Back to Journals » International Journal of Nanomedicine » Volume 19
Plant-Derived Exosome-Like Nanoparticles: Emerging Nanosystems for Enhanced Tissue Engineering
Authors Feng H, Yue Y , Zhang Y, Liang J, Liu L, Wang Q, Feng Q, Zhao H
Received 8 November 2023
Accepted for publication 26 January 2024
Published 7 February 2024 Volume 2024:19 Pages 1189—1204
DOI https://doi.org/10.2147/IJN.S448905
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Eng San Thian
Hui Feng,1 Yang Yue,1 Yan Zhang,1 Jingqi Liang,1 Liang Liu,1 Qiong Wang,1 Qian Feng,2 Hongmou Zhao1
1Department of Foot and Ankle Surgery, Honghui Hospital of Xi’an Jiaotong University, Xi’an City, Shaanxi, 710054, People’s Republic of China; 2Key Laboratory of Biorheological Science and Technology Ministry of Education College of Bioengineering, Chongqing University, Chongqing, 400044, People’s Republic of China
Correspondence: Hongmou Zhao, Department of Foot and Ankle Surgery, Honghui Hospital of Xi’an Jiaotong University, Xi’an City, Shaanxi, 710054, People’s Republic of China, Email [email protected] Qian Feng, Key Laboratory of Biorheological Science and Technology Ministry of Education College of Bioengineering, Chongqing University, Chongqing, 400044, People’s Republic of China, Email [email protected]
Abstract: Tissue engineering holds great potential for tissue repair and rejuvenation. Plant-derived exosome-like nanoparticles (ELNs) have recently emerged as a promising avenue in tissue engineering. However, there is an urgent need to understand how plant ELNs can be therapeutically applied in clinical disease management, especially for tissue regeneration. In this review, we comprehensively examine the properties, characteristics, and isolation techniques of plant ELNs. We also discuss their impact on the immune system, compatibility with the human body, and their role in tissue regeneration. To ensure the suitability of plant ELNs for tissue engineering, we explore various engineering and modification strategies. Additionally, we provide insights into the progress of commercialization and industrial perspectives on plant ELNs. This review aims to highlight the potential of plant ELNs in regenerative medicine by exploring the current research landscape and key findings.
Keywords: plant, exosome-like nanoparticles, nanomedicine, tissue engineering, cartilage, wound, bone
Introduction
Regenerative medicine has undergone a remarkable transformation with the emergence of tissue engineering, a promising field that offers enormous potential for the restoration and regeneration of damaged tissues.1–3 By integrating principles from biology, engineering, and materials science, tissue engineering has pioneered the development of functional substitutes that possess the ability to replace or rejuvenate injured or diseased tissues.4
In the rapidly evolving domain of nanomedicine, the selection of nanomaterials is crucial in determining the efficacy and safety of therapeutic modalities. Plant-based nanomaterials have emerged as highly compelling candidates within diverse biocompatible systems, principally due to their exceptional attributes.5,6 Foremost among these attributes is the innate biocompatibility inherent in plant-derived materials. This quality plays a crucial role in mitigating the tendency for immunogenic responses and toxic manifestations, commonly observed with synthetic analogs.7 Furthermore, the renewable and sustainable essence of these materials aligns closely with contemporary imperatives for eco-friendly practices in healthcare. The synthesis of plant-based nanomaterials typically entails fewer deleterious chemicals and is characterized by a diminished environmental footprint relative to certain synthetic counterparts.7,8 Beyond their ecological merits, plant-based nanomaterials inherently harbor therapeutic properties, including anti-inflammatory and antioxidant activities, synergizing with their role in drug delivery.9 With considerations of scalability and potentially streamlined regulatory pathways, plant-based nanomaterials hold promise for large-scale manufacturing and clinical translation.
Plant-derived exosome-like nanoparticles (ELNs) represent a compelling alternative in nanomedicine, exhibiting distinct characteristics and merits when compared with other biomaterials commonly employed in biomedical applications. Notably, these ELNs stand out due to their inherent bioactivity stemming from the diverse repertoire of bioactive molecules naturally present in plants. This intrinsic bioactivity sets them apart from relatively inert biomaterials like Polyvinyl Alcohol (PVA) or Poly(ε-caprolactone) (PCL).5,10 Moreover, plant-derived ELNs demonstrate high biocompatibility and low immunogenicity, a crucial advantage over materials such as Ultra-High Molecular Weight Polyethylene (UHMWPE) that may elicit undesirable immunogenic responses.6,11 A significant strength is the precision of targeting and drug delivery facilitated by these ELNs, as they can be engineered to encapsulate therapeutic cargo with a natural affinity to specific cell types. This targeted delivery capability distinguishes them from materials like Gelatin/GelMA, which may necessitate additional modifications for achieving similar precision.7,12 Embracing sustainability, plant-derived ELNs align with eco-friendly practices, in contrast to the environmental concerns associated with the production and degradation of synthetic materials like PCL. The versatility and tunability of plant-derived ELNs, akin to Chitosan, enable tailored compositions for specific applications, while their mimicry of natural exosomes enhances efficacy in biological processes and cellular interactions.
As the excellent developments in bioengineering and biotechnology during the past years, plant ELNs has emerged as a promising solution in regenerative medicine.13,14 In this review, we aim to illuminate the properties, immunological implications, role in tissue regeneration, engineering methodologies, and wide-ranging applications of plant ELNs in the field of tissue engineering (Figure 1). By offering a comprehensive analysis of the current state of advances and addressing the significant challenges, this review aims to contribute to the deeper understanding and further application of plant ELNs in tissue engineering.
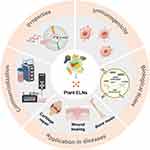 |
Figure 1 Schematic representation highlighting the characteristics, immunological implications, contributions to tissue regeneration, engineering approaches, and diverse applications of plant ELNs in the domain of tissue engineering. Reprinted from Dad HA, Gu TW, Zhu AQ, Huang LQ, Peng LH. Plant exosome-like nanovesicles: emerging therapeutics and drug delivery nanoplatforms. Mol Ther. 2021;29(1):13–31. Copyright 2021, with permission from Elsevier. Creative Commons15 and reprinted from Kim J, Li S, Zhang S, Wang J. Plant-derived exosome-like nanoparticles and their therapeutic activities. Asian J Pharm Sci. 2022;17(1):53–69. Copyright 2022, with permission from Elsevier. Creative Commons.16 Recreated with Medpeer (https://www.medpeer.cn/). |
Properties and Characteristics of Plant ELNs
Plant ELNs exhibit a delicate and intricate composition, characterized by a lipid bilayer that envelops a diverse array of bioactive molecules. These nanoparticles (NPs) comprise proteins, lipids, nucleic acids, and metabolites. With a size range of 30 to 150 nanometers, they demonstrate exceptional versatility, rendering them ideal for intercellular transport.17 The diverse composition of plant ELNs enables them to encapsulate and transport a wide range of cargoes, facilitating their engagement in numerous biological processes.
Structural and Functional Properties of Plant ELNs
Plant-derived ELNs possess a wide range of properties and characteristics that make them highly attractive for tissue engineering applications.18 Understanding these features is crucial for fully utilizing their potential in the field.
