Back to Journals » Risk Management and Healthcare Policy » Volume 13
Pharmacovigilance Perception and Knowledge Among Pharmacists and Interns in Saudi Arabia
Authors Alshayban D, Mahmoud MA , Islam MA, Alshammari S , Alsulaiman D
Received 6 December 2019
Accepted for publication 10 January 2020
Published 24 January 2020 Volume 2020:13 Pages 55—61
DOI https://doi.org/10.2147/RMHP.S241265
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Marco Carotenuto
Dhfer Alshayban,1 Mansour Adam Mahmoud,2 Md Ashraful Islam,1 Shouq Alshammari,1 Duaa Alsulaiman3
1Department of Pharmacy Practice, College of Clinical Pharmacy, Imam Abdulrahman Bin Faisal University, Dammam 31441, Saudi Arabia; 2Department of Clinical and Hospital Pharmacy, College of Pharmacy, Taibah University, Al-Madinah Al-Munawarah, Saudi Arabia; 3Pharmacy Division, King Fahad University Hospital, Alkhobar, Saudi Arabia
Correspondence: Mansour Adam Mahmoud
Department of Clinical and Hospital Pharmacy, College of Pharmacy, Taibah University, Al-Madinah Al-Munawarah, Saudi Arabia
Email [email protected]
Aim: The aim of this study was to determine and compare the level of knowledge and perception of ADRs reporting and pharmacovigilance among interns and hospital pharmacists in different health-care settings in Saudi Arabia.
Methods: A cross-sectional study was conducted among pharmacists and pharmacy interns in different hospitals in Saudi Arabia. A total of 315 participants completed the self-administered and validated questionnaire during the period from August 2018 to March 2019.
Results: There was poor perception and knowledge of pharmacovigilance and ADRs reporting among pharmacists as well as intern pharmacists. However, pharmacists had better knowledge score compared to interns (P=0.043). Most of the respondents believed that ADRs reporting is important. The majority of both interns and pharmacists stated that they did not receive adequate education about pharmacovigilance during their undergraduate or internship program.
Conclusion: There is a gap in knowledge and perception about pharmacovigilance among practicing pharmacists and new pharmacy graduates. Drug safety fundamentals and policies should be taught to undergraduate pharmacy students in Saudi Arabia.
Keywords: pharmacovigilance, adverse drugs effects, safety, pharmacists, Saudi Arabia
Introduction
Pharmacovigilance is defined as the activity and science relating to collection, detection, monitoring, assessment, and prevention of adverse drug effects with pharmaceutical products.1 The word “pharmacovigilance” is derived from the Greek word pharmakon(drug) and the Latin word vigilare (monitor or keep an eye on). Pharmacovigilance is a process of identifying the hazards related to pharmaceutical products.1
Pharmacovigilance plays an essential role in patient outcomes. Adverse drug reactions (ADRs) are defined by the World Health Organization (WHO) as “any noxious, unintended, and undesired effect of a drug, which occurs at doses used in humans for prophylaxis, diagnosis, or therapy.”2 Data from a systematic review reported that ADRs were associated with 15% of admissions to hospitals and prolonged hospitalizations and they are considered as the fourth to sixth leading cause of mortality in the USA.3 Patients who experienced any type of ADRs are hospitalized nearly 8–12 days longer than patients who did not experience ADRs.4 Any medication usage can be associated with undesirable consequences.5 The Center for Health Policy Research states that more than 50% of the approved medications in the USA are linked to some types of undesirable effects that were not discovered prior to the new medication approval process. Therefore, it is very important to improve the role of pharmacists in post-medication exposure and marketing surveillance.3
A proper spontaneous reporting system for ADRs is a basic component for comprehensive post-marketing monitoring of drug-induced risks and the study of drug safety, where there are inadequate data due to the many limitations of premarketing clinical trials.6,7 Although spontaneous reporting systems are simple to use and inexpensive, their potency is tightly linked to the actual reporting rate by health-care providers. Under-reporting is the main intrinsic and actual disadvantage.7 Consequently, health-care providers' knowledge of pharmacovigilance and ADRs reporting systems could impact their attitude towards good patient care and safety.8 Proper training for health-care providers, most importantly pharmacists, can improve the number of reporting ADRs. Previous studies showed that inadequate perception and awareness of ADRs reporting may eventually affect the reporting rate.1,8
Studies have been conducted inside and outside Saudi Arabia to examine the knowledge and perceptions of pharmacy students and staff about pharmacovigilance.10–14 These studies have a significant role in guiding policymakers to prioritize their interventions, because they clearly demonstrate the need to increase awareness of the importance of pharmacovigilance and reporting adverse effects. Pharmacists and interns must understand their pivotal role in pharmacovigilance and the surveillance of the safe use of medicines. There has been no study focusing on the knowledge of pharmacy interns about pharmacovigilance and reporting side effects. Therefore, this study was aimed at assessing the knowledge and perception of pharmacovigilance among pharmacists and interns in Saudi Arabia. More specifically, this study attempted to answer two questions: Do the interns know better than pharmacists about pharmacovigilance? Do pharmacy colleges in Saudi Arabia need to offer specific classes in medication safety?
