Back to Journals » Drug Design, Development and Therapy » Volume 18
Pharmacokinetic Comparison Between a Fixed-Dose Combination of Atorvastatin/Omega-3-Acid Ethyl Esters and the Corresponding Loose Combination in Healthy Korean Male Subjects
Authors Khwarg J , Lee S , Jang IJ , Kang WH, Lee HJ, Kim KY , Jeong KS , Won C, Choi YW, Ha DC, Jung R, Han MG, Jung WT, Nam KY, Kim YS, Yu KS , Oh J
Received 18 September 2023
Accepted for publication 3 January 2024
Published 8 February 2024 Volume 2024:18 Pages 395—406
DOI https://doi.org/10.2147/DDDT.S435885
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Manfred Ogris
Juyoung Khwarg,1 Soyoung Lee,1,2 In-Jin Jang,1 Won-Ho Kang,3 Hye Jung Lee,4 Kyu Yeon Kim,4 Ki-Sun Jeong,4 Chongho Won,4 Youn Woong Choi,5 Dae Chul Ha,5 RaeHoon Jung,5 Min-Gu Han,5 Won Tae Jung,6 Kyu-Yeol Nam,6 YeSeul Kim,6 Kyung-Sang Yu,1 Jaeseong Oh1,7
1Department of Clinical Pharmacology and Therapeutics, Seoul National University College of Medicine and Hospital, Seoul, Republic of Korea; 2Integrated Major in Innovative Medical Science, Seoul National University Graduate School, Seoul, Republic of Korea; 3R&D Center, Korea United Pharm. Inc., Seoul, Republic of Korea; 4Caleb Multilab, Inc., Seoul, Republic of Korea; 5R&D Center, Korea United Pharm.Inc., Sejong, Republic of Korea; 6Global R&D, Korea United Pharm. Inc., Seoul, Republic of Korea; 7Department of Pharmacology, Jeju National University College of Medicine, Jeju, Republic of Korea
Correspondence: Jaeseong Oh, Department of Pharmacology, Jeju National University College of Medicine, 15, Aran 13-gil, Jeju-si, Jeju-do, 63241, Republic of Korea, Email [email protected]
Purpose: Statins are widely used in combination with omega-3 fatty acids for the treatment of patients with dyslipidemia. The aim of this study was to compare the pharmacokinetic (PK) profiles of atorvastatin and omega-3-acid ethyl esters between fixed-dose combination (FDC) and loose combination in healthy subjects.
Methods: A randomized, open-label, single-dose, 2-sequence, 2-treatment, 4-period replicated crossover study was performed. Subjects were randomly assigned to one of the 2 sequences and alternately received four FDC soft capsules of atorvastatin/omega-3-acid ethyl esters (10/1000 mg) or a loose combination of atorvastatin tablets (10 mg × 4) and omega-3-acid ethyl ester soft capsules (1000 mg× 4) for four periods, each period accompanied by a high-fat meal. Serial blood samples were collected for PK analysis of atorvastatin, eicosapentaenoic acid (EPA), and docosahexaenoic acid (DHA). PK parameters were calculated by a non-compartmental analysis. The geometric mean ratio (GMR) and its 90% confidence interval (CI) of the FDC to the loose combination were calculated to compare PK parameters.
Results: A total of 43 subjects completed the study as planned. The GMR (90% CI) of FDC to loose combination for maximum concentration (Cmax) and area under the time-concentration curve from zero to the last measurable point (AUClast) were 1.0931 (1.0054– 1.1883) and 0.9885 (0.9588– 1.0192) for atorvastatin, 0.9607 (0.9068– 1.0178) and 0.9770 (0.9239– 1.0331) for EPA, and 0.9961 (0.9127– 1.0871) and 0.9634 (0.8830– 1.0512) for DHA, respectively. The intra-subject variability for Cmax and AUClast of DHA was 30.8% and 37.5%, respectively, showing high variability. Both the FDC and the loose combination were safe and well tolerated.
Conclusion: The FDC of atorvastatin and omega-3-acid ethyl esters showed comparable PK characteristics to the corresponding loose combination, offering a convenient therapeutic option for the treatment of dyslipidemia.
