isolation of high-purity and high-stability exosomes from ginseng. Front Plant Sci. 2023;13:
48. Jackson KK, Mata C, Marcus RK. A rapid capillary-channeled polymer (C-CP) fiber spin-down tip approach for the isolation of plant-derived extracellular vesicles (PDEVs) from 20 common fruit and vegetable sources. Talanta. 2023;252:
49. Yang M, Liu XY, Luo QQ, Xu LL, Chen FX. An efficient method to isolate lemon derived extracellular vesicles for gastric cancer therapy. J Nanobiotechnol. 2020;18(1):
50. Kirbas OK, Bozkurt BT, Asutay AB, et al. Optimized Isolation of Extracellular Vesicles From Various Organic Sources Using Aqueous Two-Phase System. Sci Rep. 2019;9:
51. Han JM, Song HY, Lim ST, Kim KI, Seo HS, Byun EB. Immunostimulatory Potential of Extracellular Vesicles Isolated from an Edible Plant, Petasites japonicus, via the Induction of Murine Dendritic Cell Maturation. Int J Mol Sci. 2021;22(19):
52. Jung MK, Mun JY. Sample Preparation and Imaging of Exosomes by Transmission Electron Microscopy. J Vis Exp. 2018;(431):
53. Tatischeff I, Larquet E, Falcon-Perez JM, Turpin P-Y, Kruglik SG. Fast characterisation of cell-derived extracellular vesicles by nanoparticles tracking analysis, cryo-electron microscopy, and Raman tweezers microspectroscopy. J Extracell Vesicles. 2012;1. doi:10.3402/jev.v1i0.19179
54. Hardij J, Cecchet F, Berquand A, et al. Characterisation of tissue factor-bearing extracellular vesicles with AFM: comparison of air-tapping-mode AFM and liquid Peak Force AFM. J Extracell Vesicles. 2013;2. doi:10.3402/jev.v2i0.21045
55. Ye SY, Li WZ, Wang HY, Zhu L, Wang C, Yang YL. Quantitative Nanomechanical Analysis of Small Extracellular Vesicles for Tumor Malignancy Indication. Adv Sci. 2021;8(18):
56. Thakur A, Xu C, Li WK, et al. In vivo liquid biopsy for glioblastoma malignancy by the AFM and LSPR based sensing of exosomal CD44 and CD133 in a mouse model. Biosens Bioelectron. 2021;191:
57. Williams C, Rodriguez-Barrueco R, Silva JM, et al. Double-stranded DNA in exosomes: a novel biomarker in cancer detection. Cell Res. 2014;24(6):766–769. doi:10.1038/cr.2014.44
58. Sharma S, LeClaire M, Gimzewski JK. Ascent of atomic force microscopy as a nanoanalytical tool for exosomes and other extracellular vesicles. Nanotechnology. 2018;29(13):
59. Szatanek R, Baj-Krzyworzeka M, Zimoch J, Lekka M, Siedlar M, Baran J. The Methods of Choice for Extracellular Vesicles (EVs) Characterization. Int J Mol Sci. 2017;18(6):18. doi:10.3390/ijms18061153
60. Chan MY, Dowling QM, Sivananthan SJ, Kramer RM. Particle Sizing of Nanoparticle Adjuvant Formulations by Dynamic Light Scattering (DLS) and Nanoparticle Tracking Analysis (NTA). In: Fox CB, editor. Vaccine Adjuvants: Methods and Protocols. Totowa, Nj 07512-1165 USA: Humana Press Inc, 999 Riverview Dr, Ste 208; 2017:239–252.