Structurally, plant ELNs are composed of lipid bilayers that enclose a cargo-rich interior, providing exceptional stability to the NPs and protecting the encapsulated cargo from degradation.19 Moreover, they exhibit a diverse array of surface proteins and molecules that actively contribute to their biological activities, playing a crucial role in cell targeting and interaction.20,21 These surface features enhance the efficacy of plant ELNs in tissue engineering.
In addition to their structural properties, the functional properties of plant ELNs significantly contribute to their therapeutic effectiveness. These NPs inherently possess biocompatibility, allowing them to safely and effectively interact with recipient cells without triggering detrimental immunogenic responses.16,22 This biocompatibility is particularly important in tissue engineering applications where the materials should not cause harm to the host.
Furthermore, plant ELNs demonstrate exceptional capabilities in encapsulating and delivering various bioactive molecules (Figure 2).23,24 They can efficiently load and transport nucleic acids, proteins, lipids, and other therapeutic agents.25 This cargo-loading capability enables precise and controlled release of these agents, which is highly advantageous in tissue engineering.22 By controlling the release of therapeutic molecules, plant ELNs can target specific areas and provide sustained and localized therapy.
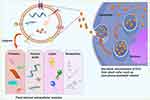 |
Figure 2 Schematic representations illustrating the structure of plant ELNs and their cargo transfer capabilities. Plant ELNs are capable of transferring a variety of cargoes to recipient cells, including small RNAs, proteins, lipids, and other pharmacologically active metabolites. Reproduced from Xu Z, Xu Y, Zhang K, et al. Plant-derived extracellular vesicles (PDEVs) in nanomedicine for human disease and therapeutic modalities. J Nanobiotechnol. 2023;21(1):114. Creative Commons.24 |
Methods of Isolation, Purification and Characterization of Plant ELNs
Significant advances have been made in the isolation and purification methods of plant ELNs, resulting in the acquisition of high-quality and homogeneous populations of NPs.26 These methods involve extracting ELNs from plant tissues or cell cultures, followed by meticulous separation approaches such as differential centrifugation, ultrafiltration, or precipitation methods.27 The choice of isolation method depends on factors such as desired yield, purity, and specific downstream applications, ensuring the procurement of well-defined plant ELN populations.
Accurate and comprehensive characterization of plant ELNs is crucial in understanding their physical properties and assessing their suitability for specific tissue engineering applications. Advanced characterization techniques are employed to explore various aspects of ELNs, including their size distribution, morphology, and cargo loading efficiency.28 Techniques such as dynamic light scattering (DLS) and nanoparticle tracking analysis (NTA) are used to determine the size distribution and concentration of ELNs in solution. Transmission electron microscopy (TEM) is particularly valuable for its high-resolution imaging capabilities, providing insights into the morphology and structural intricacies of individual ELNs.29 Moreover, methodologies like flow cytometry and Western blotting are utilized to analyze the surface proteins and cargo composition of plant ELNs.30 The isolation and purification methods of plant ELNs have undergone significant advances, ensuring the production of high-quality nanoparticle populations. Characterization techniques such as DLS, NTA, TEM, flow cytometry, and Western blotting are employed to comprehensively evaluate the physical and molecular properties of plant ELNs.
Immunological Considerations and Biocompatibility of Plant ELNs
The immunological considerations and biocompatibility of plant ELNs are crucial factors that require extensive investigation for their successful integration into tissue engineering applications. It is essential to understand the immunogenicity and immune response elicited by plant ELNs to ensure their safe and effective utilization in regenerative medicine. When introducing any foreign material into the body, including NPs, it is important to assess their potential to induce an immune response. The immune system plays a critical role in recognizing and eliminating foreign substances, which can result in inflammation or adverse reactions.31 Therefore, thorough investigation is necessary to determine the immunogenicity of plant ELNs and the subsequent immune response they may trigger. Biocompatibility is another essential aspect that should be examined when considering the integration of plant ELNs into tissue engineering applications.32 In the context of plant ELNs, it involves assessing their compatibility with recipient cells and tissues, as well as their potential to induce cytotoxicity or unwanted cellular responses. By thoroughly investigating the immunological considerations and biocompatibility of plant-derived ELNs, we can gain insights into their safety profile and compatibility with living systems.
Immunomodulatory Properties of Plant ELNs in Tissue Engineering
The immunomodulatory properties of plant-derived ELNs have attracted significant interest in tissue engineering.28 These properties are well-documented to regulate the behavior of immune cells and modulate inflammatory responses, which is crucial for promoting tissue regeneration while minimizing adverse immune reactions.33
Plant ELNs have been found to actively interact with various immune cell populations, including macrophages,34 dendritic cells,35 and T cells.36 These interactions can lead to phenotypic changes in immune cells, altering their activation state and cytokine secretion profiles. Notably, plant ELNs have the ability to modulate immune cell polarization, favoring an anti-inflammatory phenotype.34 This capacity to promote an anti-inflammatory environment is critical for tissue regeneration by mitigating excessive inflammation, which can impede the healing process. For instance, Xiong et al introduced a multifunctional hydrogel which can be conveniently packaged in a syringe for in situ local injections, providing long-term coverage to accelerate wound healing.37 The accelerated healing is achieved through the synergistic effects of two key components: magnesium ions and ginseng-derived ELNs (Figure 3). By sustained release of these ELNs, the hydrogel provides a favorable immune microenvironment for diabetic wound healing through reprogramming macrophages polarization. Importantly, this plant ELNs-based strategy addresses the challenge of coordinating neurogenesis and angiogenesis and offers potential benefits for enhanced wound healing.
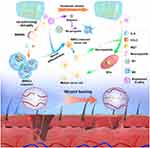 |
Figure 3 Schematic illustration of the beneficial role of Plant ELNs-based hydrogel in promotion of wound repair. Reproduced with permission from Xiong Y, Lin Z, Bu P, et al. A whole-course-repair system based on neurogenesis-angiogenesis crosstalk and macrophage reprogramming promotes diabetic wound healing. Adv Mater. 2023;35(19):e2212300.37 © 2023 The Authors. Advanced Materials published by Wiley-VCH GmbH. Creative Commons. |
Moreover, plant ELNs can influence immune cell migration and communication.38 These NPs possess targeting capabilities that allow them to selectively interact with immune cells, thereby modulating their recruitment to injured or diseased tissues. By regulating immune cell migration, plant ELNs facilitate the homing and retention of beneficial immune cells at the site of tissue damage, thereby enhancing the regenerative process. In a study by Sahin et al, it was observed that wheat-derived ELNs have beneficial effects on various cellular processes.39 The researchers found that wheat-derived ELNs promote the production of collagen type I, as well as the proliferation and migration of fibroblasts and immune cells.39 Furthermore, they discovered that wheat-derived ELNs exhibit anti-apoptotic activity in human dermal fibroblasts and human keratinocytes.39 Additionally, these ELNs were found to induce angiogenesis in human umbilical vein endothelial cells.39 These findings suggest that wheat-derived ELNs have the potential for periodontal soft tissue regeneration and angiogenesis.
The immunomodulatory properties of plant ELNs also extend to the adaptive immune response.40 Studies have shown that plant ELNs can influence T cell activation and proliferation, thereby modulating the balance between effector and regulatory T cell populations.41 This modulation has significant implications for tissue engineering, as an appropriate balance of immune responses is crucial for successful tissue regeneration and integration of engineered constructs.