Keywords: pharmacokinetics, cardiovascular disease
Graphical Abstract:
Introduction
Dyslipidemia is defined as an imbalance in the lipid profile, including total cholesterol, low-density lipoprotein cholesterol (LDL-C), triglyceride (TG), and high-density lipoprotein cholesterol (HDL-C), and is a clinically significant risk factor for atherosclerotic cardiovascular diseases (ASCVDs).1,2 The management of dyslipidemia and prevention of ASCVDs primarily focuses on maintaining LDL-C under certain levels through lifestyle modifications and lipid-lowering therapies.3–7 According to current guidelines, patients with a very high risk of ASCVDs are recommended to achieve LDL-C levels of less than 55 mg/dL6,7 or achieve 50% or greater reduction from baseline.5
Statins are currently used as first-line therapies for dyslipidemia and the prevention of ASCVDs.5,7 By inhibiting 3-hydroxy-3-methylglutaryl-coenzyme A reductase (HMG-CoA reductase), statins lower LDL-C levels by inhibiting the cholesterol synthesis pathway. Although the management of dyslipidemia primarily focuses on lowering LDL-C levels, lipid profiles of TG, very-low-density lipoprotein (VLDL), and HDL-C may not improve sufficiently in patients treated with statin monotherapy.
In case of combined hyperlipidemia, combination therapy with statins and omega-3 fatty acids may lower TGs in greater amounts and reduce the risk of ASCVDs.8 The mechanism by which omega-3 fatty acid administration lower TGs is not fully understood, but it has been suggested that omega-3 fatty acid administration contributes to hepatic lipogenesis suppression by increasing fatty acid oxidation.9,10 In a large-scale clinical study, combination therapy with omega-3 fatty acids and simvastatin significantly improved the non-HDL-C profile compared to simvastatin alone in hypertriglyceridemic patients.11 Another large-scale clinical trial in Japan demonstrated a significant reduction in major coronary events following combination therapy with statins and omega-3 fatty acids in patients with hypercholesterolemia.12
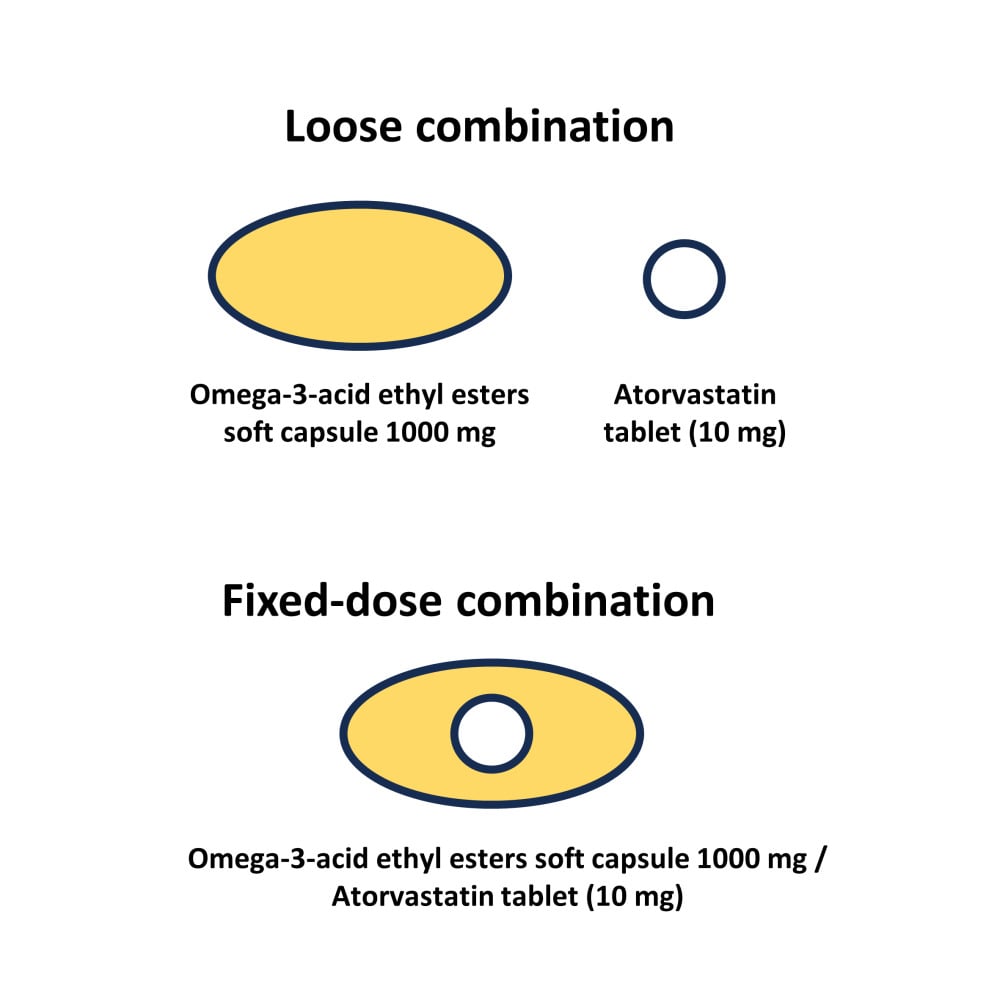
For patients taking both atorvastatin and omega-3-acid ethyl esters, a fixed-dose combination (FDC) of atorvastatin and omega-3-acid ethyl esters (ATMEG CombiGel Softcap, Korea United Pharm. Inc., Korea) was developed to improve compliance and convenience. While the possibility of drug interactions between atorvastatin and omega-3-acid ethyl esters has been reported,13 the efficacy and safety of ATMEG CombiGel have been confirmed through a Phase 3 study.14 The FDC formulation of atorvastatin and omega-3-acid ethyl esters contains atorvastatin tablet inside the omega-3-acid ethyl esters capsule. By applying the multilayer barrier coating technology developed by Korean United Pharm. Inc. (Seoul, Korea), the combination of different (solution and tablet) formulations was possible. With this approach, the pill size was maintained as a single omega-3-acid ethyl esters capsule, offering an advantage to patients who have difficulty swallowing larger pills.
The aim of this study was to compare the pharmacokinetic (PK) and safety profile of atorvastatin and omega-3-acid ethyl esters between the FDC and the corresponding loose combination in healthy Korean subjects.