61. Vestad B, Llorente A, Neurauter A, et al. Size and concentration analyses of extracellular vesicles by nanoparticle tracking analysis: a variation study. J Extracell Vesicles. 2017;6(1):1–11. doi:10.1080/20013078.2017.1344087
62. Kim A, Ng WB, Bernt W, Cho NJ. Validation of Size Estimation of Nanoparticle Tracking Analysis on Polydisperse Macromolecule Assembly. Sci Rep Feb. 2019;9:
63. Zhang MZ, Viennois E, Prasad M, et al. Edible ginger-derived nanoparticles: a novel therapeutic approach for the prevention and treatment of inflammatory bowel disease and colitis-associated cancer. Biomaterials. 2016;101:321–340. doi:10.1016/j.biomaterials.2016.06.018
64. Ju SW, Mu JY, Dokland T, et al. Grape Exosome-like Nanoparticles Induce Intestinal Stem Cells and Protect Mice From DSS-Induced Colitis. Mol Ther. 2013;21(7):1345–1357. doi:10.1038/mt.2013.64
65. Xiong HW, Huang ZP, Lin QY, et al. Surface Plasmon Coupling Electrochemiluminescence Immunosensor Based on Polymer Dots and AuNPs for Ultrasensitive Detection of Pancreatic Cancer Exosomes. Anal Chem. 2022;94(2):837–846. doi:10.1021/acs.analchem.1c03535
66. Bellassai N, D’Agata R, Jungbluth V, Spoto G. Surface Plasmon Resonance for Biomarker Detection: advances in Non-invasive Cancer Diagnosis. Front Chem. 2019;7:
67. Stanly C, Fiume I, Capasso G, Pocsfalvi G. Isolation of Exosome-Like Vesicles from Plants by Ultracentrifugation on Sucrose/Deuterium Oxide (D2O) Density Cushions. Methods Mol Biol. 2016;1459:259–269. doi:10.1007/978-1-4939-3804-9_18
68. Thery C, Witwer KW, Aikawa E, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7(1):
69. Ramirez MI, Amorim MG, Gadelha C, et al. Technical challenges of working with extracellular vesicles. Nanoscale. 2018;10(3):881–906. doi:10.1039/c7nr08360b
70. Osteikoetxea X, Balogh A, Szabo-Taylor K, et al. Improved Characterization of EV Preparations Based on Protein to Lipid Ratio and Lipid Properties. PLoS One. 2015;10(3):
71. Mihaly J, Deak R, Szigyarto IC, Bota A, Beke-Somfai T, Varga Z. Characterization of extracellular vesicles by IR spectroscopy: fast and simple classification based on amide and C-H stretching vibrations. Biochimica Et Biophysica Acta-Biomembranes. 2017;1859(3):459–466. doi:10.1016/j.bbamem.2016.12.005
72. Teng Y, Ren Y, Sayed M, et al. Plant-Derived Exosomal MicroRNAs Shape the Gut Microbiota. Cell Host Microbe. 2018;24(5):637–+. e8. doi:10.1016/j.chom.2018.10.001
73. Zhang M, Viennois E, Xu C, Merlin D. Plant derived edible nanoparticles as a new therapeutic approach against diseases. Research Support, U S Gov’t, Non-P H S Tissue Barriers. 2016;4(2):e1134415. doi:10.1080/21688370.2015.1134415
74. Thakur A, Parra DC, Motallebnejad P, Brocchi M, Chen HJ. Exosomes: small vesicles with big roles in cancer, vaccine development, and therapeutics. Bioact Mater. 2022;10:281–294. doi:10.1016/j.bioactmat.2021.08.029
75. Kowal J, Arras G, Colombo M, et al. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc Natl Acad Sci U S A. 2016;113(8):E968–E977. doi:10.1073/pnas.1521230113
76. Pocsfalvi G, Turiak L, Ambrosone A, et al. Protein biocargo of citrus fruit-derived vesicles reveals heterogeneous transport and extracellular vesicle populations. J Plant Physiol. 2018;229:111–121. doi:10.1016/j.jplph.2018.07.006
77. Rutter BD, Innes RW. Extracellular Vesicles Isolated from the Leaf Apoplast Carry Stress-Response Proteins. Plant Physiol. 2017;173(1):728–741. doi:10.1104/pp.16.01253
78. Garaeva L, Kamyshinsky R, Kil Y, et al. Delivery of functional exogenous proteins by plant-derived vesicles to human cells in vitro. Sci Rep. 2021;11(1):
