Back to Journals » Clinical, Cosmetic and Investigational Dentistry » Volume 10
Physicochemical changes in enamel submitted to pH cycling and bleaching treatment
Authors Eskelsen E, Catelan A, Hernades NMA, Soares LES , Cavalcanti AN, Aguiar FHB, Suzy Liporoni PC
Received 21 August 2018
Accepted for publication 17 October 2018
Published 12 December 2018 Volume 2018:10 Pages 281—286
DOI https://doi.org/10.2147/CCIDE.S184683
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Christopher E. Okunseri
Evania Eskelsen,1 Anderson Catelan,1 Natália Maria Aparecida Pinto Hernades,2 Luís Eduardo Silva Soares,3 Andrea Nóbrega Cavalcanti,4 Flávio Henrique Baggio Aguiar,2 Priscila Christiane Suzy Liporoni1
1Department of Dentistry, University of Taubaté, Taubaté, São Paulo, Brazil; 2Department of Restorative Dentistry, Piracicaba Dental School, University of Campinas, Piracicaba, São Paulo, Brazil; 3Laboratory of Biomedical Vibrational Spectroscopy, Research and Development Institute, University of Vale do Paraíba, São José dos Campos, São Paulo, Brazil; 4Department of Restorative Dentistry, School of Medicine and Public Health of Bahia and Federal University of Bahia, Salvador, Bahia, Brazil
Objective: The purpose of this study was to assess the hardness, mineral content, surface roughness, and micromorphology of sound and slightly demineralized enamels, before and after bleaching procedure using 10% carbamide peroxide.
Methods: Sixty bovine dental blocks were randomly divided into the following two groups: 30 slabs were submitted to three cycles of pH and 30 slabs were noncycled. Hardness (n=10) was measured using the microhardness tester with Knoop indenter under a 50 g load for 5 seconds. The calcium/phosphate (Ca/P) ratio (n=10) was obtained using a micro-energy-dispersive X-ray fluorescence (μ-EDXRF) spectrometer. The measurement of roughness average (n=10) was performed using a surface roughness tester. Specimens were bleached 6 hours/day during 21 days, and then, physicochemical properties were re-evaluated. Two additional specimens were carried out to evaluate surface micromorphology using the scanning electron microscopy. Data were statistically analyzed by ANOVA and Tukey’s test (α=0.05).
Results: Sound and slightly demineralized enamels showed no difference in Ca/P ratio after dental bleaching according to the µ-EDXRF analysis, but the Ca/P ratio decreased after bleaching for slightly demineralized enamel. Bleaching treatment decreased the hardness and increased the surface roughness, causing micromorphology alterations.
Conclusion: Therefore, bleaching procedure promoted change in bovine enamel surface and increased the demineralization of slightly demineralized enamel but not affected the mineral content of sound enamel.
Keywords: tooth bleaching, dental enamel, chemical properties, pH cycling, microhardness, roughness
Introduction
Morphological alterations arising from interaction between bleaching agent and tooth structure have been reported.1 Sound enamel treated with hydrogen peroxide can present hardness reduction,2–4 mineral loss,4–9 as well as increased roughness6,10,11 and porosity on its surface.7,11,12 However, results are controversial since other studies did not observe difference in enamel components3,12 and hardness13,14 after bleaching using the low concentration of hydrogen peroxide.
Most studies involving dental bleaching are limited to assays and conditions in which the change in mineral content is measured by indirect methods, such as hardness and roughness.11,15 However, methodologies have been adopted to measure alterations in tooth hard tissues after bleaching treatment, quantifying their mineral content (especially calcium and phosphate), using micro-energy-dispersive X-ray fluorescence (μ-EDXRF) spectrometer, scanning electron microscopy (SEM), Fourier-transform Raman spectrometry, and Fourier-transform infrared spectrometry.3,5–9,12
Although bleaching treatment is indicated by dentist only for patients with sound teeth, clinically pH changes occur in the oral cavity after meal, so that alteration in the mineral content of teeth occurs daily.16,17 Thus, this study was analyzed the chemical, mechanical, and morphological alterations in sound and slightly demineralized enamels submitted to dental bleaching. The null hypotheses tested showed that 1) bleaching agent would not cause mineral loss and change in hardness, roughness, and micromorphology of sound enamel and 2) it would not exacerbate on previously slightly demineralized enamel.