Materials and Methods
This descriptive cross-sectional study was conducted among pharmacists and intern pharmacists in Saudi Arabia from August 2018 to March 2019 using a pretested and validated questionnaire which was administered through interviews or self-administered through online distribution. Participants were eligible if they graduated with a pharmacy degree or they are current pharmacy interns who deal with medications, and are willing to provide consent to participate in the study during the allocated data collection period. Pharmacy undergraduate students and those unwilling to provide consent for participation were excluded.
Sample Size
Adequate sample size remains a major concern when conducting medical research, due to the unknown size of the population. Additionally, for online surveys, the researcher may need to enroll more participants in practice to account for potential missing/non-response error, because the researcher has no control over respondent response.15 “Power study” is a very useful and frequently used tool to calculate sample size in the field of medical research. For the current paper the, “power study” method was performed using a Web-based sample size calculator using the necessary information retrieved from published articles in Saudi Arabia.16 Moreover, sample size adjustment was done for missing/non-response using the following formula. 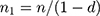
where n is required sample size as per formula, n1 is adjusted sample size and d is the potential missing or non-response rate. A minimum number of 230 samples can achieve more than 90% power when we consider only 1% margin of error and 30% missing/non-response error rate.
Data Instrument Development and Quality Assurance
After an extensive literature review, an initial draft of a questionnaire was designed in the English language.8–13 To ensure the study objectives are met, the structured questionnaire included information about attitude, knowledge, and practice on ADRs reporting and pharmacovigilance. From the initial set of 34 questions, the final questionnaire comprised a total of 28 questions and was divided into three domains: the first domain included 7 questions (with 2 of 9 questions excluded) concerned with demographic data such as degree program and gender; the second one included 10 questions (with 3 of 13 questions excluded) related to knowledge about ADRs reporting and pharmacovigilance; and the final domain consisted of 11 questions (with 1 of 12 questions excluded), designed to determine the perception of ADRs reporting. The questionnaire was piloted to ensure face validity and that all the necessary corrections had been addressed, and the piloted samples were excluded from the final analysis. To ensure data reliability, a number of adjustments were made during the design and data collection periods, including a clearly documented data collection process and a research procedure that was followed according to the data collection protocol; all of the data were gathered on one occasion to reduce the capture responses from participants as well as to reduce unintentional response bias.
Content Validity and Reliability Analysis
The development of the questionnaire was carried out by a panel of seven experts including three clinical pharmacists (different departments, including pharmacy practice), two medical professionals (general practitioners), one social scientist, and one biostatistician. The content validity index (CVI) was calculated using the Yusoff16 CVI calculation method for knowledge and perception. The I-CVI/Ave score was reported for a knowledge domain of 0.87 and for a perception domain of 0.91. According to Lynn,17 the recommended score for CVI is 0.83 or more, so the obtained score was acceptable for the current study. In addition, reliability was tested by Cronbach’s alpha and knowledge and perception values of 0.68 and 0.66 were obtained, respectively.
Variable Measurement
Data were collected through an online survey (QuestionPro), and a high response rate was observed (71.05%: 481 out of 677). Knowledge and perception were two dependent variables. To assess knowledge, a 10-item (K1 to K10) questionnaire was utilized. To avoid response bias, an inverse item (K10) was included and “false” responses were considered good knowledge. Only the correct answers were considered as good knowledge for the first two items (K1 and K2). For the other items (K3 to K9), the responses “agree” and “strongly agree” were considered as good knowledge. Perception was assessed by an 11-item (P1 to P11) questionnaire. To assess good perception, the correct answer was reflected for the first item (P1) while “agree” and “strongly agree” were reflected for the rest of the items (P2 to P11). For data analysis, knowledge and perception were divided into two groups, good (knowledge/perception) and poor (knowledge/perception). Knowledge and perception scores were also calculated for further analysis. A higher score represents a higher level of knowledge and perception about pharmacovigilance. Information was also collected about some socio-demographic variables, including gender, age, work experience and place, educational degree and institute, and nationality.