79. Pinedo M, de la Canal L, Lousa CD. A call for Rigor and standardization in plant extracellular vesicle research. J Extracell Vesicles. 2021;10(6):
80. Liu Y, Wu S, Koo YJ, et al. Characterization of and isolation methods for plant leaf nanovesicles and small extracellular vesicles. Nanomed-Nanotechnol Biol Med. 2020;29:
81. Bartel D. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–297. doi:10.1016/s0092-8674(04)00045-5
82. Vasudevan S, Tong YC, Steitz JA. Switching from repression to activation: microRNAs can up-regulate translation. Science. 2007;318(5858):1931–1934. doi:10.1126/science.1149460
83. Wang BM, Zhuang XY, Deng ZB, et al. Targeted Drug Delivery to Intestinal Macrophages by Bioactive Nanovesicles Released from Grapefruit. Mol Ther. 2014;22(3):522–534. doi:10.1038/mt.2013.190
84. Zu MH, Xie DC, Canup BSB, et al. ‘Green’ nanotherapeutics from tea leaves for orally targeted prevention and alleviation of colon diseases. Biomaterials. 2021;279:
85. Zhuang X, Deng Z-B, Mu J, et al. Ginger-derived nanoparticles protect against alcohol-induced liver damage. J Extracell Vesicles. 2015;4:28713. doi:10.3402/jev.v4.28713
86. Deng ZB, Rong Y, Teng Y, et al. Broccoli-Derived Nanoparticle Inhibits Mouse Colitis by Activating Dendritic Cell AMP-Activated Protein Kinase. Proce Paper Mol Ther. 2017;25(7):1641–1654. doi:10.1016/j.ymthe.2017.01.025
87. Fan FY, Sang LX, Jiang M. Catechins and Their Therapeutic Benefits to Inflammatory Bowel Disease. Molecules. 2017;22(3):
88. Raimondo S, Naselli F, Fontana S, et al. Citrus limon-derived nanovesicles inhibit cancer cell proliferation and suppress CML xenograft growth by inducing TRAIL-mediated cell death. Oncotarget. 2015;6(23):19514–19527. doi:10.18632/oncotarget.4004
89. Raimondo S, Saieva L, Cristaldi M, Monteleone F, Fontana S, Alessandro R. Label-free quantitative proteomic profiling of colon cancer cells identifies acetyl-CoA carboxylase alpha as antitumor target of Citrus limon-derived nanovesicles. J Proteomics. 2018;173:1–11. doi:10.1016/j.jprot.2017.11.017
90. Alvarez-Erviti L, Seow YQ, Yin HF, Betts C, Lakhal S, Wood MJA. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol. 2011;29(4):341–U179. doi:10.1038/nbt.1807
91. Guo MF, Wu F, Hu GR, et al. Autologous tumor cell-derived microparticle-based targeted chemotherapy in lung cancer patients with malignant pleural effusion. Sci Transl Med. 2019;11(474):
92. Zhang MZ, Wang XY, Han MK, Collins JF, Merlin D. Oral administration of ginger-derived nanolipids loaded with siRNA as a novel approach for efficient siRNA drug delivery to treat ulcerative colitis. Nanomedicine. 2017;12(16):1927–1943. doi:10.2217/nnm-2017-0196
93. Teng Y, Mu JY, Hu X, et al. Grapefruit-derived nanovectors deliver miR-18a for treatment of liver metastasis of colon cancer by induction of M1 macrophages. Oncotarget. 2016;7(18):25683–25697. doi:10.18632/oncotarget.8361
94. Niu WB, Xiao Q, Wang XJ, et al. A Biomimetic Drug Delivery System by Integrating Grapefruit Extracellular Vesicles and Doxorubicin-Loaded Heparin-Based Nanoparticles for Glioma Therapy. Nano Lett. 2021;21(3):1484–1492. doi:10.1021/acs.nanolett.0c04753
95. Wang QL, Zhuang XY, Mu JY, et al. Delivery of therapeutic agents by nanoparticles made of grapefruit-derived lipids. Nat Commun. 2013;4(11):1867. doi:10.1038/ncomms2886
96. Sundaram K, Miller DP, Kumar A, et al. Plant-Derived Exosomal Nanoparticles Inhibit Pathogenicity of Porphyromonas gingivalis. Iscience. 2019;21:308. doi:10.1016/j.isci.2019.10.032