Methods
Study design
This is a laboratorial study that evaluated the hardness, mineral content, surface roughness, and micromorphology of sound and slightly demineralized bovine enamels, before and after bleaching procedure using 10% carbamide peroxide. The studied factors were as follows: pH cycling process (yes: slightly demineralized enamel; no: sound enamel) and timespan study (before and after bleaching procedure).
Specimen preparation
Eighty extracted bovine incisors stored in 0.1% thymol solution were used within 1 month after extraction. Roots were separated from the crowns using a water-cooled low-speed diamond saw (Isomet 1000; Buehler Inc., Lake Bluff, IL, USA). Then, crowns were sectioned mesiodistally and buccolingually to obtain a square slab (4 mm ×4 mm ×2 mm) from each crown. Buccal enamel surface was flattened for 30 seconds with 600-, 1,200-, and 2,500-grit aluminum oxide wet abrasive papers using a polishing machine (APL-4; Arotec, Cotia, São Paulo, Brazil) and sonicated in distilled water for 5 minutes (MA610/9A; Marconi Equipamentos Para Laboratrios Ltda, Piracicaba, São Paulo, Brazil).
Slabs were immersed in artificial saliva18 containing 1.5 mM Ca, 0.9 mM P, 150 mM KCl, 0.1 mM Tris, and pH 7 for 30 days and changed daily. Saliva storage standardizes mineral content, by providing constant ion concentration and minimizing further ionic changes between the slab and environment. Then, each slab was coated with two layers of nail varnish (Revlon Inc., New York, NY, USA), except the flattened enamel surface, and it was embedded in polystyrene resin (Valglass, São José dos Campos, São Paulo, Brazil) with exposing the enamel surface.
Knoop hardness number (KHN)
Initial hardness was measured using a microhardness tester (HMV-2T E; Shimadzu Corporation, Tokyo, Japan) with a Knoop diamond indenter under a 50 g load for 5 seconds.15 The following three indentations were carried out on specimen surface: one at center and the remaining two at a distance of approximately 100 µm from central location. Of the 80 dental slabs, the 60 slabs that had the closest average hardness (KHN) values were selected.
Of the 60 dental slabs selected, 30 specimens were randomly subjected to pH cycling model and 30 specimens were not pH cycled (n=10). During the experiment, specimens that were not pH cycled were immersed in distilled water (pH 7; Byofórmula, São José dos Campos, São Paulo, Brazil) and changed daily to not interfere with mineral content.
pH cycling
The pH-cycled specimens were submitted to three demineralization–remineralization cycles at 37°C. Each cycle was composed by 6-hour immersion in demineralization solution (1.5 mM Ca, 0.9 mM P, 0.05 mM acetate buffer, 0.07 ppm F, pH 5) followed by 18 hours immersion in remineralization solution (1.5 mM Ca, 0.9 mM P, 0.1 mM Tris, 10 ppm F, pH 7). Solutions were renewed prior to begin each cycle.
Measurements and bleaching treatment
Hardness, calcium/phosphate (Ca/P) ratio, surface roughness, and surface micromorphology were carried out before and after bleaching treatment. The 0.016 mL of 10% carbamide peroxide (Opalescence PF; Ultradent Products Inc., South Jordan, UT, USA) containing 0.25% of fluorine was applied on enamel surface (approximately 1 mm of thickness) using a micropipette (Eppendorf AG, Hamburg, Germany) for 6 hours/day during 21 days at 37°C and 100% relative humidity. After bleaching session, specimens were stored for 18 hours in distilled water (pH 7) until next session. The pH of bleaching gel (pH 6.87) was measured using a digital pH meter (Equilam, Diadema, São Paulo, Brazil).