Data Management
Data cleaning is an important step before the final data analyzing procedure.18 For the current study data were checked and cleaned for incomplete information and also for extreme values using an informal technique.19,20 As an online survey was involved, missing cases were inevitable; some were treated using a statistical method (the last observation carried forward method)21 and some were excluded from the final analysis (Figure 1).
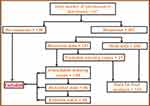 |
Figure 1 Data cleaning procedure. |
Statistical Analysis
Statistical analyses were carried out using Statistical Package for Social Scientists (IBM SPSS version 23.0) and Excel software. Descriptive statistics and cross-tabulation were used to describe the study variables, and frequencies with their corresponding percentages are presented. An independent sample t-test was utilized to compare knowledge and perception score between interns and pharmacists. For statistical significance, any value of p<0.05 was considered an acceptable range of type-I error.
Results
After excluding missing and abnormal cases from the data set, a total of 315 samples were analyzed for the current study. The majority of the participants (74.3%) were hospital pharmacists. More than half of the respondents were female (58.7%), while 41.3% were male. Regarding work experience, half (51.4%) of the respondents were interns (without having any previous experience), whereas 31.7% and 16.8% of respondents have work experience of up to 5 years and 6 or more years, respectively (Table 1).
When good knowledge about pharmacovigilance was compared between interns and pharmacists, it was found that among all items, pharmacists had better knowledge than interns. The maximum proportion of good knowledge (91.0% pharmacists’ vs 86.4% interns) was reported for item-1 (pharmacovigilance is the practice of monitoring the effects of medical drugs after they have been licensed for use, especially in order to identify and evaluate previously unreported adverse reactions). However, the lowest proportion of good knowledge for item-10 (Type A ADRs are uncommon and unpredictable, depending on the known pharmacology of the drug; they are independent of dose and only affect few people) was reported (30.3% pharmacists vs 28.4 interns) (Table 2).
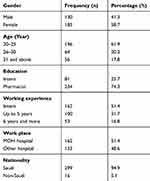 |
Table 1 Descriptive Statistics for Demographic Variables (n=315) |
 |
Table 2 Comparison of Good Knowledge for Different Items Between Interns and Pharmacists in Saudi Arabia (n=315) |
Table 3 represents the data of perception comparison. Results showed that among items 7 to 10, interns reported higher proportion of good perception about pharmacovigilance than pharmacists (item-7: 56.8% vs 50.0%, item-8: 74.1% vs 64.5%, item-9: 85.2% vs 81.6%, and item-10: 80.2% vs 78.6%, respectively), whereas for the rest of the items (item-1: 22.2% vs 32.1%, item-2: 29.6% vs 35.9%, item-3: 86.4% vs 87.2%, item-4: 30.9% vs 35.5%, item-5: 39.5% vs 49.6%, item-6: 38.3% vs 41.0% and item-11: 14.8% vs 16.2%) a lower proportion of good perception was demonstrated for interns than for pharmacists (Table 3).
 |
Table 3 Comparison of Good Perception for Different Items Between Intern and Pharmacists in Saudi Arabia (n=315) |
Knowledge and perception scores ware compared using an independent sample t-test. Results demonstrated that in regards to knowledge score, pharmacists have significantly higher knowledge score (MD=1.44; p<0.05) than interns (mean score: pharmacist, 32.67 (SD: 5.34) vs intern 31.22 (SD: 6.01)). In terms of perception, although pharmacists showed a higher mean score than interns (mean score: 36.10 (SD: 6.03) vs 35.49 (SD: 5.72)), no statistically significant difference was found (Table 4).