To fully understand the immunomodulatory potential of plant ELNs, comprehensive long-term studies are necessary. These studies should evaluate the persistence of plant ELNs within the body, their potential to induce long-lasting immune responses, and their overall impact on tissue regeneration and integration. Long-term observations will provide insights into the durability of the immunomodulatory effects of plant ELNs, ensuring their suitability for sustained tissue engineering applications.
Strategies to Enhance Biocompatibility and Minimize Immune Reactions
In recent years, significant efforts have been made to enhance the biocompatibility of plant ELNs and minimize immune reactions to maximize their potential in tissue engineering applications.12 These efforts involve various strategies, including surface engineering techniques and optimization of the physicochemical properties of plant ELNs.
Surface engineering plays a crucial role in improving the interaction between plant ELNs and host cells and tissues, thereby reducing the risk of immune recognition and activation.42 Coating plant ELNs with biocompatible materials such as polymers or lipids offers several advantages. Firstly, it provides a protective layer that shields the nanoparticles from immune surveillance and minimizes their interaction with immune cells.42 This protective coating enhances the stability and biocompatibility of plant ELNs, allowing them to efficiently traverse physiological barriers. Moreover, specific ligands can be conjugated to the surface of the NPs to enhance their target specificity, enabling selective interactions with desired cell types or tissues.43 This targeted approach minimizes off-target effects and improves the efficacy of plant ELNs in tissue regeneration.
Optimizing the physicochemical properties of plant ELNs is crucial for improving their biocompatibility and reducing immune responses.44 Parameters such as size, charge, and surface chemistry can be modulated to achieve optimal interactions with the biological environment. Controlling the size of plant ELNs is critical as it influences their circulation time, cellular uptake, and biodistribution.45 By optimizing the size within an appropriate range, plant ELNs can exhibit prolonged circulation, enhanced cellular internalization, and improved bioavailability at the target site. Furthermore, manipulating the surface charge of plant ELNs can affect their interaction with proteins and cells, thereby influencing their immunogenicity and biocompatibility.44 Neutral or slightly negative surface charges are often preferred to minimize non-specific interactions and immune cell activation.44 Additionally, surface chemistry modifications, such as introducing hydrophilic moieties or functional groups, can enhance the stability, dispersibility, and cellular uptake of plant ELNs, contributing to improved biocompatibility.16
Role of Plant ELNs in Tissue Regeneration
Plant ELNs have emerged as promising tools in tissue regeneration due to their unique properties and capabilities. These NPs, which are similar to exosomes in structure and function, hold great potential for promoting tissue repair and regeneration.46 They can deliver bioactive molecules, modulate inflammatory responses, promote cell-to-cell communication, induce angiogenesis, support scaffold-free tissue engineering, and protect against oxidative stress. Further research and development in this field hold the potential to unlock the full regenerative potential of plant ELNs for various tissue engineering applications.
Biological Activities of Plant ELNs in Promoting Tissue Regeneration
Plant ELNs play a vital role in tissue regeneration through their multifaceted effects on cellular and molecular processes. One of the key mechanisms is their ability to deliver a diverse range of bioactive molecules to target cells and tissues.15 These bioactive molecules can include growth factors, cytokines, and nucleic acids, which are crucial for regulating cellular activities and tissue repair.15 By delivering these molecules, plant ELNs can promote cell proliferation, migration, and tissue-specific differentiation, thereby facilitating the regeneration of damaged or diseased tissues.
In addition to their role as delivery vehicles, plant ELNs exhibit inherent immunomodulatory properties that contribute to tissue regeneration.28 When introduced into the regenerative microenvironment, these NPs interact with immune cells such as macrophages, dendritic cells, and T cells. Through these interactions, plant ELNs can modulate immune cell behavior and influence the inflammatory response.
Excessive inflammation can impede tissue regeneration, while a controlled and balanced immune response is essential for successful tissue repair.47,48 Plant ELNs help regulate the immune response by promoting a shift towards an anti-inflammatory environment.16 They can influence immune cell polarization, promoting the activation of anti-inflammatory immune cells and suppressing pro-inflammatory responses. This modulation of the immune response helps to mitigate excessive inflammation, which can otherwise hinder the regenerative process. For instance, Teng et al reported the treatment with ginger-derived ELNs containing aly-miR396a-5p could significantly alleviate the lung inflammation.49 Mechanistically, ELNs-mediated delivery of aly-miR396a-5p and rlcv-miR-rL1-28-3p inhibits the expression of Nsp12 and spike genes, respectively, thereby preventing the overactivation of inflammation (Figure 4). These findings highlight the potential significance of ginger-derived ELNs in addressing lung inflammation and suggest ELNs as a promising therapeutic alternative for combating excessive inflammation.
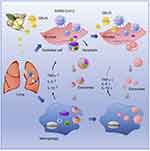 |
Figure 4 Schematic illustration of ginger-derived ELNs in alleviation of lung inflammation. Reproduced from Mol Ther, Volume 29(8), Teng Y, Xu F, Zhang X, et al. Plant-derived exosomal microRNAs inhibit lung inflammation induced by exosomes SARS-CoV-2 Nsp12. 2424–2440.49 Copyright 2021, with permission from Elsevier. Creative Commons. |
Furthermore, the interaction of plant ELNs with immune cells can have broader effects on tissue regeneration.50 These interactions can influence the secretion of cytokines and growth factors by immune cells, which in turn can impact cellular activities such as proliferation, migration, and extracellular matrix (ECM) synthesis.50 By modulating immune cell behavior and the secretion of bioactive factors, plant ELNs actively contribute to the establishment of a pro-regenerative environment within the tissue.
Overall, plant ELNs exhibit diverse and multifaceted effects on tissue regeneration. They act as delivery vehicles for bioactive molecules, promoting cellular activities necessary for tissue repair. Additionally, their inherent immunomodulatory properties help regulate the immune response, providing an environment conducive to tissue regeneration. The combined effects of bioactive molecule delivery and immunomodulation contribute to the overall regenerative potential of plant ELNs and make them valuable tools in tissue engineering and regenerative medicine.
Interaction of Plant ELNs with Recipient Cells and Their Effects on Cellular Behavior
Plant ELNs play a crucial role in tissue regeneration by efficiently interacting with recipient cells and delivering their cargo. When plant ELNs come into contact with target cells, they can be taken up through mechanisms like endocytosis or membrane fusion.46 This allows the bioactive cargo carried by the ELNs to be released directly into the cytoplasm of the recipient cells. The effects of plant ELNs on cellular behavior are diverse and context-dependent, adapting to the specific needs of tissue regeneration.51
One important effect of plant ELNs is their ability to stimulate cell proliferation. By delivering growth factors and other bioactive molecules that promote cell replication, plant ELNs can support the proliferation of damaged or diseased cells, aiding in tissue repair.51 Additionally, plant ELNs can induce cell differentiation, providing guidance to stem cells or progenitor cells towards specific lineages for tissue regeneration. This differentiation-promoting effect is crucial for the formation of functional tissues.14
Moreover, plant ELNs have been found to ameliorate inflammation via regulation of the cellular behavior of recipient cells.37 By influencing cellular signaling pathways and interactions with the extracellular matrix, plant ELNs can promote cell movement and alleviate the inflammation around the site of tissue damage. In a recent study, Liu et al extracted ELNs from garlic chives and other Allium vegetables, and investigated their effects on the NLRP3 inflammasome in primary macrophages.52 Garlic chive-derived ELNs (GC-ELNs) demonstrated potent anti-NLRP3 inflammasome activity in cell culture, which was further validated in a murine acute liver injury model and diet-induced obesity. Omics analysis of GC-ELNs was performed to identify the active components responsible for their anti-NLRP3 inflammasome function. GC-ELNs were found to be membrane-enclosed nanoparticles containing lipids, proteins, and RNAs (Figure 5). They effectively inhibited NLRP3 inflammasome activation downstream pathways, including caspase-1 autocleavage, cytokine release, and pyroptotic cell death in primary macrophages. Notably, GC-ELNs specifically targeted the NLRP3 inflammasome, with minimal impact on the activation of other inflammasomes. Local administration of GC-ELNs alleviated NLRP3 inflammasome-mediated inflammation in a chemical-induced acute liver injury model. Furthermore, oral or intravenous administration of GC-ELNs led to their accumulation in specific tissues, effectively suppressing NLRP3 inflammasome activation and chronic inflammation in diet-induced obese mice. These findings highlight GC-ELNs as promising therapeutic strategies for improving NLRP3 inflammasome-driven diseases, offering new avenues for the treatment of these complex inflammatory disorders.