Materials and Methods
This study (NCT05085184) was approved by the institutional review board (IRB) of Seoul National University Hospital (IRB number: H-2107-247-1241) and the Korea Ministry of Food and Drug Safety (MFDS). This study was conducted from 6 September to 10 December 2021. All study procedures were conducted in accordance with the principles of the Korean Good Clinical Practices and the ethical principles of the Declaration of Helsinki. Participants fully understood the objectives, expected results, and possible risks of this study and signed informed consent forms before enrollment.
Study Population
Healthy Korean adults aged between 19 and 45 years with a body mass index (BMI) of 18.0 to 27.0 kg/m2 were eligible to participate in this study. The enrolled subjects had no clinically significant abnormalities considering medical history, vital signs, clinical laboratory tests, 12-lead electrocardiogram, and physical examinations. Subjects were excluded if they had any clinically significant hypersensitivity to atorvastatin, other HMG-CoA reductase inhibitors, omega-3 fatty acids or fish; history or family history of myopathy or hereditary muscular disorders; or current or history of obstructive bile disorders.
Study Design
A randomized, open-label, single-dose, 2-sequence, 2-treatment, 4-period replicated crossover study was performed. A replicated crossover design was applied owing to the highly variable characteristics of atorvastatin and omega-3 fatty acids.15,16 Subjects were randomly assigned to one of the 2 sequences (Figure 1) and received FDC soft capsules of atorvastatin/omega-3-acid ethyl esters (10/1000 mg × 4) or a loose combination of atorvastatin tablets (Lipitor, Pfizer Ltd., 10 mg × 4) and omega-3-acid ethyl ester soft capsules (Omacor, Kuhnil Pharmaceutical, Co. Ltd., 1000 mg × 4) with a high-fat meal (≥900 kcal and ≥35% lipid content) in each period. The study dose was selected to evaluate the pharmacokinetics of atorvastatin and omega-3 fatty acids at the highest approved dosage. Wash-out between periods was 7 days, which was longer than 5 times the previously reported half-lives of atorvastatin and omega-3 fatty acids.16,17 PK blood samples for atorvastatin were collected at 0 (predose) and 0.5, 1, 2, 3, 4, 5, 6, 8, 12, 24, 36, and 48 h postdose in each period. To adjust the baseline for the endogenous compounds of EPA and DHA, serial predose blood samples were also collected. PK blood samples for EPA were collected at −15, −14, −13, −12, −11, 0 (predose), 0.5, 1, 2, 3, 4, 5, 6, 7, 8, 10, 12, 24, 48, 72, and 96 h postdose in each period. PK blood samples for DHA were collected at −15, −14, −13, −12, −11, 0 (predose), 0.5, 1, 2, 3, 4, 5, 6, 7, 8, 10, and 12 h postdose in each period. The blood samples were centrifuged at 1912 g for 10 minutes and stored at −70 °C until bioanalysis. To minimize the confounding effect of omega-3 fatty acids from food intake, study participants were hospitalized during the entire study period, and meals provided during the study were prepared without fish and consisted of a standard diet with restricted EPA and DHA content.
