Back to Journals » Clinical Ophthalmology » Volume 16
Photodynamic Therapy-Induced Acute Exudative Maculopathy (PAEM): Prevalence, Impact and Management Strategies
Authors Sumnicht AJ, Chalam KV , Sierpina DI
Received 28 April 2022
Accepted for publication 31 August 2022
Published 27 September 2022 Volume 2022:16 Pages 3145—3154
DOI https://doi.org/10.2147/OPTH.S359302
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Scott Fraser
Andrew J Sumnicht, Kakarla V Chalam, David I Sierpina
Loma Linda University Eye Institute, Loma Linda University, Loma Linda, CA, USA
Correspondence: David I Sierpina, 11370 Anderson St., Suite #2900, Loma Linda, CA, 92354, USA, Tel +1 909-558-2182, Fax +1 909-558-2506, Email [email protected]
Abstract: Photodynamic therapy (PDT) has a niche role in treating various choroidal pathologies. PDT-induced acute exudative maculopathy (PAEM) is an uncommon complication of PDT that results in exudative retinal detachment and mild to severe decrease in vision. Successful management strategies include observation, local or systemic corticosteroids, and intravitreal anti-vascular endothelial growth factor (anti-VEGF) injections. Most cases return to visual acuity near baseline. This review summarizes what is known about PAEM to date including etiology, prevalence, management strategies, and outcomes. We conclude that management of PAEM must take into consideration various patient-specific factors. Treatment with corticosteroids or anti-VEGF agents may expedite time to recovery, though lack of randomized controlled trials preclude firm conclusions regarding a standardized approach to managing this complication of PDT.
Keywords: photodynamic therapy, exudative maculopathy, serous detachment
Introduction
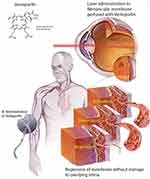
Photodynamic therapy (PDT) is a treatment used in ophthalmology for various choroidal pathologies. Selected patients receive an intravenous infusion of a photosensitizing porphyrin-based agent and then target lesions are exposed to a set wavelength of light at pre-specified parameters (Figures 1 and 2). The standard full fluence technique developed in the TAP trial involves 83 seconds of exposure to 689nm light at 50 J/cm2 and an irradiance of 600mW/cm2 five minutes following a 10-minute infusion of 6mg/m2 body surface area of Verteporfin (Visudyne, CIBA Vision Corp, Duluth, GA) at previously identified target lesions in the patient’s choroid.1 An example of a treatment plan using indocyanine green angiography-guided PDT to treat PCV can be found in Figure 3.
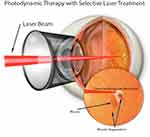 |
Figure 2 Graphic depiction of light focused on a choroidal neovascular membrane in the application of photodynamic therapy. |
PDT underwent extensive investigation in the 1980s, initially as a novel technique used to treat malignant tumors.2 Its earliest successful application to the eye was reported in 1984 in seven patients with malignant uveal melanoma.3 PDT was developed on the basis of using porphyrin-based intravenous solutions as photosensitizers that concentrate in tissues, then activating the molecule using a specific wavelength of light to induce thrombosis in the target area.4 Its application to amelanotic iris melanomas was further studied in a rabbit model in 1985,5 and it was shown to be effective in the treatment of preretinal neovascularization in a rabbit model in 1991.6 PDT eventually underwent Phase II clinical trials in treating subfoveal choroidal neovascular membrane (CNVM) with promising results in 1997.7 The TAP trial further demonstrated the benefit of PDT in patients with neovascular age-related macular degeneration (nAMD) with a predominantly classic form of CNVM.1 Ophthalmologic application of PDT typically stems from protocols developed from the TAP trial and the verteporfin in PDT (VIP) study group.8 A half fluence energy application (25 J/cm2) has been favored in recent investigations and clinical practice, and is particularly well-studied in central serous chorioretinopathy (CSCR)9,10 due to an association with better vision retention and lower risk of severe and permanent vision loss from macular ischemia.11