97. Lei C, Mu JY, Teng Y, et al. Lemon Exosome-like Nanoparticles-Manipulated Probiotics Protect Mice from C. diff Infection. Iscience. 2020;23(10):
98. Song HL, Canup BSB, Ngo VL, Denning TL, Garg P, Laroui H. Internalization of Garlic-Derived Nanovesicles on Liver Cells is Triggered by Interaction With CD98. Acs Omega. 2020;5(36):23118–23128. doi:10.1021/acsomega.0c02893
99. Liu BL, Lu YZ, Chen XY, et al. Protective Role of Shiitake Mushroom-Derived Exosome-Like Nanoparticles in D-Galactosamine and Lipopolysaccharide-Induced Acute Liver Injury inMice. Nutrients. 2020;12(2):
100. Zhu N, Zhang DY, Wang WL, et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Eng J Med. 2020;382(8):727–733. doi:10.1056/NEJMoa2001017
101. Xiao J, Feng SY, Wang X, et al. Identification of exosome-like nanoparticle-derived microRNAs from 11 edible fruits and vegetables. Peerj. 2018;6:
102. Kalarikkal SP, Sundaram GM. Edible plant-derived exosomal microRNAs: exploiting a cross-kingdom regulatory mechanism for targeting SARS-CoV-2. Toxicol Appl Pharmacol. 2021;414:
103. Zhou L-K, Zhou Z, Jiang X-M, et al. Absorbed plant MIR2911 in honeysuckle decoction inhibits SARS-CoV-2 replication and accelerates the negative conversion of infected patients. Cell Discovery. 2020;6(1):
104. Teng Y, Xu FY, Zhang XC, et al. Plant-derived exosomal microRNAs inhibit lung inflammation induced by exosomes SARS-CoV-2 Nsp12. Mol Ther. 2021;29(8):2424–2440. doi:10.1016/j.ymthe.2021.05.005
105. Raghu G, Rochwerg B, Zhang Y, et al. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline: treatment of Idiopathic Pulmonary Fibrosis An Update of the 2011 Clinical Practice Guideline. Am J Respir Crit Care Med. 2015;192(2):E3–E19. doi:10.1164/rccm.201506-1063ST
106. Li XY, Liang Z, Du JC, et al. Herbal decoctosome is a novel form of medicine. Sci China-Life Sci. 2019;62(3):333–348. doi:10.1007/s11427-018-9508-0
107. Du JC, Liang Z, Xu JT, et al. Plant-derived phosphocholine facilitates cellular uptake of anti-pulmonary fibrotic HJT-sRNA-m7. Sci China-Life Sci. 2019;62(3):309–320. doi:10.1007/s11427-017-9026-7
108. Tang W, Fan WP, Lau J, Deng LM, Shen ZY, Chen XY. Emerging blood-brain-barrier-crossing nanotechnology for brain cancer theranostics. Chem Soc Rev. 2019;48(11):2967–3014. doi:10.1039/c8cs00805a
109. Sun TM, Zhang YS, Pang B, Hyun DC, Yang MX, Xia YN. Engineered Nanoparticles for Drug Delivery in Cancer Therapy. Angewandte Chem Int Edition. 2014;53(46):12320–12364. doi:10.1002/anie.201403036
110. Zhuang XY, Teng Y, Samykutty A, et al. Grapefruit-derived Nanovectors Delivering Therapeutic miR17 Through an Intranasal Route Inhibit Brain Tumor Progression. Mol Ther. 2016;24(1):96–105. doi:10.1038/mt.2015.188
111. Xu XH, Yuan TJ, Dad HA, et al. Plant Exosomes As Novel Nanoplatforms for MicroRNA Transfer Stimulate Neural Differentiation of Stem Cells In Vitro and In Vivo. Nano Lett. 2021;21(19):8151–8159. doi:10.1021/acs.nanolett.1c02530
112. Zhang XY, Lucas AM, Veturi Y, et al. Large-scale genomic analyses reveal insights into pleiotropy across circulatory system diseases and nervous system disorders. Nat Commun. 2022;13(1):
113. Hao DC, Xiao PG. Impact of Drug Metabolism/Pharmacokinetics and their Relevance Upon Traditional Medicine-based Cardiovascular Drug Research. Curr Drug Metab. 2019;20(7):556–574. doi:10.2174/1389200220666190618101526
114. Liu T, Qiu Z, Qiu Y, Chen Y, Hu S, Liu D. A preliminary study on protective mechanism of ginseng root exosomes against doxorubicin induced myocardial injury. Chine Traditional Herbal Drugs. 2021;52(12):3514–3521.
