Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 11 » Issue 1
Physical exercise is effective in preventing cigarette smoke-induced pulmonary oxidative response in mice
Authors Nesi R, Soares de Souza P, Pedroso dos Santos G, Thirupathi A, Menegalli B, Lock Silveira PC, Acordi da Silva L, Valença SDS, Aurino de Pinho R
Received 7 August 2015
Accepted for publication 21 December 2015
Published 22 March 2016 Volume 2016:11(1) Pages 603—610
DOI https://doi.org/10.2147/COPD.S93958
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Russell
Renata Tiscoski Nesi,1 Priscila Soares de Souza,1 Giulia Pedroso dos Santos,1 Anand Thirupathi,1 Bruno T Menegali,1 Paulo Cesar Lock Silveira,1 Luciano Acordi da Silva,1 Samuel Santos Valença,2 Ricardo Aurino Pinho1
1Laboratory of Exercise Biochemistry and Physiology, Graduate Program in Health Sciences, Health Sciences Unit, Universidade do Extremo Sul Catarinense, Criciúma, SC, Brazil; 2Biomedical Science Institute, Federal University of Rio de Janeiro, Rio de Janeiro, Brazil
Abstract: Reactive oxygen species (ROS) are important in the pathogenesis of pulmonary injury induced by cigarette smoke (CS) exposure, and physical exercise (Ex) is useful in combating impaired oxidative process. We verified the preventive effects of Ex on lung oxidative markers induced by smoking. In this study, 36 mice (C57BL-6, 30–35 g) were split into four groups: control, CS, Ex, and CS plus Ex. Ex groups were given prior physical training in water (2×30 min/d, 5 days/wk, 8 weeks). After training, the CS groups were subjected to passive exposure to four cigarettes, 3 × per day, for 60 consecutive days. After 24 hours from the last exposure, CS animals were sacrificed, and lung samples were collected for further analysis. Left lung sample was prepared for histological analysis, and right lung was used for biochemical analysis (superoxide, hydroxyproline, lipid peroxidation [thiobarbituric acid reactive species], protein carbonylation [carbonyl groups formation], superoxide dismutase [SOD], catalase [CAT], and glutathione peroxidase [GPx] activities). Group comparisons were evaluated by analysis of variance (ANOVA). Results were expressed as mean ± standard deviation, with P<0.05 considered significantly different. Preventive Ex impeded histological changes and increased the enzymatic defense system (SOD and GPx) by reducing oxidative damage in lipids and proteins. This preventive effect of prior physical Ex alleviates damage caused by CS exposure.
Keywords: exercise, cigarette smoke, COPD, free radicals, oxidative stress
Introduction
Cigarette smoke (CS), a complex mixture of more than 7,000 chemical compounds and oxidants according to the Department of Health and Human Services of USA,1 is an important etiological factor in the development of pulmonary disease as the major cause of chronic obstructive pulmonary disease (COPD).2,3 Reactive oxygen species (ROS) and reactive nitrogen species (RNS), and carbon-centered radicals are constituents in both the tar and gas phases of smoke.3 These are produced by reactive compounds present in smoke, which include reactive aldehydes, quinones, and benzo(a)pyrene,4 inducing oxidative burden by disturbing the oxidant–antioxidant balance, thereby causing lung inflammatory cellular damage.5 This process is reversed by an efficient defense system that acts by activating antioxidant enzymes, endogenous antioxidants, or by antioxidant supplementation.4,6
Although the benefits of physical exercise (Ex) on pulmonary function are limited, physical training is important in preventing and treating pulmonary diseases. Moderate training improves lung immune system7 and cardiopulmonary capacity.8 In addition, regular aerobic physical Ex can improve the defense system against excessive ROS production in pulmonary disease.
Oxidative stress caused by cigarette smoke results in alveolar wall deterioration leading to airway enlargement.9 Moreover, increased oxidative stress can trigger proinflammatory cytokines, affecting smoker’s lungs; it is involved, under inflammatory conditions, in tissue injury deterioration (such as debilitation of airway epithelium), thus playing a role in the pathogenesis of inflammatory lung diseases.10–12
Parameters of oxidative stress on pulmonary function are altered following inhalation of CS.13,14 Other studies show several mechanisms with preventive or therapeutic effects available to reduce cigarette damage.5,15–17 To completely assess the preventive effects of Ex on lung inflammation induced by CS, the effects of prior Ex combat oxidative damage or redox unbalance induced by CS still need to be studied. Thus, in this study, we hypothesize that physical training could decrease negative effects of CS on oxidative stress parameters in lungs of mice after long-term CS exposure.