PDT has been a key treatment in the setting of pachychoroid conditions and benign vascular intraocular tumors by itself or in combination with anti-vascular endothelial growth factor (VEGF) intravitreal injections.12–14 PDT has demonstrated efficacy in the treatment of a variety of conditions including CSCR, polypoidal choroidal vasculopathy (PCV), nAMD, choroidal hemangioma, retinal capillary hemangioma, and CNVM related to pathologic myopia.1,11,14–17
In particular, the use of PDT in the treatment of PCV has been the subject of intense scrutiny over the last decade as this condition’s prevalence across demographic groups and misidentification as typical nAMD were increasingly recognized.18–21 The LAPTOP study showed superior visual outcomes using intravitreal ranibizumab given monthly for 3 months followed by pro re nata (PRN) dosing compared with PDT treatments alone in patients with PCV.13 Combination therapy was evaluated in the EVEREST I and EVEREST II studies, which demonstrated a superior response to PRN intravitreal ranibizumab in combination with PDT compared to PRN ranibizumab alone in terms of both polyp regression, as well as visual acuity.14,22 In contrast, there was an 85% rate of improved vision in the PLANET study where PCV was treated with treat and extend intravitreal aflibercept alone, and there was no clear benefit to using PDT as rescue therapy when injections failed.23
PDT carries a risk of three to five days of photosensitivity along with the possibility of nausea, dry eye, flank pain and joint pain. Vision-threatening complications of PDT include choroidal ischemia, RPE tear, RPE atrophy, secondary CNVM, subretinal hemorrhage, and PDT-induced acute exudative maculopathy (PAEM).24,25
PAEM is a complication that is uncommonly reported, though when it does occur may result in a significant acute decrease in visual acuity. PAEM has been fairly well discussed in the literature, though many studies are descriptive, and reports focused on treatment of PAEM are low in power due to the low prevalence of the condition. This review aims to discuss what is known about PAEM, outcomes of treatments for PAEM in the literature, and calls for future investigation on the topic of PAEM.
PDT-Induced Acute Exudative Maculopathy
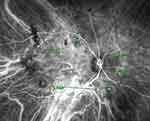
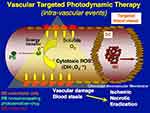
PAEM is described as a transient increase in subretinal fluid within the first several days following PDT, usually detected when the patient experiences an acute decrease in vision following treatment. It is proposed that increased subretinal exudation following PDT occurs via excessive action of its intended mechanism. An acute increase in subretinal exudation one day following PDT in a patient with PCV can be seen in Figure 4. Schmidt-Erfurth et al demonstrated that PDT obliterates vessels in the choriocapillaris in the treatment areas through an inflammatory and pro-thrombotic mechanism.26 Cytokines including VEGF and reactive oxygen species are released and lead to increased local vascular permeability, thrombosis with subsequent ischemia and further inflammation (Figure 5).26–28 It has also been proposed by Husain et al that PDT causes retinal pigment epithelium pump dysfunction which can further exacerbate fluid accumulation.29
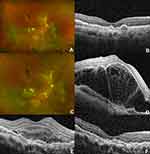 |
Figure 4 Patient from Figure 3 with a diagnosis of polypoidal choroidal vasculopathy. (A) Fundus photograph and (B) optical coherence tomography (OCT) of the macula of the right eye prior to photodynamic therapy (PDT). (C) Fundus photo one day after PDT. A blunted foveal reflex consistent with subretinal fluid is present. (D) OCT of the macula one day after PDT demonstrating severe serous retinal detachment, at which point treatment was initiated with 1 mg/kg oral prednisone daily. (E) OCT of the macula of the right eye with improvement in subretinal fluid at day 8. 1 cc of 40mg/cc Kenalog was injected into the sub-tenon space at this visit. (F) OCT of the macula of the right eye demonstrating resolved subretinal fluid at day 16 after PDT. |
Following treatment of PAEM, histopathologic analysis and optical coherence tomographic angiography (OCT-A) provide evidence of recanalization of blood vessels following PDT that is complete between one week and one month.26,36 This may be one mechanism by which resolution of subretinal fluid resolves with observation alone in some reports.