µ-EDXRF measurement
Quantitative element analysis of Ca/P ratio was performed using a micro-X-ray fluorescence spectrometer by micro-energy-dispersive (µ-EDX-1300; Shimadzu Corporation), equipped with a rhodium X-ray tube and a Si (Li) detector cooled by liquid nitrogen. The equipment was coupled to a computer system for data processing, and the measurements of 15 kV energy of scans were carried out with a count rate of 100 live seconds per point in three different spot zones. Equipment was adjusted using a certified commercial reagent of stoichiometric hydroxyapatite (synthetic Ca10(PO4)6(OH)2, grade 99.999%, lot 10818HA; Sigma-Aldrich Co., St Louis, MO, USA) as reference. Measurements were collected using the fundamental parameters of characteristic X-ray emission of the elements Ca and P. The element O was used as chemical balance, and energy calibration was performed using internal standards for light elements.
Surface roughness
Specimens were individually positioned in a surface roughness tester (Surftest 401; Mitutoyo, Kawasaki, Japan) to measure the roughness average (Ra) values. Three readings in different parts of the specimen were obtained, and roughness data (µm) were determined. The extension of each reading was 2.85 mm, using a cutoff of 0.8 mm.
Surface micromorphology
Two additional specimens of each group at each time were prepared for SEM (JSM-5310; JEOL, Tokyo, Japan) analysis. Specimens were sputter coated with gold in a vacuum evaporator (MED 010; Balzers, Balzer, Liechtenstein), and photomicrographs of surface micromorphology were obtained at 2,000× magnification.
Statistical analysis
Normality of data was previously analyzed by Kolmogorov–Smirnov test, and normal distributions were observed. So data were statistically analyzed using two-way repeated measures ANOVA and Tukey’s test to determine significant differences (α=0.05).
Results
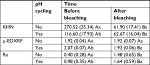
Table 1 shows the mean values of Knoop hardness, µ-EDXRF, and surface roughness for each group before and after bleaching treatment. Surface micromorphology images are presented in Figure 1A–D. Hardness testing revealed significantly lower KHN values after bleaching procedure for sound and slightly demineralized enamels. Noncycled group showed significantly higher KHN compared to pH-cycled group prior to bleaching treatment. KHN values obtained after bleaching were statistically similar for both groups.
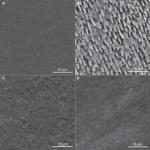  | Figure 1 Images of sound enamel (A), enamel after pH cycling model (B), sound enamel after bleaching treatment (C), and bleached enamel previously submitted to pH cycling (D) at 2,000× magnification. |
The µ-EDXRF analysis showed that sound enamel showed no significant difference between Ca/P ratio before and after bleaching procedure, while pH-cycled group showed lower ratio after bleaching. Slightly demineralized enamel presented higher Ca/P ratio compared to sound enamel prior to bleaching, but it was similar after bleaching treatment.
Bleaching procedure increased the surface roughness for pH-cycled and noncycled groups. Slightly demineralized enamel showed a significantly higher Ra value compared to sound enamel prior to bleaching. However, after bleaching treatment, Ra values were statistically similar for both groups.
Figure 1 represents photomicrographs obtained using SEM. Figure 1A shows sound enamel with polished surface. The 10% carbamide peroxide promoted minor alterations in enamel morphology (Figure 1C). The pH cycling resulted in surface demineralization characteristics (Figure 1B). Similar characteristics were observed in enamel previously submitted to pH cycling followed by bleaching treatment (Figure 1D) and in enamel that was only bleached (Figure 1C).
Discussion
Bleaching treatment using carbamide peroxide has been related to morphological changes and increased porosities in enamel.2,13 In the present investigation, no difference in the Ca/P ratio was observed for sound enamel after dental bleaching according to µ-EDXRF analysis, but Ca/P ratio was decreased for slightly demineralized enamel. In addition, bleaching procedure decreased the hardness and increased the surface roughness. Thus, 10% carbamide peroxide promoted alterations in enamel surface morphology, decreased the hardness, increased the surface roughness, and exacerbated the mineral loss in slightly demineralized enamel; therefore, the two null hypotheses were rejected.