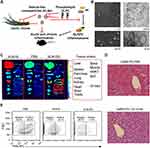 |
Figure 5 A. Schematic illustration of the important role of GC-ELNs in alleviating overactive inflammation. B. Representative SEM and ultrastructure TEM images of GC-ELNs. C. Representative images of mouse tissues under Licor Odyssey Clx image system. D. Representative images of liver sections with hematoxylin and eosin (H&E) staining. E. GC-ELNs suppress immune cell infiltration in mice. Reproduced from Liu B, Li X, Yu H, et al. Therapeutic potential of garlic chive-derived vesicle-like nanoparticles in NLRP3 inflammasome-mediated inflammatory diseases. Theranostics. 2021;11(19):9311–9330.52 Copyright 2023, Ivyspring International Publisher. Creative Commons. |
The bioactive cargo carried by plant ELNs is instrumental in mediating their effects on recipient cells.19 Growth factors within the cargo can activate cellular signaling pathways that are involved in cell proliferation and differentiation.52 Nucleic acids, such as microRNAs, can regulate gene expression, influencing cellular processes relevant to tissue regeneration.53 Additionally, the lipid components of plant ELNs can modulate cellular membrane properties and signaling cascades, further impacting cellular behavior.38
The composition and surface characteristics of plant ELNs also play a role in their interaction with recipient cells.28,35 The surface proteins and lipids of plant ELNs can engage with receptors on the cell membrane, initiating signaling events and internalization processes. Moreover, the physical properties of plant ELNs, such as their size, charge, and surface modifications, can affect their cellular uptake and distribution within tissues, thereby influencing their therapeutic efficacy.28
Application of Plant ELNs in Promotion of Different Tissue Regeneration
Plant ELNs have shown promising applications in promoting the regeneration of various tissues due to their unique properties and capabilities. Their ability to deliver bioactive molecules, modulate cellular behavior, and provide a favorable microenvironment makes them valuable tools in tissue engineering and regenerative medicine (Table 1). By harnessing the unique properties of plant ELNs, we can develop innovative strategies to enhance tissue regeneration and improve patient outcomes.
 |
Table 1 Application of Plant ELNs in Different Tissue Regeneration |
Plant ELNs in Bone Tissue Engineering
Bone tissue engineering has emerged as a promising approach for repairing and regenerating damaged or lost bone tissue, and plant ELNs offer unique properties that make them attractive for promoting bone regeneration.27 The small size, biocompatibility, and cargo-carrying capabilities of plant ELNs contribute to their potential in this field.
Plant ELNs have demonstrated their ability to enhance osteogenic differentiation and mineralization of bone-forming cells [.14 They can deliver bioactive molecules such as bone morphogenetic proteins (BMPs) and growth factors to target cells, stimulating their differentiation into osteoblasts, which are responsible for bone formation. The cargo delivered by plant ELNs not only promotes osteogenic differentiation but also supports the synthesis of essential ECM components, including collagen and calcium phosphate minerals, which contribute to the formation of structural bone tissue. For instance, Hwang et al have investigated the effects of yam-derived ELNs (YNVs) on bone regeneration in mice with osteoporosis induced by ovariectomy.14 In this study, YNVs were successfully isolated and characterized. The researchers observed that YNVs stimulate the proliferation, differentiation, and mineralization of osteoblasts, which are responsible for bone formation. They also found increased expression of bone differentiation markers such as osteopontin (OPN), alkaline phosphatase (ALP), and collagen type I (COLI) in the presence of YNVs. Interestingly, YNVs do not contain saponins like diosgenin and dioscin, which are known to have osteogenic activity in yams. Instead, the researchers discovered that the osteogenic activity of YNVs is mediated through the activation of the BMP-2/p-p38-dependent Runx2 pathway. Furthermore, the researchers conducted in vivo experiments using osteoporotic mice and found that YNVs promote longitudinal bone growth and increase mineral density in the tibia. These positive effects on bone were accompanied by significant increases in osteoblast-related parameters. Moreover, when orally administered, YNVs were transported through the gastrointestinal tract and absorbed in the small intestine. Histological analysis and toxicity tests showed excellent systemic biosafety of YNVs, including no adverse effects on the liver and kidneys (Figure 6).
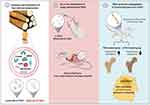 |
Figure 6 Schematic representation illustrating the isolation, administration, and osteogenic functions of yam-derived ELNs in vitro and in vivo. Reproduced from J Control Release, Volume 355, Hwang JH, Park YS, Kim HS, et al. Yam-derived exosome-like nanovesicles stimulate osteoblast formation and prevent osteoporosis in mice. 84–198.14 Copyright 2023, with permission from Elsevier. |
In addition to their cargo delivery capabilities, plant ELNs exhibit inherent immunomodulatory properties, which are crucial for successful bone tissue engineering.54 Inflammatory responses and immune reactions play significant roles in the healing process of bone fractures or defects.55,56 Plant ELNs have shown the ability to modulate immune responses by suppressing excessive inflammation and creating a pro-regenerative environment.22,57 Through their regulation of immune cell behavior and cytokine secretion, plant ELNs help reduce the risk of adverse immune reactions that could hinder bone regeneration.20,58,59
The physical characteristics of plant ELNs also contribute to their application in bone tissue engineering.60,61 Their nanoscale size enables efficient cellular uptake and penetration into the bone microenvironment. Additionally, surface modifications can be made to improve the binding affinity of plant ELNs to bone-related cells and extracellular matrix components, enhancing their integration within the bone tissue.60 This can involve functionalizing plant ELNs with bone-targeting ligands or peptides that promote selective binding to bone cells and enhance their osteoinductive potential. Furthermore, plant ELNs have been shown to induce angiogenesis, the formation of new blood vessels, which is crucial for establishing a functional vascular network within the regenerating bone.14 The bioactive cargo carried by plant ELNs, including angiogenic growth factors, promotes the recruitment and proliferation of endothelial cells, initiating the formation of new blood vessels.