Material and methods
Reagents
All the reagents were purchased from Sigma Chemicals (St Louis, MO, USA).
Sample
Thirty-six male, 3- to 4-month-old C57BL-6 mice, weighing 30–35 g were obtained from Universidade do Extremo Sul Catarinense (Criciúma, Santa Catarina, Brazil). The mice were randomly assigned to four groups (n=9 each group) based on, C (control), CS (cigarette smoke), Ex (exercise), and Ex + CS (exercise plus cigarette smoke). Food and water were available ad libitum. The room was kept at 70% humidity, 20°C±2°C and 12 hours light/dark cycle. The study and all the procedures were approved by the institutional committee of ethics in research resolution (Comitê de ética em pesquisa [CEP] da Universidade do Extremo Sul Catarinense – UNESC) and followed National and International standards of care for animal welfare.
Ex protocol
All groups were exercised in a swimming pool specially designed for mice. Animals were given an adaptation period (10 min/d) of 1 week to adapt to new environment with water temperature of 32°C. After the adaptation, the Ex groups began the swimming program (5 d/wk) for 8 weeks, lasting 2×30 minutes with 5 minutes interval. The untrained animals were kept in a swimming pool without water for 8 weeks, similar to the Ex-trained groups.
CS exposure and sacrifice
Animals were exposed to 12 commercial filtered cigarettes (tar 8 mg and nicotine 0.6 mg) per day, 7 days/week for 60 days, as previously described.18,19 Briefly, animals were placed in an inhalation chamber (40 cm long, 30 cm wide, and 25 cm high) inside an exhaustion chapel. A cigarette was coupled to a plastic 50 mL syringe, so that puffs were aspirated and subsequently injected into the exposure chamber. Animals were kept in this smoke-air condition (±3%) for 6 minutes with 1 minute of exhaustion between each cigarette, resulting in 72 minutes of CS exposure from 12 cigarettes with a total particulate matter of 300 mg/m3 in the chamber. After 24 hours from the last exposure, animals were subjected to euthanasia by cervical displacement, and the lungs were immediately removed, processed, sampled, stored, and frozen at −80°C until analysis.
Physical Ex intensity
Blood lactate (BL) level was determined after the last session of Ex by analyzing 50 μL of tail capillary blood, using a commercial kit according to manufacturer’s instructions (Roche, Mannheim, Germany).
Histological assessment
The left lung was perfused, through the main bronchus, with a fixative solution (10% neutral-buffered formalin) at a pressure of 25 cm H2O. After that, it was immersed immediately and maintained in fixative solution for 12–24 hours for paraffin inclusion. Tissue blocks placed in formalin, dehydrated in a graded series of ethanol, and embedded in paraffin were cut into 4 μm-thickness serial sections and were stained with hematoxylin–eosin staining.20
Hydroxyproline assay
Hydroxyproline content in the sample was determined by a colorimetric method as described earlier.21 Initially, the sample (30 mg) was hydrolyzed in 1 mL of HCl (6N) and 250 μl of the sample was incubated with 500 mL of 0.05 M chloramine-T for 20 minutes at room temperature in test tubes. After that, the mixture was incubated with 500 mL of 3.17 M perchloric acid for 5 minutes at room temperature. Finally, the mixture was incubated with 500 mL of 20% dimethylbenzaldehyde for 20 minutes at 60°C. The color developed by the reaction was read spectrophotometrically at 557 nm, and the results were expressed as mg of hydroxyproline per gram of tissue.
Superoxide anion assay
Levels of anion superoxide were measured following a previously described method.22 Submitochondrial particles (SMP) of the lung were isolated by differential centrifugation, and the level of superoxide was estimated by measuring adrenaline oxidation in a buffer containing SMP, succinate (as an electron transfer chain initiator), and catalase. The color developed by the reaction was read spectrophotometrically at 780 nm and expressed as nmol/min/mg protein.