 |
Table 4 Comparison of Knowledge and Perception Between Intern and Pharmacists About Pharmacovigilance in Different Health-Care Settings, Saudi Arabia |
Discussion
The majority of the study population knew the correct definition of pharmacovigilance (PV). This might be because of the continuing education activities conducted by the top hospital management and supervised and monitored by the Saudi Food and Drug Authority (SFDA). The SFDA has drug safety coordinators in hospitals who aim to raise awareness and improve ADRs reporting. In addition, in the last few years the SFDA conducted annual conferences on drug safety. In this year (2019) the SFDA received the highest number of ADRs reports since its establishment. Pharmacists must transfer their knowledge to the public to increase their awareness of ADR reporting. A study assessing PV knowledge among the public in Saudi Arabia found that only 15.7% of the public knows about the term “pharmacovigilance” and only 8.6% acknowledge awareness of the national PV center.22
In Saudi Arabia, ADRs reporting is regulated by the National Pharmacovigilance Center which is part of the SFDA. A study conducted in 2013 in the eastern region of Saudi Arabia found that 10% of community pharmacists were aware of the existence of the ADRs reporting system.23 Another study performed in Riyadh in 2014 reported that only 22% of community pharmacists knew where and how ADRs reports are submitted.11 A multicenter study conducted in three regions of Saudi Arabia reported that 67% of health-care professionals (physicians, pharmacists, nurses, and interns) were not aware of the existence of a National Pharmacovigilance Center.12 There is a major improvement in the knowledge of pharmacists about the right regulatory body to which ADRs reports should be submitted. In the current study, more than three-quarters of the interns, as well as pharmacists, were aware of the fact that ADR reports should be submitted to the National Pharmacovigilance Center. However, technical details on the process of reporting ADRs were known to half of the interns and slightly more than half of the pharmacists. In addition, more than half of the participants believed that they are not prepared with the knowledge and skills to report ADRs. Therefore, more efforts are needed from SFDA to clarify this issue to health-care professionals. Also, ADRs reporting forms should be simple to use, because this was one of the challenges and reasons for underreporting of ADRs in a qualitative study performed among health-care professionals in Riyadh.24 More interns compared to pharmacists in this study believed that ADRs reporting should be mandatory. Interns are the future pharmacists and their positive perception is a good sign that much improvement in pharmacovigilance practice is anticipated in the coming decades. Similar to a recent study12 16% of the participants believed that only serious ADRs should be reported.
Adverse drug events (ADEs) are harmful events that are preventable if they occur due to error, whereas ADRs are noxious and unintended events that are non-preventable. Interestingly, only about a quarter of interns and pharmacists could not differentiate between ADRs and ADEs. Improvements were seen in ADRs reporting in this study (22.2% of interns and 32.1% of pharmacists) compared to previous studies where 12.5%11 of community pharmacists and 26.8% of hospital pharmacists had reported ADRs.25 These improvements are justified by the enhancement of the mechanism of ADRs reporting and the availability of both online and paper reporting forms in the majority of the hospitals in Saudi Arabia. In addition, the ADRs reporting process is part of the requirements for national and international accreditations for hospitals.
Strength and Limitations of the Study
The major strength of this study is that it focused on an issue that has not been adequately studied, especially in Saudi Arabia. The comparative nature of this study differentiates it from other published studies on pharmacovigilance. However, there are some limitations. The main limitation of this study that it is a cross-sectional study, so the causality could not be warranted. Secondly, the study was based on a self-reported questionnaire, so personal bias may have affected the results.
Conclusion and Recommendation
Based on this survey, it was found that there are poor awareness and knowledge of ADRs reporting among pharmacists as well as pharmacy interns. However, most of the responders believe that ADRs reporting is important and must be done by pharmacists. Therefore, it is suggested to add a pharmacovigilance course in the curriculum of pharmacy colleges in Saudi Arabia. It is also recommended that education and training programs are implemented for practicing pharmacists to enhance knowledge and perception regarding pharmacovigilance and ADRs reporting.
Acknowledgments
We would like to acknowledge the KFUH authority for giving permission to collect information from pharmacists and interns and the Department of Pharmacy Practice for the research approval.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Said ASA, Hussain N. Adverse drug reaction reporting practices among United Arab emirates pharmacists and prescribers. Hosp Pharm. 2017;52(5):361–366.
2. Organization WH. The safety of medicines in public health programmes: pharmacovigilance an essential tool; 2006
3. Bouvy JC, De Bruin ML, Koopmanschap MA. Epidemiology of adverse drug reactions in Europe: a review of recent observational studies. Drug Saf. 2015;38(5):437–453. doi:10.1007/s40264-015-0281-0
4. Giardina C, Cutroneo PM, Mocciaro E, et al. Adverse drug reactions in hospitalized patients: results of the FORWARD (facilitation of reporting in hospital ward) study. Front Pharmacol. 2018;9(350). doi:10.3389/fphar.2018.00350
5. De Sarro GB. Retrospective analysis of adverse drug reactions to bronchodilators observed in two pulmonary divisions of Catanzaro, Italy. Pharmacol Res. 2003;47(6):493–499. doi:10.1016/S1043-6618(03)00003-3
6. Lemay J, Alsaleh FMB, Al-Buresli L, Al-Mutairi M, Abahussain EA, Bayoud TB. Reporting of adverse drug reactions in primary care settings in Kuwait: a comparative study of physicians and pharmacists. Med Principles Pract. 2018;27:30–38. doi:10.1159/000487236
7. Vessal G, Mardani Z, Mollai M. Knowledge, attitudes, and perceptions of pharmacists to adverse drug reaction reporting in Iran. Harm World Sci. 2009;31(2):183–187. doi:10.1007/s11096-008-9276-6.