After bleaching procedure, sound and pH-cycled enamels showed no difference in hardness as observed by studies using early artificial caries lesions.16,19,20 Fluorine present in bleaching gel is likely to have contributed to this fact, since it has been reported that fluorine is more effective in inhibiting the demineralization than increasing the remineralization,21 regardless of administration route.22 Probably as related by a previous study,23 which incorporated fluorine ions to 10% carbamide peroxide, the fluorine contributes to the remineralization of demineralized enamel. In addition, fluorine effectiveness can be boosted by bleaching gel components, which remove proteins adhered to enamel prisms, allowing these clean prisms to interact with fluoride ions, increasing the remineralization capacity of enamel.24
Bleached enamel showed hardness reduction as reported by previous studies,14,17,22,25 which may be related to the composition of whitening product, such as hydrogen peroxide concentration, activators, thickeners, and pH.2,23 Bleaching gel used in this investigation had pH near neutral (6.87) and the hydrogen peroxide concentration of approximately 3.6%. Therefore, hardness decrease can probably be related to carbopol present in gel composition, which is an acid polymer that can cause demineralization of enamel surface2,26 and interact synergically with free radicals, increasing mineral loss caused by other components.27
Interaction between bleaching agent and hydroxyapatite results in the following reaction:
Ca10(PO4)6(OH)2 +8H+ →10Ca2+ +6HPO42– +2H2O,
in which the analysis of calcium element and phosphate group are good indicators of enamel demineralization.28 The present study not revealed difference in the Ca/P ratio for sound enamel after dental bleaching. This finding corroborates with other studies3,12,29 in which the levels of calcium and phosphorus were not altered after bleaching procedure, probably because it has a slightly acid, nearly neutral pH.
In contrast, difference in the Ca/P ratio before and after cariogenic challenge was related to previous studies30,31 as observed in the present investigation, such difference concerning the mineral content after pH cycling might be caused by ions’ dissolution from hydroxyapatite partially diluted on enamel surface, while its surface remains intact. Another factor to be considered is that only Ca and P elements were analyzed using µ-EDXRF. The O element was used as balance, due to the internal calibration of equipment by synthetic hydroxyapatite as previously reported.6 Considering that tooth enamel is composed of other chemical elements and that impurities (as carbonate) can also be present,21 the rapid loss of these components at carious early stage can indirectly influence the Ca/P ratio.32 Since, there are O reduction and consequently a quantitative increase in other elements to form the stoichiometric balance.
Comparison of the Ca/P ratio between sound and pH-cycled enamels after bleaching treatment showed no difference, which probably indicates that carbamide peroxide was not a direct factor on change in enamel components.16 This increase in the mineral content of pH-cycled group after bleaching treatment could probably be related to fluoride present in the composition of bleaching agent, which may have favored enamel remineralization.24
Micromorphological changes in enamel surface are associated with the increase in roughness, porosity, and erosion areas. After pH cycling, a significant increase in surface roughness of enamel was observed. Furthermore, bleaching promoted the increased surface roughness for sound6,10,11 and slightly demineralized enamels,10 which related to subproducts of peroxide oxidation reaction. Urea is able to penetrate into interprismatic regions of enamel, increasing its permeability and causing structural changes due to the dissociation of hydrogen bonds between the NH and CO groups and due to the protein-denaturing ability.2 However, urea raises the pH of bleaching gel, promoting a reduction in the adverse effects.33 Some studies34,35 that assessed unbleached and bleached enamels observed no difference in surface roughness; this discrepancy may be related to different methodologies used, such as bleaching agent, saliva presence, exposure time to whitening product, and others.
The pH cycling model promoted alterations in enamel surface in all analyses performed. Dental bleaching using 10% carbamide peroxide increased the roughness and decreased the hardness in sound and pH-cycled enamels, but it influenced the Ca/P ratio only for slightly demineralized enamel, indicating higher loss of calcium than phosphate. Surface morphological changes were evident after pH cycling and bleaching procedure using 10% carbamide peroxide for both groups. Comparing the images 1B (after pH cycling) and 1D (bleached after pH cycling), a removal of a mineral surface previously demineralized was occurred.