Plant ELNs in Cartilage Regeneration
Cartilage regeneration is a significant challenge in tissue engineering due to the limited regenerative capacity of cartilage, which is attributed to its avascular nature and low cellularity.62 Overcoming this challenge requires the establishment of a suitable microenvironment that supports the growth and differentiation of chondrocytes, the primary cells responsible for cartilage formation.63 Plant ELNs offer potential solutions to address this challenge by delivering specific cues to guide chondrogenic differentiation and promote extracellular matrix production.64
Studies have demonstrated that ELNs can effectively deliver growth factors to chondrocytes, stimulating their differentiation into chondrocyte-like cells and enhancing the synthesis of cartilage-specific matrix components like collagen and proteoglycans.65 By delivering these bioactive molecules, plant ELNs contribute to the formation of a favorable microenvironment for cartilage regeneration, facilitating the growth and maturation of new cartilage tissue.64
In addition to their role in delivering growth factors, plant ELNs possess immunomodulatory properties that can benefit cartilage regeneration.64 Inflammatory responses in the joint can negatively impact cartilage tissue and impede regeneration. ELNs have been found to modulate inflammatory responses by reducing the secretion of pro-inflammatory cytokines and promoting an anti-inflammatory environment.66 This immunomodulatory effect helps mitigate the detrimental effects of inflammation on cartilage tissue, promoting a more favorable environment for regeneration to occur.
Another critical aspect of successful cartilage regeneration is the delivery and retention of therapeutic agents within the cartilage tissue.67,68 Plant ELNs can serve as carriers for therapeutic molecules, overcoming challenges such as poor penetration and limited retention within the avascular cartilage. Due to their small size, plant ELNs can penetrate deep into the cartilage matrix, ensuring efficient delivery of therapeutic cargo. Additionally, their cargo delivery capabilities enable sustained and controlled release of bioactive molecules, ensuring a prolonged exposure of chondrocytes to the therapeutic agents.64 This targeted and sustained delivery of therapeutic cargo enhances the efficacy of cartilage regeneration approaches, promoting better outcomes in terms of tissue repair and functional restoration.
Plant ELNs in Wound Healing and Skin Tissue Engineering
Plant-derived ELNs have emerged as a promising tool for enhancing wound healing and advancing skin tissue engineering approaches.37,69,70 Their unique properties and capabilities contribute to their effectiveness in promoting tissue repair and regeneration.
One of the key roles of plant ELNs in wound healing is their ability to regulate important cellular processes involved in tissue repair.53 By delivering bioactive molecules such as growth factors and microRNAs, plant ELNs can initiate signaling pathways that promote cell migration, proliferation, and differentiation. These processes are critical for wound closure, re-epithelialization, neurogenesis, and angiogenesis. Neurogenesis is particularly important in wound healing as it ensures an adequate supply of neuropeptides to the healing tissue, facilitating its regeneration. Plant ELNs can enhance neurogenesis by delivering bioactive factors to the wound site, stimulating the neural differentiation of mesenchymal stem cells (MSCs). In a prior study, ginseng-derived exosomes (G-Exos) were investigated for their potential to enhance the neural differentiation of MSCs. The researchers demonstrated, for the first time, that G-Exos are capable of stimulating the neural differentiation of MSCs by transferring specific miRNAs to the MSCs efficiently (Figure 7). In vitro assays showed that G-Exos were able to transfer plant-derived miRNAs to mammalian MSCs, leading to enhanced neural differentiation.53 Furthermore, in an in vivo setting, a photo-cross-linkable hydrogel loaded with chemokines and G-Exos was used.53 This hydrogel demonstrated strong efficacy in recruiting MSCs and directing their neural differentiation. The combined effect of the chemokines and G-Exos in the hydrogel promoted the migration and differentiation of MSCs into functional neural cells. The findings of this study suggest that G-Exos can serve as promising nanoplatforms for transferring plant-derived ELNs to mammalian stem cells, such as MSCs, to enhance their neural differentiation and wound healing. This research holds significant potential for the field of neural regenerative medicine, providing new insights into the development of therapeutic strategies for neural diseases and tissue regeneration.
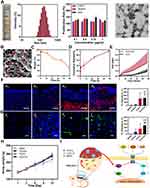 |
Figure 7 A. Image showing G-Exo bands (highlighted by a red rectangle), size distribution of G-Exos obtained from the 30/45% sucrose gradient interfaces, viability of BMSCs when exposed to G-Exos, and scanning electron microscopy depiction of the G-Exos. B. SEM image depicting CLD-C-E (G-Exos indicated by the red arrow). Scale bar = 200 nm. C. Degradation observed in CLD-C-E. D. CXCL12 release profile from CLD-C-E. E. BMSC recruitment towards CLD-C-E at the wound site. F. Immunofluorescence staining and fluorescent intensity of CD90 (in red) in the healed skin on day 2: (e1) blank group, (e2) G-Exo group, (e3) CLD-C group, and (e4) CLD-C-E group. Blue represents DAPI, scale bar = 50 μm. G. Immunofluorescence staining and fluorescent intensity of nestin (in green) in the healed skin on day 12: (f1) blank group, (f2) G-Exo group, (f3) CLD-C group, and (f4) CLD-C-E group. Blue indicates DAPI, scale bar = 20 μm. H. Bodyweight of the treated-mice at different time points. I. Schematic illustration of the underlying mechanisms of the neural differentiation and development, mediated by G-Exos. *** p < 0.001 compared to the blank group, ** p < 0.01 relative to the blank group, * p < 0.05 in comparison to the blank group, $$ p < 0.01 compared to the G-Exo group, $ p < 0.05 in comparison to the G-Exo group. Reproduce with permission.53 Copyright 2021, American Chemical Society. |
In addition to their role in promoting cellular processes, plant ELNs also possess anti-inflammatory properties, which are crucial for managing the inflammatory response during wound healing.37 Excessive inflammation can delay the healing process and contribute to chronic wounds.71,72 Plant ELNs can modulate the activity of immune cells and regulate the secretion of pro-inflammatory cytokines, thus promoting a more balanced and controlled inflammatory environment.37 By reducing inflammation, plant ELNs provide a favorable milieu for wound healing, enabling the progression of subsequent stages of tissue repair.
Furthermore, the engineering of plant ELNs can enhance their performance in skin tissue engineering.37 Surface modifications of plant ELNs can be employed to improve their stability, adhesion to the wound site, and controlled release of therapeutic cargo. By functionalizing the surface of plant ELNs with specific ligands or polymers, their targeting capabilities and interaction with skin cells can be optimized.16 These modifications enable the enhanced delivery of bioactive molecules to the desired target cells, maximizing the therapeutic effect of plant ELNs in wound healing.
Perspective and Challenges
The commercialization of plant ELNs for tissue engineering applications has obtained significant attention in recent years.73 These NPs possess unique properties and demonstrate promising therapeutic potential, leading to increased interest in translating them from the laboratory to clinical applications.12
The progress in commercializing plant ELN-based strategies for tissue engineering has been driven by various factors. Firstly, the growing body of scientific evidence showing the effectiveness of plant ELNs in promoting tissue regeneration has generated substantial interest from the industry.12,25 Preclinical studies and in vitro experiments have yielded promising results, laying the foundation for the development of commercial products that harness the therapeutic capabilities of plant ELNs.57 These studies have demonstrated the ability of plant ELNs to deliver bioactive molecules, modulate cellular responses, and enhance tissue repair processes.