8. Güner MD, Ekmekci PE. Healthcare professionals’ pharmacovigilance knowledge and adverse drug reaction reporting behavior and factors determining the reporting rates. J Drug Assess. 2019;8(1):13–20. doi:10.1080/21556660.2019.1566137.
9. Elkalmi RM, Hassali MA, Ibrahim MI, Widodo RT, Efan QM, Hadi MA. Pharmacy students’ knowledge and perceptions about pharmacovigilance in Malaysian public universities. Am J Pharm Educ. 2011;75(5):96. doi:10.5688/ajpe75596.
10. Rajiah K, Maharajan MK, Nair S. Pharmacy students’ knowledge and perceptions about adverse drug reactions reporting and pharmacovigilance. Saudi Pharm J. 2016;24(5):600–604. doi:10.1016/j.jsps.2015.03.021
11. Mahmoud MA, Alswaida Y, Alshammari T, Khan TM, Alrasheedy HMA, Aljadhey H. Community pharmacists’ knowledge, behaviors and experiences about adverse drug reaction reporting in Saudi Arabia. Saudi Pharm J. 2014;22(5):411–418. doi:10.1016/j.jsps.2013.07.005
12. AlShammari TM, Almoslem MJ. Knowledge, attitudes & practices of healthcare professionals in hospitals towards the reporting of adverse drug reactions in Saudi Arabia: a multi-centre cross sectional study. Saudi Pharma J. 2018;26(7):925–931. doi:10.1016/j.jsps.2018.04.012.
13. Almandil NB. Healthcare professionals’ awareness and knowledge of adverse drug reactions and pharmacovigilance. Saudi Med J. 2016;37(12):1359–1364. doi:10.15537/smj.2016.12.17059.
14. Al-Arifi MN. Community pharmacist perception and attitude toward ethical issues at community pharmacy setting in central Saudi Arabia. Saudi Pharm J. 2014;22(4):315–325. doi:10.1016/j.jsps.2013.08.003.
15. Sakpal TV. Sample size estimation in clinical trial. Perspect Clin Res. 2010;1(2):67–69.
16. Yusoff MSB. ABC of content validation and content validity index calculation. Educ Med J. 2019;11(2):49–54. doi:10.21315/eimj2019.11.2.6
17. Lynn MR. Determination and quantification of content validity. Nurs Res. 1986;35(6):382–385. doi:10.1097/00006199-198611000-00017
18. Osborne JW. Best Practices in Data Cleaning: A Complete Guide to Everything You Need to Do Before and After Collecting Your Data. Sage; 2013.
19. Dunn OJ, Clark VA. Applied Statistics: Analysis of Variance and Regression. New York: Wiley; 1987: 7974.
20. Islam A, Islam N, Bharati P, Aik S, Hossain G. Socio-economic and demographic factors influencing nutritional status among early childbearing young mothers in Bangladesh. BMC Womens Health. 2016;16(1):58. doi:10.1186/s12905-016-0338-y.
21. Lang T. Documenting research in scientific articles: guidelines for authors: reporting multivariate analyses. Chest. 2007;131(2):628. doi:10.1378/chest.06-2088
22. Sales I, Aljadhey H, Albogami Y, Mahmoud MA. Public awareness and perception toward adverse drug reactions reporting in Riyadh, Saudi Arabia. Saudi Pharma J. 2017;25(6):868–872. doi:10.1016/j.jsps.2017.01.004.
23. Khan TM. Community pharmacists’ knowledge and perceptions about adverse drug reactions and barriers towards their reporting in Eastern region, Alahsa, Saudi Arabia. Ther Adv Drug Saf. 2013;4(2):45–51. doi:10.1177/2042098612474292.
24. Aljadhey H, Mahmoud MA, Alshammari TM, et al. A qualitative exploration of the major challenges facing pharmacovigilance in Saudi Arabia. Saudi Med J. 2015;36(9):1097–1102. doi:10.15537/smj.2015.9.12125
25. Aldryhim AY, Alomair A, Alqhtani M, et al. Factors that facilitate reporting of adverse drug reactions by pharmacists in Saudi Arabia. Expert Opin Drug Saf. 2019;18(8):745–752. doi:10.1080/14740338.2019.1632287
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