115. De Robertis M, Sarra A, D’Oria V, et al. Blueberry-Derived Exosome-Like Nanoparticles Counter the Response to TNF-alpha-Induced Change on Gene Expression in EA.hy926 Cells. Biomolecules. 2020;10(5). doi:10.3390/biom10050742
116. Perut F, Roncuzzi L, Avnet S, et al. Strawberry-Derived Exosome-Like Nanoparticles Prevent Oxidative Stress in Human Mesenchymal Stromal Cells. Biomolecules. 2021;11(1):
117. Baldini N, Torreggiani E, Roncuzzi L, Perut F, Zini N, Avnet S. Exosome-like Nanovesicles Isolated from Citrus limon L. Exert Anti-oxidative Effect. Curr Pharm Biotechnol. 2018;19(11):877–885. doi:10.2174/1389201019666181017115755
118. Kim B, Kim HJ, Cha YS. The protective effects of steamed ginger on adipogenesis in 3T3-L1 cells and adiposity in diet-induced obese mice. Nutr Res Pract. 2021;15(3):279–293. doi:10.4162/nrp.2021.15.3.279
119. Rothhammer V, Quintana FJ. The aryl hydrocarbon receptor: an environmental sensor integrating immune responses in health and disease. Nat Rev Immunol. 2019;19(3):184–197. doi:10.1038/s41577-019-0125-8
120. Wang C, Xu CX, Krager SL, Bottum KM, Liao DF, Tischkau SA. Aryl Hydrocarbon Receptor Deficiency Enhances Insulin Sensitivity and Reduces PPAR-alpha Pathway Activity in Mice. Environ Health Perspect. 2011;119(12):1739–1744. doi:10.1289/ehp.1103593
121. Jaeger C, Khazaal AQ, Xu CX, Sun MW, Krager SL, Tischkau SA. Aryl Hydrocarbon Receptor Deficiency Alters Circadian and Metabolic Rhythmicity. J Biol Rhythms. 2017;32(2):109–120. doi:10.1177/0748730417696786
122. Jaeger C, Xu CX, Sun MW, Krager S, Tischkau SA. Aryl hydrocarbon receptor-deficient mice are protected from high fat diet-induced changes in metabolic rhythms. Chronobiol Int. 2017;34(3):318–336. doi:10.1080/07420528.2016.1256298
123. Xu CX, Wang C, Zhang ZM, et al. Aryl hydrocarbon receptor deficiency protects mice from diet-induced adiposity and metabolic disorders through increased energy expenditure. Int J Obes. 2015;39(8):1300–1309. doi:10.1038/ijo.2015.63
124. Kumar A, Ren Y, Sundaram K, et al. miR-375 prevents high-fat diet-induced insulin resistance and obesity by targeting the aryl hydrocarbon receptor and bacterial tryptophanase (tnaA) gene. Theranostics. 2021;11(9):4061–4077. doi:10.7150/thno.52558
125. Akao Y, Kuranaga Y, Heishima K, et al. Plant hvu-MIR168-3p enhances expression of glucose transporter 1 (SLC2A1) in human cells by silencing genes related to mitochondrial electron transport chain complex I. J Nutritional Biochem. 2022;101:
126. Zhang L, Hou DX, Chen X, et al. Exogenous plant MIR168a specifically targets mammalian LDLRAP1: evidence of cross-kingdom regulation by microRNA. Cell Res. 2012;22(1):107–126. doi:10.1038/cr.2011.158
127. Lang C, Karunairetnam S, Lo KR, et al. Common Variants of the Plant microRNA-168a Exhibit Differing Silencing Efficacy for Human Low-Density Lipoprotein Receptor Adaptor Protein 1 (LDLRAP1). Research Support non-U S Gov’t MicroRNA. 2019;8(2):166–170. doi:10.2174/2211536608666181203103233
128. Luo Y, Wang PJ, Wang X, et al. Detection of dietetically absorbed maize-derived microRNAs in pigs. Sci Rep. 2017;7:
129. Berger E, Colosetti P, Jalabert A, et al. Use of Nanovesicles from Orange Juice to Reverse Diet-Induced Gut Modifications in Diet-Induced Obese Mice. Mol Therapy Methods Clin Dev. 2020;18:880–892. doi:10.1016/j.omtm.2020.08.009
130. Zhao X, Shi AH, Ma Q, et al. Nanoparticles prepared from pterostilbene reduce blood glucose and improve diabetes complications. J Nanobiotechnol. 2021;19(1):
131. Gouda W, Hafiz NA, Mageed L, et al. Effects of nano-curcumin on gene expression of insulin and insulin receptor. Bulletin National Res Centre. 2019;43(1):56.
132. Abdel-Mageid AD, Abou-Salem MES, Salaam N, El-Garhy HAS. The potential effect of garlic extract and curcumin nanoparticles against complication accompanied with experimentally induced diabetes in rats. Phytomedicine. 2018;43:126–134. doi:10.1016/j.phymed.2018.04.039
133. Chin AR, Fong MY, Somlo G, et al. Cross-kingdom inhibition of breast cancer growth by plant miR159. Cell Res. 2016;26(2):217–228. doi:10.1038/cr.2016.13
134. Potesta M, Roglia V, Fanelli M, et al. Effect of microvesicles from Moringa oleifera containing miRNA on proliferation and apoptosis in tumor cell lines. Cell Death Discovery. 2020;6(1):
135. Hajialyani M, Tewari D, Sobarzo-Sanchez E, Nabavi SM, Farzaei MH, Abdollahi M. Natural product-based nanomedicines for wound healing purposes: therapeutic targets and drug delivery systems. Int J Nanomedicine. 2018;13:5023–5043. doi:10.2147/ijn.S174072
136. Sahin F, Kocak P, Gunes MY, Ozkan I, Yildirim E, Kala EY. In Vitro Wound Healing Activity of Wheat-Derived Nanovesicles. Appl Biochem Biotechnol. 2019;188(2):381–394. doi:10.1007/s12010-018-2913-1
137. clinicaltrials.gov [homepage on the Internet]. Bethesda, MD: National Library of Medicine. Available from: https://clinicaltrials.gov/.