Antioxidant enzymes activities
Superoxide dismutase (SOD) activity was determined according to the method described by McCord and Fridovich.23 Enzymatic activity was estimated by adrenaline auto-oxidation inhibition, read at 480 nm in a spectrophotometer. Enzyme activity was expressed as U/mg protein. Catalase (CAT) activity was determined according to the method described by Aebi.24 The quadriceps muscle was sonicated in a 50 mM phosphate buffer solution, and the resulting suspension was centrifuged at 3,000× g for 10 minutes. The supernatant was used for the enzyme assay. CAT activity was measured using the rate of decrease in hydrogen peroxide absorbance at 240 nm and expressed as U/mg protein. Glutathione peroxidase (GPx) activity was measured by monitoring the oxidation of nicotinamide adenine dinucleotide phosphate (NADPH) at 340 nm in the presence of H2O2.25 Enzyme activity was expressed as U/mg protein.
Oxidative damage markers
Measurement of 2-thiobarbituric acid reactive substances (TBARS) was performed using the method described by Draper and Hadley.26 Briefly, the lung tissue (10 mg/tissue) was mixed with 1 mL of 10% trichloroacetic acid and 1 mL of 0.67% thiobarbituric acid; subsequently, these mixtures were heated in a boiling water bath for 15 minutes. TBARS were determined by measuring absorbance at 532 nm, and the results are expressed as nmol TBARS/mg protein. Oxidative damage to proteins was determined according to the method described by Levine et al.27 Protein carbonyl content was measured by the formation of protein hydrazone derivatives using 2,4-dinitrophenylhydrazine (DNPH). These derivatives were sequentially extracted with 10% (v/v) trichloroacetic acid followed by treatment with ethanol/ethyl acetate, 1:1 (v/v) and re-extraction with 10% trichloroacetic acid. The resulting precipitate was dissolved in 6 M urea hydrochloride. The difference of the absorbance values between DNPH samples and the blank was used to calculate the nanomoles of DNPH incorporated per milligram of protein. Results are shown for each sample read at 370 nm in a spectrophotometer.
Protein content
Protein content of lung homogenates was assayed using bovine serum albumin as a standard, according to the method described by Lowry et al.28 Folin phenol reagent (phosphomolybdic–phosphotungstic reagent) was added to bind to the protein. The bound reagent was slowly reduced, changing from yellow to blue. Absorbance was read at 750 nm.
Statistical analyses
Means ± SD were calculated, and multiple comparisons were performed by using one-way analysis of variance (ANOVA) with Tukey post hoc tests, and P-value <0.05 was considered significant. Statistical Package for the Social Sciences (SPSS; SPSS Inc., Chicago, IL, USA) version 18.0 was used for statistical analysis.
Results
Effects of physical Ex
Our results showed lactate levels of 6.9±0.7 mmol/L in the untrained C group and 4.2±0.4 mmol/L in trained group in the final stage of the last day of Ex in relation to pre-exercise (2.9±0.3 and 2.2±0.5 nmol/L, respectively).
Histological effect of CS exposure and/or physical Ex on lungs
The lungs of C mice were histologically normal, with parenchyma consisting of alveoli connected to alveolar ducts, separated from each other only by thin alveolar septa (Figure 1A), whereas, in the mice exposed to CS a marked macrophage inflammatory infiltration was observed in airspaces in many alveoli as well as areas with disruption of alveolar septa and enlarged airspace (Figure 1B). CS exposure induced histological changes, and physical training impeded this progression, attenuating the macrophage infiltration in airspaces (Figure 1C) and ameliorating the aspects of the elastic fibers (Figure 1F).
Effect of CS exposure and/or physical Ex on lung hydroxyproline content
The comparison of hydroxyproline contents among the four groups is shown in Figure 2. CS exposure produced a significant increase in the hydroxyproline levels as compared to the C group (P<0.05). The physical training did not alter the values of hydroxyproline. However, it impeded the increase in values when exposed to CS (P<0.05).
Effect of CS exposure and/or physical Ex on lung superoxide production
As seen in Figure 3, CS exposure produced a significant increase in the superoxide production as compared to the C group (P<0.05). Physical training did not alter these values and impeded the increase in values when exposed to CS (P<0.05).