Furthermore, advances in manufacturing techniques and scale-up processes have significantly contributed to commercialization efforts. Researchers have optimized the isolation and purification methods of plant ELNs to ensure high yield and reproducibility.22 This optimization has made industrial-scale production of plant ELNs more feasible, allowing for large-scale manufacturing that can meet the demands of the market. The ability to produce plant ELNs in sufficient quantities is crucial for commercialization, as it enables widespread availability and cost-effectiveness of these products.
However, several challenges still need to be addressed to achieve successful commercialization. One significant challenge is the standardization of production processes to ensure consistency in the quality and characteristics of plant ELNs. Variations in plant sources, growth conditions, and extraction protocols can impact the composition and functionality of the NPs.15,46,74 Therefore, establishing standardized protocols and implementing robust quality control measures are essential to ensure the reproducibility and reliability of plant ELN-based products. By defining specific parameters and methodologies, these standardized protocols ensure consistent product quality and facilitate comparisons between different batches.
Ensuring the safe application of ELNs in nanomedicine and tissue regeneration necessitates a comprehensive understanding of their toxicological aspects. Critical considerations encompass biodegradability, clearance dynamics, and cytotoxicity. Assessments of cytotoxic effects on various cell types are imperative to ascertain that ELNs do not induce cell damage or death. Additionally, potential inflammatory responses require scrutiny, with monitoring of cytokine release and evaluation of immune cell activation. Concerns extend to organ toxicity, as systemic distribution prompts investigations into whether ELNs accumulate in specific organs and if such accumulation adversely affects organ function. Hemocompatibility assessments are essential to ensure that ELNs do not induce hemolysis or interfere with blood coagulation. Genotoxicity evaluations are crucial to establish that ELNs do not induce DNA damage or mutations. Long-term assessments are necessary to gauge cumulative toxicity over extended periods. Surface modifications, such as coatings with biocompatible materials, offer a strategy to enhance ELN biocompatibility and mitigate potential toxic effects.
Conclusion
In summary, ELNs exhibit significant promise in tissue engineering due to their distinct properties and therapeutic potential. Despite challenges in production, standardization, and regulatory approval, the field is advancing rapidly. The development of these nanoparticles necessitates continuous efforts, collaboration, and investment for their full realization in clinical applications. By overcoming existing challenges, plant ELNs could substantially influence tissue engineering, enhancing the prospects of advanced regenerative therapies for patients in need.
Abbreviations
ELNs, plant-derived exosome-like nanoparticles; NPs, nanoparticles; DLS, dynamic light scattering; NTA, nanoparticle tracking analysis; TEM, transmission electron microscopy; ECM, extracellular matrix; GC-ELNs, Garlic chive-derived ELNs; BMPs, bone morphogenetic proteins; OPN, osteopontin; ALP, alkaline phosphatase; COLI, collagen I; G-Exos, ginseng-derived exosomes; MSCs, mesenchymal stem cells.
Acknowledgments
The authors would like to thank Medpeer for its picture source assistance during the preparation of this manuscript.
Funding
This study is supported by grants from Scientific research project of Xi’an Jiaotong University (xzy012022130) and Xi’an Talent Plan Project (XAYC210060).
Disclosure
The authors declare that there are no conflicts of interest for this work.
References
1. Kharaziha M, Baidya A, Annabi N. Rational design of immunomodulatory hydrogels for chronic wound healing. Adv Mater. 2021;33(39):e2100176. doi:10.1002/adma.202100176
2. Kim SJ, Kim EM, Yamamoto M, Park H, Shin H. Engineering multi-cellular spheroids for tissue engineering and regenerative medicine. Adv Healthc Mater. 2020;9(23):e2000608. doi:10.1002/adhm.202000608
3. Matai I, Kaur G, Seyedsalehi A, Mcclinton A, Laurencin CT. Progress in 3D bioprinting technology for tissue/organ regenerative engineering. Biomaterials. 2020;226:119536. doi:10.1016/j.biomaterials.2019.119536
4. Sorushanova A, Delgado LM, Wu Z, et al. The collagen suprafamily: from biosynthesis to advanced biomaterial development. Adv Mater. 2019;31(1):e1801651. doi:10.1002/adma.201801651
5. Hou T, Sankar Sana S, Li H, et al. Development of plant protein derived tri angular shaped nano zinc oxide particles with inherent antibacterial and neurotoxicity properties. Pharmaceutics. 2022;14(10):2155. doi:10.3390/pharmaceutics14102155
6. Mamidi N, Delgadillo RMV, Gonzalez-Ortiz A. Engineering of carbon nano-onion bioconjugates for biomedical applications. Mater Sci Eng C Mater Biol Appl. 2021;120:111698. doi:10.1016/j.msec.2020.111698
7. Mamidi N, Villela Castrejon J, Gonzalez-Ortiz A. Rational design and engineering of carbon nano-onions reinforced natural protein nanocomposite hydrogels for biomedical applications. J Mech Behav Biomed Mater. 2020;104:103696. doi:10.1016/j.jmbbm.2020.103696
8. Mamidi N, Zuniga AE, Villela-Castrejon J. Engineering and evaluation of forcespun functionalized carbon nano-onions reinforced poly (epsilon-caprolactone) composite nanofibers for pH-responsive drug release. Mater Sci Eng C Mater Biol Appl. 2020;112:110928. doi:10.1016/j.msec.2020.110928
9. Kumar R, Butreddy A, Kommineni N, et al. Lignin: drug/gene delivery and tissue engineering applications. Int J Nanomed. 2021;16:2419–2441. doi:10.2147/IJN.S303462
10. Mamidi N, Ijadi F, Norahan MH. Leveraging the recent advancements in gelma scaffolds for bone tissue engineering: an assessment of challenges and opportunities. Biomacromolecules. 2023. doi:10.1021/acs.biomac.3c00279
11. Mamidi N, Gonzalez-Ortiz A, Romo IL, Barrera EV. Development of functionalized carbon nano-onions reinforced zein protein hydrogel interfaces for controlled drug release. Pharmaceutics. 2019;11(12):621. doi:10.3390/pharmaceutics11120621
12. Karamanidou T, Tsouknidas A. Plant-derived extracellular vesicles as therapeutic nanocarriers. Int J Mol Sci. 2021;23(1):191. doi:10.3390/ijms23010191
13. Han J, Wu T, Jin J, et al. Exosome-like nanovesicles derived from phellinus linteus inhibit mical2 expression through cross-kingdom regulation and inhibit ultraviolet-induced skin aging. J Nanobiotechnol. 2022;20(1):455. doi:10.1186/s12951-022-01657-6
14. Hwang JH, Park YS, Kim HS, et al. Yam-derived exosome-like nanovesicles stimulate osteoblast formation and prevent osteoporosis in mice. J Control Release. 2023;355:184–198. doi:10.1016/j.jconrel.2023.01.071
15. Dad HA, Gu TW, Zhu AQ, Huang LQ, Peng LH. Plant exosome-like nanovesicles: emerging therapeutics and drug delivery nanoplatforms. Mol Ther. 2021;29(1):13–31. doi:10.1016/j.ymthe.2020.11.030
16. Kim J, Li S, Zhang S, Wang J. Plant-derived exosome-like nanoparticles and their therapeutic activities. Asian J Pharm Sci. 2022;17(1):53–69. doi:10.1016/j.ajps.2021.05.006
17. Teng Y, Ren Y, Sayed M, et al. Plant-derived exosomal microRNAs shape the gut microbiota. Cell Host Microbe. 2018;24(5):637–52 e8. doi:10.1016/j.chom.2018.10.001
18. Cong M, Tan S, Li S, et al. Technology insight: plant-derived vesicles-how far from the clinical biotherapeutics and therapeutic drug carriers? Adv Drug Deliv Rev. 2022;182:114108. doi:10.1016/j.addr.2021.114108
19. Ou X, Wang H, Tie H, et al. Novel plant-derived exosome-like nanovesicles from Catharanthus roseus: preparation, characterization, and immunostimulatory effect via TNF-alpha/NF-kappaB/PU.1 axis. J Nanobiotechnol. 2023;21(1):160. doi:10.1186/s12951-023-01919-x
20. Chen Q, Zu M, Gong H, et al. Tea leaf-derived exosome-like nanotherapeutics retard breast tumor growth by pro-apoptosis and microbiota modulation. J Nanobiotechnology. 2023;21(1):6. doi:10.1186/s12951-022-01755-5
21. Sundaram K, Miller DP, Kumar A, et al. Plant-derived exosomal nanoparticles inhibit pathogenicity of porphyromonas gingivalis. iScience. 2019;21:308–327. doi:10.1016/j.isci.2019.10.032
22. Lin Q, Qu M, Zhou B, et al. Exosome-like nanoplatform modified with targeting ligand improves anti-cancer and anti-inflammation effects of imperialine. J Control Release. 2019;311–312:104–116. doi:10.1016/j.jconrel.2019.08.037
23. Wang Y, Wei Y, Liao H, et al. Plant exosome-like nanoparticles as biological shuttles for transdermal drug delivery. Bioengineering. 2023;10(1):1.