138. Whitehead CA, Luwor RB, Morokoff AP, Kaye AH, Stylli SS. Cancer exosomes in cerebrospinal fluid. Transl Cancer Res. 2017;6:S1352–S1370. doi:10.21037/tcr.2017.08.31
139. Grimolizzi F, Monaco F, Leoni F, et al. Exosomal miR-126 as a circulating biomarker in non-small-cell lung cancer regulating cancer progression. Sci Rep. 2017;7:
140. Gaurav I, Thakur A, Kumar G, et al. Delivery of Apoplastic Extracellular Vesicles Encapsulating Green-Synthesized Silver Nanoparticles to Treat Citrus Canker. Nanomaterials. 2023;13(8):1306. doi:10.3390/nano13081306
141. van der Meel R, Fens M, Vader P, van Solinge WW, Eniola-Adefeso O, Schiffelers RM. Extracellular vesicles as drug delivery systems: lessons from the liposome field. J Control Release. 2014;195:72–85. doi:10.1016/j.jconrel.2014.07.049
142. Loureiro JA, Andrade S, Duarte A, et al. Resveratrol and Grape Extract-loaded Solid Lipid Nanoparticles for the Treatment of Alzheimer’s Disease. Molecules. 2017;22(2):
143. Vashisht M, Rani P, Onteru SK, Singh D. Curcumin Encapsulated in Milk Exosomes Resists Human Digestion and Possesses Enhanced Intestinal Permeability in Vitro. Appl Biochem Biotechnol. 2017;183(3):993–1007. doi:10.1007/s12010-017-2478-4
144. Sun DM, Zhuang XY, Xiang XY, et al. A Novel Nanoparticle Drug Delivery System: the Anti-inflammatory Activity of Curcumin Is Enhanced When Encapsulated in Exosomes. Mol Ther. 2010;18(9):1606–1614. doi:10.1038/mt.2010.105
145. Kim MS, Haney MJ, Zhao Y, et al. Development of exosome-encapsulated paclitaxel to overcome MDR in cancer cells. Nanomed-Nanotechnol Biol Med. 2016;12(3):655–664. doi:10.1016/j.nano.2015.10.012
146. Wahlgren J, Karlson TD, Brisslert M, et al. Plasma exosomes can deliver exogenous short interfering RNA to monocytes and lymphocytes. Nucleic Acids Res. 2012;40(17):
147. Wan Y, Wang LX, Zhu CD, et al. Aptamer-Conjugated Extracellular Nanovesicles for Targeted Drug Delivery. Cancer Res. 2018;78(3):798–808. doi:10.1158/0008-5472.Can-17-2880
148. Sato YT, Umezaki K, Sawada S, et al. Engineering hybrid exosomes by membrane fusion with liposomes. Sci Rep. 2016;6:
149. Fuhrmann G, Serio A, Mazo M, Nair R, Stevens MM. Active loading into extracellular vesicles significantly improves the cellular uptake and photodynamic effect of porphyrins. J Control Release. 2015;205:35–44. doi:10.1016/j.jconrel.2014.11.029
150. Li ZF, Wang HZ, Yin HR, Bennett C, Zhang HG, Guo PX. Arrowtail RNA for Ligand Display on Ginger Exosome-like Nanovesicles to Systemic Deliver siRNA for Cancer Suppression. Sci Rep. 2018;8:
151. Nemati M, Singh B, Mir RA, et al. Plant-derived extracellular vesicles: a novel nanomedicine approach with advantages and challenges. Cell Commun Signal. 2022;20(1):
152. Homayun B, Lin XT, Choi HJ. Challenges and Recent Progress in Oral Drug Delivery Systems for Biopharmaceuticals. Pharmaceutics. 2019;11(3):
153. Naidoo V, Mdanda S, Ntshangase S, et al. Brain penetration of ketamine: intranasal delivery VS parenteral routes of administraion. J Psychiatr Res. 2019;112:7–11. doi:10.1016/j.jpsychires.2019.02.003
154. Wiklander OPB, Brennan MA, Lotval J, Breakefield XO, El Andaloussi S. Advances in therapeutic applications of extracellular vesicles. Sci Transl Med. 2019;11(492):
155. Greening DW, Xu R, Ji H, Tauro BJ, Simpson RJ. A Protocol for Exosome Isolation and Characterization: evaluation of Ultracentrifugation, Density-Gradient Separation, and Immunoaffinity Capture Methods. In: Posch A, editor. Proteomic Profiling: Methods and Protocols. 999 Riverview Dr, Ste 208, Totowa, Nj 07512-1165 USA: Humana Press Inc; 2015:179–209.
156. Yang CH, Zhang MZ, Merlin D. Advances in plant-derived edible nanoparticle-based lipid nano-drug delivery systems as therapeutic nanomedicines. J Mat Chem B. 2018;6(9):1312–1321. doi:10.1039/c7tb03207b
157. Cong MH, Tan SY, Li SM, et al. Technology insight: plant-derived vesicles-How far from the clinical biotherapeutics and therapeutic drug carriers? Review. Adv Drug Deliv Rev. 2022;182:
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.