Effect of CS exposure and/or physical Ex on lung antioxidant enzymes (SOD, CAT, and GPx)
As seen in Figure 4A, the SOD activity was higher in the lung homogenates (P<0.05) in CS group when compared to C group. However, Ex plus CS group increased the SOD production in relation to C and when compared with CS group (P<0.05). The CAT activity (Figure 4B) was also increased in the CS group (P<0.05), whereas in the Ex groups, exposure did not alter the values in relation to C (P<0.05). The GPx activity (Figure 4C) was increased in Ex and Ex plus CS groups in relation to C and CS groups (P<0.05).
Effect of CS exposure and/or physical Ex on oxidative damage markers
As seen in Figure 4A and B, the CS group show an elevated TBARS level and carbonyl groups content in lung homogenates (P<0.05) when compared to the C group, and these values were reduced with preventive physical training (P<0.05).
Discussion
Preventive physical training reduced pulmonary lesions and oxidative stress markers in lungs of rats exposed to CS. There is some substantial evidence to support the positive effect of physical Ex as a therapeutic resource in treating pulmonary diseases.16,29–32 However, no research has verified the effects of preventive physical training on oxidative parameters in the respiratory disease induced by CS. This is the first study to show that regular physical Ex prevents the progression of alterations in lung parenchyma and oxidative damage induced by CS.
In addition to many other factors, effects of physical training are associated with the intensity of the Ex. Anaerobic threshold is a term referring to the oxygen consumption during high-intensity Ex above which the lactate production rate exceeds that of lactate removal, thus causing increased lactate levels in tissues.33 After Ex, when the lactate levels are reduced, there is an improvement of the physical conditioning due to the muscle oxidative metabolism. The data indicated significantly higher lactate levels (P<0.05) in untrained animals than in the Ex group – evidence of Ex-induced muscle adaptation.
CS is an inflammatory agent, and several studies have shown that this exposure provokes alterations in lung architecture34 and also increases the hydroxyproline content.19 This evidence was in accordance with our findings; however, the prior physical training was not sufficient in reducing both histological alterations and collagen content, although it impeded these alterations from progressing.
The pulmonary morphological alterations in our study are probably associated with several factors, although ROS and oxidative stress play an important role in these processes.33 Oxidative stress is involved in the progression of lung tissue injury induced by CS and alters the role of airway/airspace epithelium.10,13 Pulmonary damage appears to be a consequence of a primary inflammatory lesion characterized by accumulation of alveolar macrophages and neutrophils in the lower respiratory tract.34 Activated inflammatory cells, which accumulate in the lower airways, release harmful amounts of ROS that result in parenchymal injury, and interstitial and alveolar damage.9,11,20,35
Ex, therefore, is an important agent in preventing and treating pulmonary disease. However, the training model did not significantly alter the histological parameters but impeded the lesion progression. In this case, the preventive effect of Ex can be associated with resistance of tissues to smoke-induced morphological alterations. We suggest that the resistance of tissues to morphological damage is related to the antioxidant system upregulation caused by preventive exercise. This hypothesis is sustained in other studies that show the direct effect of physical training on lung function and lung parenchyma.5–7,33,36 Although the histological results obtained are not clear, this current study shows a positive effect of preventive training on oxidative stress parameters.