24. Xu Z, Xu Y, Zhang K, et al. Plant-derived extracellular vesicles (PDEVs) in nanomedicine for human disease and therapeutic modalities. J Nanobiotechnol. 2023;21(1):114. doi:10.1186/s12951-023-01858-7
25. You JY, Kang SJ, Rhee WJ. Isolation of cabbage exosome-like nanovesicles and investigation of their biological activities in human cells. Bioact Mater. 2021;6(12):4321–4332. doi:10.1016/j.bioactmat.2021.04.023
26. Suharta S, Barlian A, Hidajah AC, et al. Plant-derived exosome-like nanoparticles: a concise review on its extraction methods, content, bioactivities, and potential as functional food ingredient. J Food Sci. 2021;86(7):2838–2850. doi:10.1111/1750-3841.15787
27. Seo K, Yoo JH, Kim J, et al. Ginseng-derived exosome-like nanovesicles extracted by sucrose gradient ultracentrifugation to inhibit osteoclast differentiation. Nanoscale. 2023;15(12):5798–5808. doi:10.1039/D2NR07018A
28. Yi Q, Xu Z, Thakur A, et al. Current understanding of plant-derived exosome-like nanoparticles in regulating the inflammatory response and immune system microenvironment. Pharmacol Res. 2023;190:106733. doi:10.1016/j.phrs.2023.106733
29. Zhang L, He F, Gao L, et al. Engineering exosome-like nanovesicles derived from asparagus cochinchinensis can inhibit the proliferation of hepatocellular carcinoma cells with better safety profile. Int J Nanomed. 2021;16:1575–1586. doi:10.2147/IJN.S293067
30. Woith E, Guerriero G, Hausman JF, et al. Plant extracellular vesicles and nanovesicles: focus on secondary metabolites, proteins and lipids with perspectives on their potential and sources. Int J Mol Sci. 2021;22(7):3719. doi:10.3390/ijms22073719
31. Cain DW, Cidlowski JA. Immune regulation by glucocorticoids. Nat Rev Immunol. 2017;17(4):233–247. doi:10.1038/nri.2017.1
32. Mu J, Zhuang X, Wang Q, et al. Interspecies communication between plant and mouse gut host cells through edible plant derived exosome-like nanoparticles. Mol Nutr Food Res. 2014;58(7):1561–1573. doi:10.1002/mnfr.201300729
33. Wang J, Zhang D, Zhu Y, Mo X, Mchugh PC, Tong Q. Astragalus and human mesenchymal stem cells promote wound healing by mediating immunomodulatory effects through paracrine signaling. Regener Med. 2022;17(4):219–232. doi:10.2217/rme-2021-0076
34. Liu J, Xiang J, Jin C, et al. Medicinal plant-derived mtDNA via nanovesicles induces the cGAS-STING pathway to remold tumor-associated macrophages for tumor regression. J Nanobiotechnol. 2023;21(1):78. doi:10.1186/s12951-023-01835-0
35. Rezaie J, Feghhi M, Etemadi T. A review on exosomes application in clinical trials: perspective, questions, and challenges. Cell Commun Signal. 2022;20(1):145. doi:10.1186/s12964-022-00959-4
36. Tasli PN. Usage of celery root exosome as an immune suppressant; Lipidomic characterization of Apium graveolens originated exosomes and its suppressive effect on PMA/ionomycin mediated CD4(+) T lymphocyte activation. J Food Bio Chem. 2022;46(12):e14393. doi:10.1111/jfbc.14393
37. Xiong Y, Lin Z, Bu P, et al. A whole-course-repair system based on neurogenesis-angiogenesis crosstalk and macrophage reprogramming promotes diabetic wound healing. Adv Mater. 2023;35(19):e2212300. doi:10.1002/adma.202212300
38. Zu M, Xie D, Canup BSB, et al. ‘Green’ nanotherapeutics from tea leaves for orally targeted prevention and alleviation of colon diseases. Biomaterials. 2021;279:121178. doi:10.1016/j.biomaterials.2021.121178
39. Sahin F, Kocak P, Gunes MY, Ozkan I, Yildirim E, Kala EY. In vitro wound healing activity of wheat-derived nanovesicles. Appl Biochem Biotechnol. 2019;188(2):381–394. doi:10.1007/s12010-018-2913-1
40. Deng CJ, Liu L, Liu LZ, et al. A secreted pore-forming protein modulates cellular endolysosomes to augment antigen presentation. FASEB J. 2020;34(10):13609–13625. doi:10.1096/fj.202001176R
41. MaC P, Gai C, Negro F, et al. Plant-derived extracellular vesicles as a delivery platform for RNA-based vaccine: feasibility study of an oral and Intranasal SARS-CoV-2 vaccine. Pharmaceutics. 2023;15(3):doi:10.3390/pharmaceutics15030974
42. Yin L, Yan L, Yu Q, et al. Characterization of the microRNA profile of ginger exosome-like nanoparticles and their anti-inflammatory effects in intestinal caco-2 cells. J Agric Food Chem. 2022;70(15):4725–4734. doi:10.1021/acs.jafc.1c07306
43. Barzin M, Bagheri AM, Ohadi M, Abhaji AM, Salarpour S, Dehghannoudeh G. Application of plant-derived exosome-like nanoparticles in drug delivery. Pharm Dev Technol. 2023;28(5):383–402. doi:10.1080/10837450.2023.2202242
44. Chen N, Sun J, Zhu Z, Cribbs AP, Xiao B. Edible plant-derived nanotherapeutics and nanocarriers: recent progress and future directions. Expert Opin Drug Deliv. 2022;19(4):409–419. doi:10.1080/17425247.2022.2053673
45. Suresh AP, Kalarikkal SP, Pullareddy B, Sundaram GM. Low pH-based method to increase the yield of plant-derived nanoparticles from fresh ginger rhizomes. ACS Omega. 2021;6(27):17635. doi:10.1021/acsomega.1c02162
46. Yanez-Mo M, Siljander PR, Andreu Z, et al. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles. 2015;4(1):27066. doi:10.3402/jev.v4.27066
47. Liu S, Yu JM, Gan YC, et al. Biomimetic natural biomaterials for tissue engineering and regenerative medicine: new biosynthesis methods, recent advances, and emerging applications. Mil Med Res. 2023;10(1):16. doi:10.1186/s40779-023-00448-w