Prevalence and Disease Characteristics
PAEM occurs in an estimated 1.4–30.4% of patients treated with PDT.33,34,37 It has been reported to occur in the setting of chronic CSCR, choroidal hemangioma, retinal capillary hemangioma, CNVM, nAMD, and PCV with the highest incidence reported in CSCR (30.4%) followed by PCV (8.45%) and nAMD (6.67%).17,30–34,36 In a 2020 report, Manayath et al described the risk factors for PAEM in a large cohort of 177 PDT treatments in 155 eyes. These include age over 65 years, PCV, spot size >3.5 mm, pre-treatment best corrected visual acuity (BCVA) 20/40 or better, first time treatment and reduced-fluence PDT.34 However, it must be noted that although only 1/8 patients with PAEM in this study received full-fluence treatment, the lack of reported baseline data regarding how many total patients in this cohort were treated with standard-fluence PDT, as well as the relative rarity of standard-fluence treatments today compared with reduced-fluence treatments allows for the possibility that PAEM may be as common or more common in full-fluence PDT. A 2022 study by Fernandez-Vigo et al examined these risk factors in 92 eyes of 75 patients with CSCR, all treated with half-fluence PDT and a spot size of 4 mm ± 500 μm and found no statistically significant risk factors for developing PAEM in their cohort.36 They did report that PAEM was associated with worse baseline VA and flow voids on OCT-A, and that there was no association with axial length or subfoveal choroidal thickness.
Criteria for diagnosis of PAEM has not been established, therefore the incidence may be over- or under-stated in the literature depending on tomographic values established by authors. Authors rely primarily on OCT to establish diagnosis, though with varying criteria. Manayath et al noted PAEM to be diagnosed at a mean of 2.9 days with mean central macular thickness (CMT) increase of 253 µm beyond baseline, though they did not establish criteria for diagnosis beyond describing an increased CMT from pre-PDT measurement in the setting of clinically worsening vision or examination findings.34 Fernandez-Vigo et al used a CMT increase of 50 µm at three days post-PDT to define PAEM in CSCR patients, with decreasing incidences reported at thresholds of 100 and 200 µm increased CMT.37 In this cohort, 25% of patients with PAEM had a CNVM compared with 8.4% of the control group.37 The extraordinarily high incidence of PAEM in CSCR reported here (30.4%) compared to Manayath et al (1.19%) is likely explained by the low CMT threshold for diagnosing PAEM, as well as the use of a large spot size (4 mm ± 500 μm) in all patients. Based on this definition of PAEM, similar recovery was noted in the patients with PAEM versus the control group using observation alone at one and three month follow up.37 Di Nicola et al report a “PDT-related transient exudative response” in 4/17 (24%) of eyes with retinal hemangioblastomas treated with PDT, without reported CMT or initiation of any treatment beyond observation.15 Therefore, further observational studies in patients receiving PDT for any indication along with well-defined criteria and/or staging for PAEM will be useful in elucidating the true incidence in various conditions. It is reasonable to speculate that many patients may have a small increase in CMT after PDT that is not visually significant, but they are not being captured because it is not standard practice to perform OCT within three days of PDT in asymptomatic patients.
OCT-A is less accessible than OCT, though may be useful in diagnosing and monitoring PAEM. A localized darkening on OCT-A, described as a flow void, occurs in the choriocapillaris at the treatment area of PDT. An early increase in flow voids beyond the target treatment may occur at a higher rate in patients who develop PAEM.37 Recanalization of these flow voids is proposed to occur by one month following treatment and may be correlated to resolution of subretinal fluid.26,36,37
In a fluorescence pharmacokinetics study by Debefve et al, aspirin was co-administered with verteporfin in chicken embryos to assess the effects of cyclo-oxygenase inhibition on vascular permeability following PDT. Enhancement of vascular leakage was found in this setting, representing another possible risk factor in patients taking aspirin or similar medications.38 Further study is warranted to identify medications that may increase risk of developing PAEM.
Prognosis
A review of diagnosed and treated cases of PAEM can be found in Table 1. The degree of acute vision loss from PAEM has been reported to be as mild as 20/30 to as severe as no light perception vision following PDT. Resolution of exudative retinal detachment has been documented to occur within two to six weeks for most cases with a final Snellen equivalent of 20/60 BCVA or better in most cases, and most cases resolve near pre-PDT BCVA with resolution of subretinal fluid. One outlier case of PAEM in the setting of retinal capillary hemangioma was noted to require vitrectomy as part of treatment and a total period of one year to recover from no light perception to 20/500 BCVA.17 Fernandez-Vigo et al observed that all patients meeting their criteria for PAEM had similar recoveries using observation alone, except for the patients who had CNVM diagnosed by OCT-A who received an anti-VEGF injection.