The results show a significant increase in the oxidant production and oxidative damage in the cigarette groups. These results are corroborated by other studies.17,19,33 However, the relevant information is that preventive physical training impeded markers (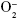 , SOD, CAT, and GPx) that were high. It is possible that the defense mechanisms are associated with an effective antioxidant system. The increased SOD activity observed in CS plus Ex group could directly attenuate lung injury by reducing superoxide concentrations in the lung extracellular matrix.5,31,37,38 This reduction may decrease stimulation of fibroblasts and diminish inflammatory cell recruitment.9,39 Although SOD activity stayed high after training, the same did not happen with the catalase activity. Thus, it is possible that other antioxidants act as defense mechanisms in the lung, such as the glutathione system. The glutathione system plays an important role in the pulmonary defense mechanism against attacks from free radicals diminishing the H2O2 content, principally by the action of GPx altering the balance of the pulmonary oxidant–antioxidant capacity.40 The results show an increase in the GPx activity in animals after training. Depending on the GPx level in the cell, it may represent an important resource against the pulmonary damage.29 In addition, several studies suggested that GSH content and GSH-dependent enzyme activities respond to training.28,41,42 This is because the submaximal physical training improved the ability to maintain the glutathione redox status in the tissue for a long time. In addition, the liberation of ROS from cigarettes has the potential to induce nuclear factor (κB) (NF-κB) through several distinct mechanisms including respiratory burst, which recruit the inflammatory cells.19 This process initiates an event cascade namely ROS generation, activation of cytokines and factors of transcription, and induces protein tyrosine kinases, all of which are an important signal for injury by CS.19 In compensation, the aerobic physical training from low or moderate intensities may alter the composition and activity of the IκBα/NF-κB pathway43 and reduce the expression of NF-κB by inflammatory cells in the lung perivascular and alveolar parenchyma compartments.7 Moderate Ex training program increases the NF-κB inhibitory proteins (IκBα and IκBβ), and this is probably associated, among other factors, with the increase in expression and activity of antioxidants enzymes and consequent reduction of oxidative stress.43 It is possible that oxidative stress and/or an imbalance in antioxidant–pro-oxidant status may directly stimulate the activity of IKK (inhibitors of NF-κB kinase), and alternatively, oxidative stress may affect the proteasome enzymatic activity that leads to the activation of NF-κB.10 Therefore, the increase in the antioxidant system and consequent reduction of the oxidative stress induced by physical training observed in the present study can be a main factor in the protection of lung against the pulmonary injury provoked by CS.
, SOD, CAT, and GPx) that were high. It is possible that the defense mechanisms are associated with an effective antioxidant system. The increased SOD activity observed in CS plus Ex group could directly attenuate lung injury by reducing superoxide concentrations in the lung extracellular matrix.5,31,37,38 This reduction may decrease stimulation of fibroblasts and diminish inflammatory cell recruitment.9,39 Although SOD activity stayed high after training, the same did not happen with the catalase activity. Thus, it is possible that other antioxidants act as defense mechanisms in the lung, such as the glutathione system. The glutathione system plays an important role in the pulmonary defense mechanism against attacks from free radicals diminishing the H2O2 content, principally by the action of GPx altering the balance of the pulmonary oxidant–antioxidant capacity.40 The results show an increase in the GPx activity in animals after training. Depending on the GPx level in the cell, it may represent an important resource against the pulmonary damage.29 In addition, several studies suggested that GSH content and GSH-dependent enzyme activities respond to training.28,41,42 This is because the submaximal physical training improved the ability to maintain the glutathione redox status in the tissue for a long time. In addition, the liberation of ROS from cigarettes has the potential to induce nuclear factor (κB) (NF-κB) through several distinct mechanisms including respiratory burst, which recruit the inflammatory cells.19 This process initiates an event cascade namely ROS generation, activation of cytokines and factors of transcription, and induces protein tyrosine kinases, all of which are an important signal for injury by CS.19 In compensation, the aerobic physical training from low or moderate intensities may alter the composition and activity of the IκBα/NF-κB pathway43 and reduce the expression of NF-κB by inflammatory cells in the lung perivascular and alveolar parenchyma compartments.7 Moderate Ex training program increases the NF-κB inhibitory proteins (IκBα and IκBβ), and this is probably associated, among other factors, with the increase in expression and activity of antioxidants enzymes and consequent reduction of oxidative stress.43 It is possible that oxidative stress and/or an imbalance in antioxidant–pro-oxidant status may directly stimulate the activity of IKK (inhibitors of NF-κB kinase), and alternatively, oxidative stress may affect the proteasome enzymatic activity that leads to the activation of NF-κB.10 Therefore, the increase in the antioxidant system and consequent reduction of the oxidative stress induced by physical training observed in the present study can be a main factor in the protection of lung against the pulmonary injury provoked by CS.
In summary, the results suggest that prior physical Ex improves the pulmonary antioxidant defense mechanism and prevents emphysema profile of the lung in mice exposed to CS. The preventive effect of the Ex on this pathway is relevant because the oxidative damage is a crucial factor of emphysema development induced by CS exposure. Thus, our results showed that the lung protection induced by the physical training was effective in impeding the establishment of oxidative stress.