Conclusion
Therefore, dental bleaching using 10% carbamide peroxide was safe and effective for sound bovine enamel, but it promotes demineralization for slightly demineralized enamel. In addition, due to superficial changes that were observed after bleaching treatment, this treatment should be performed with caution, especially in patients who excessively use acidic foods and beverages.
Disclosure
The authors report no conflicts of interest in this work.
References
Ghalili KM, Khawaled K, Rozen D, Afsahi V. Clinical study of the safety and effectiveness of a novel over-the-counter bleaching tray system. Clin Cosmet Investig Dent. 2014;6:15–19. | ||
Basting RT, Rodrigues AL, Serra MC. The effect of 10% carbamide peroxide, carbopol and/or glycerin on enamel and dentin microhardness. Oper Dent. 2005;30(5):608–616. | ||
Parreiras SO, Vianna P, Kossatz S, Loguercio AD, Reis A. Effects of light activated in-office bleaching on permeability, microhardness, and mineral content of enamel. Oper Dent. 2014;39(5):E225–E230. | ||
Klaric E, Rakic M, Sever I, Milat O, Par M, Tarle Z. Enamel and dentin microhardness and chemical composition after experimental light-activated bleaching. Oper Dent. 2015;40(4):E132–E141. | ||
Sa Y, Chen D, Liu Y, et al. Effects of two in-office bleaching agents with different pH values on enamel surface structure and color: an in situ vs. in vitro study. J Dent. 2012;40 (1):e26–e34. | ||
Attia ML, Cavalli V, do Espírito Santo AM, et al. Effects of bleaching agents combined with regular and whitening toothpastes on surface roughness and mineral content of enamel. Photomed Laser Surg. 2015;33(7):378–383. | ||
Coceska E, Gjorgievska E, Coleman NJ, et al. Enamel alteration following tooth bleaching and remineralization. J Microsc. 2016;262(3):232–244. | ||
Pinto A, Bridi EC, Amaral F, et al. Enamel mineral content changes after bleaching with high and low hydrogen peroxide concentrations: colorimetric spectrophotometry and total reflection X-ray fluorescence analyses. Oper Dent. 2017;42(3):308–318. | ||
Vieira-Junior WF, Ferraz LN, Pini N, et al. Effect of toothpaste use against mineral loss promoted by dental bleaching. Oper Dent. 2018;43(2):190–200. | ||
Basting RT, Rodrigues AL, Serra MC. Micromorphology and surface roughness of sound and demineralized enamel and dentin bleached with a 10% carbamide peroxide bleaching agent. Am J Dent. 2007;20(2):97–102. | ||
Sasaki RT, Catelan A, Bertoldo ES, et al. Effect of 7.5% hydrogen peroxide containing remineralizing agents on hardness, color change, roughness and micromorphology of human enamel. Am J Dent. 2015;28(5):261–267. | ||
de Moraes IQ, Silva LN, Porto IC, de Lima Neto CF, dos Santos NB, Fragoso LS. Effect of in-office bleaching with 35% hydrogen peroxide with and without addition of calcium on the enamel surface. Microsc Res Tech. 2015;78(11):975–981. | ||
Sasaki RT, Arcanjo AJ, Flório FM, Basting RT. Micromorphology and microhardness of enamel after treatment with home-use bleaching agents containing 10% carbamide peroxide and 7.5% hydrogen peroxide. J Appl Oral Sci. 2009;17(6):611–616. | ||
Grazioli G, Valente LL, Isolan CP, Pinheiro HA, Duarte CG, Münchow EA. Bleaching and enamel surface interactions resulting from the use of highly-concentrated bleaching gels. Arch Oral Biol. 2018;87:157–162. | ||
Maia E, Baratieri LN, Caldeira de Andrada MA, Monteiro S, Vieira LC. The influence of two home-applied bleaching agents on enamel microhardness: an in situ study. J Dent. 2008;36(1):2–7. | ||
Demarco FF, Meireles SS, Sarmento HR, Dantas RV, Botero T, Tarquinio SB. Erosion and abrasion on dental structures undergoing at-home bleaching. Clin Cosmet Investig Dent. 2011;3:45–52. | ||