 |
Table 1 Comparison of Treatments Used for Photodynamic Therapy-Induced Acute Exudative Maculopathy (PAEM) |
Management Strategies
Due to the underlying etiology of PAEM, it has been recommended that the treatment of complications arising from PDT be aimed at targeting inflammation.39 This seems to be the rationale for the use of periocular and systemic steroids in PAEM.32–34 Steroids administered systemically allow for the greatest concentrations in the choroidal circulation which is the site of this pathology in PAEM. However, in the setting of pachychoroid conditions there is a reported association between endogenous steroid concentrations and pathologic thickening of the choroid.40 Treatment of PAEM involving systemic steroids should be weighed in light of the potential for disease exacerbation and systemic side effects, though ease of discontinuation of systemic steroids is an advantage over periocular or intraocular administration. Studies of suprachoroidal administration of triamcinolone have led to recent Food and Drug Administration (FDA) approval of this route, which may allow for better access to the site of pathology in PAEM with a better side effect profile than other options for intraocular treatment.41,42 Figure 4 demonstrates resolution in subretinal fluid in a patient with PAEM in the setting of PCV managed with oral steroids and sub-Tenon triamcinolone acetonide.
Anti-VEGF administration has also been employed for the treatment of PAEM,17,31,33,34 and is reasonable considering reports of elevated VEGF expression in the setting of PDT-induced ischemia.28 This treatment may reduce the time to resolution of subretinal fluid and visual recovery compared to observation (Table 1), though reports directly comparing these options are not available. Precaution is advised for the use of anti-VEGF agents in patients at high risk for arteriothrombotic events as systemic anti-VEGF levels are known to increase following intravitreal injection of these agents.43–45
Lastly, observation has been shown to be an effective management strategy particularly specific to PAEM in the setting of CSCR.30,36 This strategy has not been reported in other conditions, though given the evidence of vascular recanalization following PDT in one to four weeks and a recovery period that is similar between PAEM in CSCR and the other described conditions,26,37 it is unclear whether adding treatment with anti-VEGF or corticosteroids provides a true benefit over observation alone.
Data describing the various treatments of this rare complication are limited, precluding firm conclusions regarding relative safety and efficacy of each option. With respect to safety, high dose systemic steroids are not innocuous, especially in patients with underlying systemic co-morbidities such as hypertension, which has a known association with PCV.46 The risks should be carefully weighed in all patients prior to initiation of systemic steroids due to the multitude of systemic side effects, particularly in cases in which there may be little clear benefit to the patient. Use of topical difluprednate mitigates this risk, and hourly application led to resolution of PAEM after very minimal fluence (12 J/cm2) PDT treatment of nAMD in a case described by Mammo and Farooghian.33 The risk-benefit ratio of systemic steroids compared to other treatment modalities is not well-defined, and further study in this area is warranted.
Conclusion
PDT is a generally safe and effective treatment for various choroidal pathologies. Despite the advent of anti-VEGF intravitreal injections, PDT still has a significant role in clinical practice today. Clinicians that use PDT should be aware of the incidence, risk factors, and management strategies for PAEM should it arise in their patients. Criteria, staging, and best practice guidelines for PAEM should be developed by professional societies to aid in diagnosis and management of this complication. PAEM should be monitored with serial OCT, examinations, and OCT-A if available until resolution of exudation is achieved. Further studies are needed to better elucidate incidence of PAEM in various conditions and to assess the true efficacy of treatment versus observation alone.
Abbreviations
PCV, polypoidal choroidal vasculopathy; nAMD, neovascular age-related macular degeneration; ICGA, indocyanine green angiography; OCT, optical coherence tomography; PED, pigment epithelial detachment; PDT, photodynamic therapy; VEGF, vascular endothelial growth factor; PAEM, PDT-induced acute exudative maculopathy; STTA, sub-Tenon triamcinolone acetonide; BCVA, best corrected visual acuity; mg, milligrams; CSC, central serous chorioretinopathy; CNVM, choroidal neovascular membrane; BCVA, best corrected visual acuity; µm, micrometers; OCT-A, optical coherence tomographic angiography; CMT, central macular thickness; mm, millimeters.