Acknowledgment
This research was supported by grants from CNPq/MCT (Brazil), CAPES/MEC (Brazil), and UNESC (Brazil).
Disclosure
The authors report no conflicts of interest in this work.
References
U.S. Department of Health and Human Services. A Report of the Surgeon General: How Tobacco Smoke Causes Disease: What It Means to You. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2010. | ||
Pryor WA, Stone K. Oxidants in cigarette smoke. Radicals, hydrogen peroxide, peroxynitrate, and peroxynitrite. Ann N Y Acad Sci. 1993;686:12–27. | ||
Church DF, Pryor WA. Free-radical chemistry of cigarette smoke and its toxicological implications. Environ Health Perspect. 1985;64:111–126. | ||
Barnes PJ. Mediators of chronic obstructive pulmonary disease. Pharmacol Rev. 2004;56(4):515–548. | ||
Stevenson CS, Koch LG, Britton SL. Aerobic capacity, oxidant stress, and chronic obstructive pulmonary disease – a new take on an old hypothesis. Pharmacol Ther. 2006;110(1):71–82. | ||
Halliwell B. Cigarette smoking and health: a radical view. J R Soc Health. 1993;113(2):91–96. | ||
Vieira RP, de Andrade VF, Duarte AC, et al. Aerobic conditioning and allergic pulmonary inflammation in mice. II. Effects on lung vascular and parenchymal inflammation and remodeling. Am J Physiol Lung Cell Mol Physiol. 2008;295(4):L670–L679. | ||
Celli B, Goldstein R, Jardim J, Knobil K. Future perspectives in COPD. Respir Med. 2005;99(Suppl B):S41–S48. | ||
Kode A, Yang SR, Rahman I. Differential effects of cigarette smoke on oxidative stress and proinflammatory cytokine release in primary human airway epithelial cells and in a variety of transformed alveolar epithelial cells. Respir Res. 2006;7:132. | ||
Rahman I, Adcock IM. Oxidative stress and redox regulation of lung inflammation in COPD. Eur Respir J. 2006;28(1):219–242. | ||
Boskabady MH, Gholami Mahtaj L. Lung inflammation changes and oxidative stress induced by cigarette smoke exposure in guinea pigs affected by Zataria multiflora and its constituent, carvacrol. BMC Complement Altern Med. 2015;15:39. | ||
Rastrick JM, Stevenson CS, Eltom S, et al. Cigarette smoke induced airway inflammation is independent of NF-κB signalling. PLoS One. 2013;8(1):e54128. | ||
Rahman I. Pharmacological antioxidant strategies as therapeutic interventions for COPD. Biochim Biophys Acta. 2012;1822(5):714–728. | ||
Valenca SS, Bezerra FS, Romana-Souza B, Paiva RO, Costa AM, Porto LC. Supplementation with vitamins C and E improves mouse lung repair. J Nutr Biochem. 2008;19(9):604–611. | ||
Lanzetti M, Bezerra FS, Romana-Souza B, et al. Mate tea reduced acute lung inflammation in mice exposed to cigarette smoke. Nutrition. 2008;24(4):375–381. | ||
Schneider CM, Hsieh CC, Sprod LK, Carter SD, Hayward R. Exercise training manages cardiopulmonary function and fatigue during and following cancer treatment in male cancer survivors. Integr Cancer Ther. 2007;6(3):235–241. | ||
Silva Bezerra F, Valenca SS, Lanzetti M, et al. Alpha-tocopherol and ascorbic acid supplementation reduced acute lung inflammatory response by cigarette smoke in mouse. Nutrition. 2006;22(11–12):1192–1201. | ||
Valenca SS, Silva Bezerra F, Lopes AA, et al. Oxidative stress in mouse plasma and lungs induced by cigarette smoke and lipopolysaccharide. Environ Res. 2008;108(2):199–204. | ||
Valenca SS, Castro P, Pimenta WA, et al. Light cigarette smoke-induced emphysema and NFκB activation in mouse lung. Int J Exp Pathol. 2006;87(5):373–381. | ||
Lanzetti M, da Costa CA, Nesi RT, et al. Oxidative stress and nitrosative stress are involved in different stages of proteolytic pulmonary emphysema. Free Radic Biol Med. 2012;53(11):1993–2001. | ||