de Fátima Carvalho Vasconcelos M, Fonseca-Gonçalves A, de França AKA, de Medeiros UV, Maia LC, Queiroz CS. An in vitro evaluation of human enamel surfaces subjected to erosive challenge after bleaching. J Esthet Restor Dent. 2017;29(2):128–136. | ||
Hara AT, Queiroz CS, Paes Leme AF, Serra MC, Cury JA. Caries progression and inhibition in human and bovine root dentine in situ. Caries Res. 2003;37(5):339–344. | ||
Burgmaier GM, Schulze IM, Attin T. Fluoride uptake and development of artificial erosions in bleached and fluoridated enamel in vitro. J Oral Rehabil. 2002;29(9):799–804. | ||
Pinto CF, Paes Leme AF, Cavalli V, Giannini M. Effect of 10% carbamide peroxide bleaching on sound and artificial enamel carious lesions. Braz Dent J. 2009;20(1):48–53. | ||
Ogaard B, Rølla G. Intra-oral models: comparison of in situ substrates. J Dent Res. 1992;71(Suppl 3):920–923. | ||
Wiegand A, Schreier M, Attin T. Effect of different fluoridation regimes on the microhardness of bleached enamel. Oper Dent. 2007;32(6):610–615. | ||
Attin T, Betke H, Schippan F, Wiegand A. Potential of fluoridated carbamide peroxide gels to support post-bleaching enamel re-hardening. J Dent. 2007;35(9):755–759. | ||
Gladwell J, Simmons D, Wright JT. Remineralization potential of a fluoridated carbamide peroxide whitening gel. J Esthet Restor Dent. 2006;18(4):206–212. | ||
Leandro GA, Attia ML, Cavalli V, do Rego MA, Liporoni PC. Effects of 10% carbamide peroxide treatment and sodium fluoride therapies on human enamel surface microhardness. Gen Dent. 2008;56(3):274–277. | ||
Basting RT, Rodrigues AL, Serra MC. The effects of seven carbamide peroxide bleaching agents on enamel microhardness over time. J Am Dent Assoc. 2003;134(10):1335–1342. | ||
Rodrigues JA, Oliveira GP, Amaral CM. Effect of thickener agents on dental enamel microhardness submitted to at-home bleaching. Braz Oral Res. 2007;21(2):170–175. | ||
Santini A, Pulham CR, Rajab A, Ibbetson R. The effect of a 10% carbamide peroxide bleaching agent on the phosphate concentration of tooth enamel assessed by Raman spectroscopy. Dent Traumatol. 2008;24(2):220–223. | ||
Duschner H, Götz H, White DJ, Kozak KM, Zoladz JR. Effects of hydrogen peroxide bleaching strips on tooth surface color, surface microhardness, surface and subsurface ultrastructure, and microchemical (Raman spectroscopic) composition. J Clin Dent. 2006;17(3):72–78. | ||
Arnold WH, Bietau V, Renner PO, Gaengler P. Micromorphological and micronanalytical characterization of stagnating and progressing root caries lesions. Arch Oral Biol. 2007;52(6):591–597. | ||
de Carvalho FG, Puppin-Rontani RM, Soares LE, Santo AM, Martin AA, Nociti-Junior FH. Mineral distribution and CLSM analysis of secondary caries inhibition by fluoride/MDPB-containing adhesive system after cariogenic challenges. J Dent. 2009;37(4):307–314. | ||
Lynch RJ, Ten Cate JM. The effect of lesion characteristics at baseline on subsequent de- and remineralisation behaviour. Caries Res. 2006;40(6):530–535. | ||
Cavalli V, Giannini M, Carvalho RM. Effect of carbamide peroxide bleaching agents on tensile strength of human enamel. Dent Mater. 2004;20(8):733–739. | ||
Cadenaro M, Breschi L, Nucci C, et al. Effect of two in-office whitening agents on the enamel surface in vivo: a morphological and non-contact profilometric study. Oper Dent. 2008;33(2):127–134. | ||
Faraoni-Romano JJ, Turssi CP, Serra MC. Concentration-dependent effect of bleaching agents on microhardness and roughness of enamel and dentin. Am J Dent. 2007;20(1):31–34. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.