Acknowledgments
The authors would like to thank the Department of Ophthalmology at Loma Linda University and the Department Chair Dr. Michael Rauser, MD for his support of research endeavors.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Treatment of age-related macular degeneration with photodynamic therapy (TAP) Study Group. Photodynamic therapy of subfoveal choroidal neovascularization in age-related macular degeneration with verteporfin: one-year results of 2 randomized clinical trials–TAP report. Arch Ophthalmol. 1999;117(10):1329–1345. doi:10.1001/archopht.117.10.1329
2. Cai WM, Yang Y, Zhang NW, et al. Photodynamic therapy in the management of cancer: an analysis of 114 cases. Adv Exp Med Biol. 1985;193:13–19.
3. Tse DT, Dutton JJ, Weingeist TA, et al. Hematoporphyrin photoradiation therapy for intraocular and orbital malignant melanoma. Arch Ophthalmol. 1984;102(6):833–838. doi:10.1001/archopht.1984.01040030653011
4. Henderson BW, Dougherty TJ, Malone PB. Studies on the mechanism of tumor destruction by photoradiation therapy. Prog Clin Biol Res. 1984;170:601–612.
5. Gomer CJ, Jester JV, Razum NJ, et al. Photodynamic therapy of intraocular tumors: examination of hematoporphyrin derivative distribution and long-term damage in rabbit ocular tissue. Cancer Res. 1985;45(8):3718–3725.
6. Wilson CA, Saloupis P, Hatchell DL. Treatment of experimental preretinal neovascularization using photodynamic thrombosis. Invest Ophthalmol Vis Sci. 1991;32(9):2530–2535.
7. Schmidt-Erfurth U, Miller J, Sickenberg M, et al. Photodynamic therapy of subfoveal choroidal neovascularization: clinical and angiographic examples. Graefes Arch Clin Exp Ophthalmol. 1998;236(5):365–374. doi:10.1007/s004170050092
8. Verteporfin In Photodynamic Therapy Study Group. Verteporfin therapy of subfoveal choroidal neovascularization in age-related macular degeneration: two-year results of a randomized clinical trial including lesions with occult with no classic choroidal neovascularization–verteporfin in photodynamic therapy report 2. Am J Ophthalmol. 2001;131(5):541–560. doi:10.1016/s0002-9394(01)00967-9
9. van Dijk EHC, Fauser S, Breukink MB, et al. Half-dose photodynamic therapy versus high-density subthreshold micropulse laser treatment in patients with chronic central serous chorioretinopathy: the PLACE trial. Ophthalmology. 2018;125(10):1547–1555. doi:10.1016/j.ophtha.2018.04.021
10. Yamashita A, Shiraga F, Shiragami C, et al. Two-year results of reduced-fluence photodynamic therapy for polypoidal choroidal vasculopathy. Am J Ophthalmol. 2013;155(1):96–102 e1. doi:10.1016/j.ajo.2012.06.027
11. Lai FH, Ng DS, Bakthavatsalam M, et al. A multicenter study on the long-term outcomes of half-dose photodynamic therapy in chronic central serous chorioretinopathy. Am J Ophthalmol. 2016;170:91–99. doi:10.1016/j.ajo.2016.07.026
12. Newman DK. Photodynamic therapy: current role in the treatment of chorioretinal conditions. Eye. 2016;30(2):202–210. doi:10.1038/eye.2015.251
13. Oishi A, Miyamoto N, Mandai M, et al. LAPTOP study: a 24-month trial of verteporfin versus ranibizumab for polypoidal choroidal vasculopathy. Ophthalmology. 2014;121(5):1151–1152. doi:10.1016/j.ophtha.2013.12.037