Woessner JF Jr. The determination of hydroxyproline in tissue and protein samples containing small proportions of this imino acid. Arch Biochem Biophys. 1961;93:440–447. | ||
Poderoso JJ, Carreras MC, Lisdero C, Riobo N, Schopfer F, Boveris A. Nitric oxide inhibits electron transfer and increases superoxide radical production in rat heart mitochondria and submitochondrial particles. Arch Biochem Biophys. 1996;328(1):85–92. | ||
McCord JM, Fridovich I. Superoxide dismutase: an enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969;244:6049–6055. | ||
Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. | ||
Flohé L, Gunzler W. Assays of glutathione peroxidase. Methods Enzymol. 1984;105(1):114–121. | ||
Draper HH, Hadley M. Malondialdehyde determination as index of lipid peroxidation. Methods Enzymol. 1990;186:421–431. | ||
Levine RL, Garland D, Oliver CN, et al. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol. 1990;186:464–478. | ||
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193(1):265–275. | ||
Teixeira KC, Soares FS, Rocha LG, et al. Attenuation of bleomycin-induced lung injury and oxidative stress by N-acetylcysteine plus deferoxamine. Pulm Pharmacol Ther. 2008;21(2):309–316. | ||
Toledo AC, Magalhaes RM, Hizume DC, et al. Aerobic exercise attenuates pulmonary injury induced by exposure to cigarette smoke. Eur Respir J. 2012;39(2):254–264. | ||
Fisher-Wellman K, Bloomer RJ. Acute exercise and oxidative stress: a 30 year history. Dyn Med. 2009;8:1. | ||
Bloomer RJ, Fisher-Wellman K. The role of exercise in minimizing postprandial oxidative stress in cigarette smokers. Nicotine Tob Res. 2009;11(1):3–11. | ||
van der Vaart H, Postma DS, Timens W, ten Hacken NH. Acute effects of cigarette smoke on inflammation and oxidative stress: a review. Thorax. 2004;59(8):713–721. | ||
Domagala-Kulawik J. Effects of cigarette smoke on the lung and systemic immunity. J Physiol Pharmacol. 2008;59(Suppl 6):19–34. | ||
Micale RT, La Maestra S, Di Pietro A, et al. Oxidative stress in the lung of mice exposed to cigarette smoke either early in life or in adulthood. Arch Toxicol. 2013;87(5):915–918. | ||
Yu YB, Liao YW, Su KH, et al. Prior exercise training alleviates the lung inflammation induced by subsequent exposure to environmental cigarette smoke. Acta Physiol (Oxf). 2012;205(4):532–540. | ||
Flo C, Lopes FD, Kasahara DI, et al. Effects of exercise training on papain-induced pulmonary emphysema in Wistar rats. J Appl Physiol. 2006;100(1):281–285. | ||
Moodie FM, Marwick JA, Anderson CS, et al. Oxidative stress and cigarette smoke alter chromatin remodeling but differentially regulate NF-κB activation and proinflammatory cytokine release in alveolar epithelial cells. FASEB J. 2004;18(15):1897–1899. | ||
Bowler RP, Crapo JD. Oxidative stress in airways: is there a role for extracellular superoxide dismutase? Am J Respir Crit Care Med. 2002;166(12 Pt 2):S38–S43. | ||
Rahman I, MacNee W. Lung glutathione and oxidative stress: implications in cigarette smoke-induced airway disease. Am J Physiol. 1999;277(6 Pt 1):L1067–L1088. | ||
Elokda AS, Nielsen DH. Effects of exercise training on the glutathione antioxidant system. Eur J Cardiovasc Prev Rehabil. 2007;14(5):630–637. | ||
Foronjy RF, Mirochnitchenko O, Propokenko O, et al. Superoxide dismutase expression attenuates cigarette smoke- or elastase-generated emphysema in mice. Am J Respir Crit Care Med. 2006;173(6):623–631. | ||
Kramer HF, Goodyear LJ. Exercise, MAPK, and NF-κB signaling in skeletal muscle. J Appl Physiol. 2007;103(1):388–395. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.