48. Xiong Y, Mi BB, Lin Z, et al. The role of the immune microenvironment in bone, cartilage, and soft tissue regeneration: from mechanism to therapeutic opportunity. Mil Med Res. 2022;9(1):65. doi:10.1186/s40779-022-00426-8
49. Teng Y, Xu F, Zhang X, et al. Plant-derived exosomal microRNAs inhibit lung inflammation induced by exosomes SARS-CoV-2 Nsp12. Mol Ther. 2021;29(8):2424–2440. doi:10.1016/j.ymthe.2021.05.005
50. Lian MQ, Chng WH, Liang J, et al. Plant-derived extracellular vesicles: recent advancements and current challenges on their use for biomedical applications. J Extracell Vesicles. 2022;11(12):e12283. doi:10.1002/jev2.12283
51. Fan SJ, Chen JY, Tang CH, Zhao QY, Zhang JM, Qin YC. Edible plant extracellular vesicles: an emerging tool for bioactives delivery. Front Immunol. 2022;13:1028418. doi:10.3389/fimmu.2022.1028418
52. Liu B, Li X, Yu H, et al. Therapeutic potential of garlic chive-derived vesicle-like nanoparticles in NLRP3 inflammasome-mediated inflammatory diseases. Theranostics. 2021;11(19):9311–9330. doi:10.7150/thno.60265
53. Xu XH, Yuan TJ, Dad HA, et al. Plant exosomes as novel nanoplatforms for microRNA transfer stimulate neural differentiation of stem cells in vitro and in vivo. Nano Lett. 2021;21(19):8151–8159. doi:10.1021/acs.nanolett.1c02530
54. Zhang Z, Yu Y, Zhu G, et al. The emerging role of plant-derived exosomes-like nanoparticles in immune regulation and periodontitis treatment. Front Immunol. 2022;13:896745. doi:10.3389/fimmu.2022.896745
55. Lin Z, Xiong Y, Meng W, et al. Exosomal PD-L1 induces osteogenic differentiation and promotes fracture healing by acting as an immunosuppressant. Bioact Mater. 2022;13:300. doi:10.1016/j.bioactmat.2021.10.042
56. Tao R, Mi B, Hu Y, et al. Hallmarks of peripheral nerve function in bone regeneration. Bone Res. 2023;11(1):6. doi:10.1038/s41413-022-00240-x
57. He B, Cai Q, Qiao L, et al. RNA-binding proteins contribute to small RNA loading in plant extracellular vesicles. Nat Plants. 2021;7(3):342–352. doi:10.1038/s41477-021-00863-8
58. He Y, He Z, Leone S, Liu S. Milk exosomes transfer oligosaccharides into macrophages to modulate immunity and attenuate adherent-invasive E. coli (AIEC) infection. Nutrients. 2021;13(9):3198. doi:10.3390/nu13093198
59. Subudhi PD, Bihari C, Sarin SK, Baweja S. Emerging role of edible exosomes-like nanoparticles (ELNs) as hepatoprotective agents. Nanotheranostics. 2022;6(4):365–375. doi:10.7150/ntno.70999
60. Li DF, Yang MF, Xu J, et al. Extracellular vesicles: the next generation theranostic nanomedicine for inflammatory bowel disease. Int J Nanomed. 2022;17:3893–3911. doi:10.2147/IJN.S370784
61. Yang B, Chen Y, Shi J. Exosome biochemistry and advanced nanotechnology for next-generation theranostic platforms. Adv Mater. 2019;31(2):1.
62. Kwon H, Brown WE, Lee CA, et al. Surgical and tissue engineering strategies for articular cartilage and meniscus repair. Nat Rev Rheumatol. 2019;15(9):550–570. doi:10.1038/s41584-019-0255-1
63. Bhattacharjee M, Coburn J, Centola M, et al. Tissue engineering strategies to study cartilage development, degeneration and regeneration. Adv Drug Deliv Rev. 2015;84:107–122. doi:10.1016/j.addr.2014.08.010
64. Yildirim M, Unsal N, Kabatas B, Eren O, Sahin F. Effect of Solanum lycopersicum and citrus limon-derived exosome-like vesicles on chondrogenic differentiation of adipose-derived stem cells. Appl Biochem Biotechnol. 2023;196(1):203–219. doi:10.1007/s12010-023-04491-0
65. Thomas BL, Eldridge SE, Nosrati B, et al. WNT3A-loaded exosomes enable cartilage repair. J Extracell Vesicles. 2021;10(7):e12088. doi:10.1002/jev2.12088
66. Ni Z, Kuang L, Chen H, et al. The exosome-like vesicles from osteoarthritic chondrocyte enhanced mature IL-1beta production of macrophages and aggravated synovitis in osteoarthritis. Cell Death Dis. 2019;10(7):522. doi:10.1038/s41419-019-1739-2
67. Xu XL, Xue Y, Ding JY, et al. Nanodevices for deep cartilage penetration. Acta Biomater. 2022;154:23–48. doi:10.1016/j.actbio.2022.10.007
68. Zhang M, Hu W, Cai C, Wu Y, Li J, Dong S. Advanced application of stimuli-responsive drug delivery system for inflammatory arthritis treatment. Mater Today Bio. 2022;14:100223. doi:10.1016/j.mtbio.2022.100223
69. Liew FF, Chew BC, Ooi J. Wound healing properties of exosomes - a review and modelling of combinatorial analysis Strategies. Curr Mol Med. 2022;22(2):165–191. doi:10.2174/1566524021666210405131238
70. Narauskaite D, Vydmantaite G, Rusteikaite J, et al. Extracellular vesicles in skin wound healing. Pharmaceuticals. 2021;14(8):811. doi:10.3390/ph14080811
71. Xiong Y, Chen L, Liu P, et al. All-in-one: multifunctional hydrogel accelerates oxidative diabetic wound healing through timed-release of exosome and fibroblast growth factor. Small. 2022;18(1):e2104229. doi:10.1002/smll.202104229
72. Xiong Y, Chen L, Yan C, et al. Circulating exosomal miR-20b-5p inhibition restores wnt9b signaling and reverses diabetes-associated impaired wound healing. Small. 2020;16(3):e1904044. doi:10.1002/smll.201904044
73. Ito Y, Taniguchi K, Kuranaga Y, et al. Uptake of microRNAs from exosome-like nanovesicles of edible plant juice by rat enterocytes. Int J Mol Sci. 2021;22(7):3749. doi:10.3390/ijms22073749
74. Pan D, Liu W, Zhu S, et al. Potential of different cells-derived exosomal microRNA cargos for treating spinal cord injury. J Orthop Translat. 2021;31:33–40. doi:10.1016/j.jot.2021.09.008
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