14. Koh A, Lee WK, Chen LJ, et al. EVEREST study: efficacy and safety of verteporfin photodynamic therapy in combination with ranibizumab or alone versus ranibizumab monotherapy in patients with symptomatic macular polypoidal choroidal vasculopathy. Retina. 2012;32(8):1453–1464. doi:10.1097/IAE.0b013e31824f91e8
15. Di Nicola M, Williams BK, Hua J, et al. Photodynamic therapy for retinal hemangioblastoma: treatment outcomes of 17 consecutive patients. Ophthalmol Retina. 2022;6(1):80–88. doi:10.1016/j.oret.2021.04.007
16. Rinaldi M, Semeraro F, Chiosi F, et al. Reduced-fluence verteporfin photodynamic therapy plus ranibizumab for choroidal neovascularization in pathologic myopia. Graefes Arch Clin Exp Ophthalmol. 2017;255(3):529–539. doi:10.1007/s00417-016-3498-4
17. Kim HM, Park KH, Woo SJ. Massive exudative retinal detachment following photodynamic therapy and intravitreal bevacizumab injection in retinal capillary hemangioma. Korean J Ophthalmol. 2015;29(2):143–145. doi:10.3341/kjo.2015.29.2.143
18. Kokame GT, deCarlo TE, Kaneko KN, et al. Anti-vascular endothelial growth factor resistance in exudative macular degeneration and polypoidal choroidal vasculopathy. Ophthalmol Retina. 2019;3(9):744–752. doi:10.1016/j.oret.2019.04.018
19. Ladas ID, Rouvas AA, Moschos MM, et al. Polypoidal choroidal vasculopathy and exudative age-related macular degeneration in Greek population. Eye. 2004;18(5):455–459. doi:10.1038/sj.eye.6700706
20. Scassellati-Sforzolini B, Mariotti C, Bryan R, et al. Polypoidal choroidal vasculopathy in Italy. Retina. 2001;21(2):121–125. doi:10.1097/00006982-200104000-00004
21. Yannuzzi LA, Wong DW, Sforzolini BS, et al. Polypoidal choroidal vasculopathy and neovascularized age-related macular degeneration. Arch Ophthalmol. 1999;117(11):1503–1510. doi:10.1001/archopht.117.11.1503
22. Koh A, Lai TYY, Takahashi K, et al. Efficacy and safety of ranibizumab with or without verteporfin photodynamic therapy for polypoidal choroidal vasculopathy: a randomized clinical trial. JAMA Ophthalmol. 2017;135(11):1206–1213. doi:10.1001/jamaophthalmol.2017.4030
23. Lee WK, Iida T, Ogura Y, et al. Efficacy and safety of intravitreal aflibercept for polypoidal choroidal vasculopathy in the PLANET study: a randomized clinical trial. JAMA Ophthalmol. 2018;136(7):786–793. doi:10.1001/jamaophthalmol.2018.1804
24. Nicholson B, Noble J, Forooghian F, et al. Central serous chorioretinopathy: update on pathophysiology and treatment. Surv Ophthalmol. 2013;58(2):103–126. doi:10.1016/j.survophthal.2012.07.004
25. Hirami Y, Tsujikawa A, Otani A, et al. Hemorrhagic complications after photodynamic therapy for polypoidal choroidal vasculopathy. Retina. 2007;27(3):335–341. doi:10.1097/01.iae.0000233647.78726.46
26. Schmidt-Erfurth U, Laqua H, Schlotzer-Schrehard U, et al. Histopathological changes following photodynamic therapy in human eyes. Arch Ophthalmol. 2002;120(6):835–844.
27. Fingar VH, Wieman TJ, Wiehle SA, et al. The role of microvascular damage in photodynamic therapy: the effect of treatment on vessel constriction, permeability, and leukocyte adhesion. Cancer Res. 1992;52(18):4914–4921.