Determination of Plasma Concentration
The plasma concentration of atorvastatin was measured by ultra-performance liquid chromatography (ACQUITY UPLC I-CLASS System; Waters, Milford, MA, USA) coupled with tandem mass spectrometry (Xevo TQ-XS triple quadrupole MS/MS system; Waters, Milford, MA, USA). The samples were separated under an ACQUITY UPLC BEH C18, 1.7 μm column (2.1 mm × 50 mm) (Waters, Milford, MA, USA). Atorvastatin-d5 was used as an internal standard (IS). The mobile phase was 10 mM ammonium formate (0.1% formic acid): acetonitrile 45:55 (v/v), with a flow rate of 0.35 mL/min. For the quality control samples, the accuracy was 98.7–100.3%, and the precision was ≤ 2.7%.
Plasma concentrations of EPA and DHA were measured by ultra-performance liquid chromatography (ACQUITY UPLC I-CLASS System; Waters, Milford, MA, USA) coupled with tandem mass spectrometry (Xevo TQ-XS triple quadrupole MS/MS system; Waters, Milford, MA, USA). The samples were separated under a Kinetex C18 100A 1.7 μm column (2.1 mm × 50 mm) (Phenomenex, Torrance, CA, USA). The mobile phase was 5 mM ammonium formate (0.2% formic acid): acetonitrile 30:70 (v/v) and had a flow rate of 0.4 mL/min. For the quality control samples of EPA and DHA, the accuracy was 100.1–104.0% and 100.2–102.1%, respectively, and the precision was ≤ 4.1% and ≤ 4.5%, respectively.
PK Assessment
The PKs were evaluated on subjects who had completed all the study procedures with no major protocol deviation. PK parameters were calculated by a non-compartmental analysis using Phoenix WinNonlin software (Version 8.2 Certara, NJ, USA). As EPA and DHA are endogenous compounds, baseline-adjusted plasma concentration was used for the calculation of the pharmacokinetic parameters of EPA and DHA. The average blood concentrations at −15, −14, −13, −12, −11, and 0 h for each period were used to adjust the baseline EPA and DHA levels. If the baseline adjusted concentration value was negative, the value was replaced with 0 when calculating the PK parameters of EPA and DHA. For concentrations of atorvastatin, concentrations below the quantification limit were considered as 0, while while concentrations after Tmax were regarded as missing data. The area under the time-concentration curve (AUC) from zero to the last measurable point (AUClast) was calculated using the linear-up/log-down trapezoidal method. The AUC from 0 to infinity (AUCinf) was calculated as AUClast + Clast/λz, where Clast is the last measurable concentration and λz is the terminal elimination rate constant estimated from linear regression of the terminal phase of the log-linear plot of the plasma concentration-time curve. The maximum plasma concentration (Cmax) and the time to reach Cmax (Tmax) were obtained from the observed concentration data. Apparent clearance (CL/F) was calculated as dose/AUCinf, and apparent volume of distribution (Vz/F) was calculated as CL/F/λz. The terminal elimination half-life (t1/2) was calculated as ln(2)/λz. The intra-subject coefficient of variation (CV, %) was calculated as 100 ×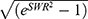 , where SWR is the within-subject standard deviation of the log transformed parameters.
, where SWR is the within-subject standard deviation of the log transformed parameters.
Safety and Tolerability Assessment
Safety and tolerability were assessed in subjects who had taken the study drug at least once. All adverse events (AEs) that occurred during this study were recorded, and the severity and relationship of the AEs with the study drug were evaluated. Monitoring of vital signs, physical examination, 12-lead ECG, and clinical laboratory tests were also performed.