28. Schmidt-Erfurth U, Schlotzer-Schrehard U, Cursiefen C, et al. Influence of photodynamic therapy on expression of vascular endothelial growth factor (VEGF), VEGF receptor 3, and pigment epithelium-derived factor. Invest Ophthalmol Vis Sci. 2003;44(10):4473–4480. doi:10.1167/iovs.02-1115
29. Husain D, Miller JW, Michaud N, et al. Intravenous infusion of liposomal benzoporphyrin derivative for photodynamic therapy of experimental choroidal neovascularization. Arch Ophthalmol. 1996;114(8):978–985. doi:10.1001/archopht.1996.01100140186012
30. Al-Awadi A, Mandelcorn ED, Somani S. Atypical transient subretinal exudation following photodynamic therapy for chronic central serous retinopathy: a case report. Can J Ophthalmol. 2017;52(1):e38–e41. doi:10.1016/j.jcjo.2016.08.001
31. Chakurkar R, Tyagi M, Pappuru RR, et al. Photodynamic therapy-induced acute exudative maculopathy in a case of choroidal haemangioma. BMJ Case Rep. 2019;12(11):e231865. doi:10.1136/bcr-2019-231865
32. Figurska M, Wierzbowska J, Robaszkiewicz J. Severe decrease in visual acuity with choroidal hypoperfusion after photodynamic therapy. Med Sci Monit. 2011;17(6):CS75–9. doi:10.12659/msm.881799
33. Mammo Z, Forooghian F. Incidence of acute exudative maculopathy after reduced-fluence photodynamic therapy. Retin Cases Brief Rep. 2017;11(3):217–220. doi:10.1097/ICB.0000000000000356
34. Manayath GJ, Ranjan R, Vidhate S, et al. Photodynamic therapy-induced acute exudative maculopathy: incidence, clinical features, and long-term outcomes. Retina. 2020;40(1):135–144. doi:10.1097/IAE.0000000000002343
35. Sumnicht AJ, Chalam KV, Alset AE, et al. The role of oral steroids in the treatment of photodynamic therapy-associated exudative maculopathy, a case report and review of the literature. Photodiagnosis Photodyn Ther. 2021;35:102390. doi:10.1016/j.pdpdt.2021.102390
36. Fernandez-Vigo JI, Moreno-Morillo FJ, Burgos-Blasco B, et al. Early vessel occlusion and recanalization after photodynamic therapy in central serous chorioretinopathy by OCT angiography. Eur J Ophthalmol. 2021;11206721211027060. doi:10.1177/11206721211027060
37. Fernandez-Vigo JI, Moreno Morillo FJ, Valverde-Megias A, et al. Acute exudative maculopathy and bacillary layer detachment in central serous chorioretinopathy patients after photodynamic therapy. Retina. 2022;42:859–866. doi:10.1097/IAE.0000000000003404
38. Debefve E, Pegaz B, Ballini JP, et al. Combination therapy using aspirin-enhanced photodynamic selective drug delivery. Vascul Pharmacol. 2007;46(3):171–180. doi:10.1016/j.vph.2006.09.006
39. Tatar O, Adam A, Shinoda K, et al. Influence of verteporfin photodynamic therapy on inflammation in human choroidal neovascular membranes secondary to age-related macular degeneration. Retina. 2007;27(6):713–723. doi:10.1097/IAE.0b013e318042d3b0
40. Wang E, Chen S, Yang H, et al. Choroidal thickening and pachychoroid in Cushing syndrome: correlation with endogenous cortisol level. Retina. 2019;39(2):408–414. doi:10.1097/IAE.0000000000001956
41. Merrill PT, Henry CR, Nguyen QD, et al. Suprachoroidal CLS-TA with and without systemic corticosteroid and/or steroid-sparing therapy: a post-hoc analysis of the phase 3 PEACHTREE clinical trial. Ocul Immunol Inflamm. 2021;1–8. doi:10.1080/09273948.2021.1954199
42. Khurana RN, Merrill P, Yeh S, et al. Extension study of the safety and efficacy of CLS-TA for treatment of macular oedema associated with non-infectious uveitis (MAGNOLIA). Br J Ophthalmol. 2021;
43. Zarbin MA. Anti-VEGF agents and the risk of arteriothrombotic events. Asia Pac J Ophthalmol. 2018;7(1):63–67.
44. Wang X, Sawada T, Sawada O, et al. Serum and plasma vascular endothelial growth factor concentrations before and after intravitreal injection of aflibercept or ranibizumab for age-related macular degeneration. Am J Ophthalmol. 2014;158(4):738–744 e1. doi:10.1016/j.ajo.2014.06.009
45. Zehetner C, Kirchmair R, Huber S, et al. Plasma levels of vascular endothelial growth factor before and after intravitreal injection of bevacizumab, ranibizumab and pegaptanib in patients with age-related macular degeneration, and in patients with diabetic macular oedema. Br J Ophthalmol. 2013;97(4):454–459. doi:10.1136/bjophthalmol-2012-302451
46. Ross RD, Gitter KA, Cohen G, et al. Idiopathic polypoidal choroidal vasculopathy associated with retinal arterial macroaneurysm and hypertensive retinopathy. Retina. 1996;16(2):105–111. doi:10.1097/00006982-199616020-00003
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.