Statistical Analysis
SAS software (Version 9.3, SAS Institute Inc., Cary, NC, USA) was used for the statistical analysis. The geometric mean ratio (GMR) and its 90% confidence interval (CI) of the FDC to the loose combination were calculated to compare the PK parameters Cmax and AUClast. The sum of Cmax and AUClast after the first and second administration of the FDC and the loose combination were used for the calculation of the GMR and its 90% CI. Analysis of variance (ANOVA) was conducted using a linear mixed model with log transformed AUClast and Cmax as dependent variables, sequence, period, and treatment as fixed effects and subjects nested within a sequence as a random effect. The GMRs and their 90% CIs were compared with the conventional bioequivalence range (0.8–1.25). The reported maximum intra-subject CVs (%) of AUClast and Cmax of omega-3-acid ethyl esters were 43.1%.16 When assuming there would be no pharmacokinetic difference between the FDC and the loose combination, the minimum number of subjects required to demonstrate the bioequivalence between the FDC and the loose combination with 80% statistical power at a 5% significance level was 30 subjects. Considering a dropout rate of 30%, a total sample size of 44 subjects was determined.
Results
Demographics
A total of 44 subjects were randomized, and 43 subjects completed the study as planned. One subject withdrew consent before the first study drug administration. All subjects were males, and the mean ± SD values for age, height, body weight, and body mass index (BMI) were 30.53 ± 6.42 years, 172.12 ± 5.65 cm, 67.90 ± 7.75 kg and 22.92 ± 2.32 kg/m2, respectively. There were no statistically significant differences in age, height, body weight, BMI, alcohol consumption or smoking status between the sequences.
Pharmacokinetics
The plasma concentration-time profile and PK parameters of atorvastatin, EPA and DHA were comparable between the FDC and loose combination (Figures 2–4, Tables 1–3). The 90% CIs of the GMRs of the FDC to the loose combination for Cmax and AUClast of atorvastatin, EPA, and DHA were all within the accepted bioequivalence range of 0.8–1.25 (Table 3). The baseline EPA and DHA levels were stable. The intra-subject CVs (%) for Cmax and AUClast of DHA were 30.8% and 37.5%, respectively, showing high variability. The corresponding values for EPA were 24.0% and 25.6%, respectively, and 27.5% and 10.4% for atorvastatin, respectively.
 |
Table 3 Geometric Mean Ratio (90% CI) of FDC to Loose Combination for Atorvastatin, EPA, and DHA |
Safety and Tolerability
A total of 19 treatment emergent adverse events (TEAEs) occurred in 10 (23.3%) subjects (Table 4). Thirteen TEAEs occurred in 6 subjects (14%) after administration of the FDC, and 6 TEAEs occurred in 5 subjects (11.6%) after administration of the loose combination. Among the 19 TEAEs, 15 TEAEs in 8 (18.6%) subjects were considered to be related to the study drug and classified as ADRs. Chapped lips, oral macule, limb mass, and neck pain were TEAEs considered unrelated to the study drug. All TEAEs were resolved without sequelae and were mild in severity, except for 1 moderate migraine. The frequency of TEAEs for both the FDC and the corresponding loose combination was also comparable. No serious adverse events were reported during the study. No clinically significant abnormalities or changes from baseline were observed in the vital signs, physical examination, 12-lead ECG, or clinical laboratory tests. Both the FDC and the loose combination were safe and well tolerated.
Discussion
This study aimed to compare the pharmacokinetic characteristics and safety of atorvastatin and omega-3-acid ethyl esters (EPA and DHA) between the FDC formulation (atorvastatin/omega-3-acid ethyl esters 10/1000 mg × 4) and the corresponding loose combination (atorvastatin tablet 10 mg × 4 and omega-3-acid ethyl esters soft capsule 1000 mg × 4) in healthy subjects. The GMR and its 90% CI of the FDC to loose combination for Cmax and AUClast of atorvastatin, EPA, and DHA were all within the bioequivalence range of 0.8–1.25, demonstrating comparable PK profiles between the FDC and loose combination. Both the FDC and the loose combination were safe and well tolerated. The multilayer barrier coating technology developed by Korean United Pharm. Inc. was applied and enabled both the omega-3 and atorvastatin substances to remain intact within the FDC. Therefore, the clinical impact of the formulation on the PK and safety profiles of the FDC would likely be minimal. This multilayer barrier coating technology could be further applied to the development of FDCs of other drug substances.
Generally, a 3-period partial replicated crossover design (RRT – RTR – TRR) or a 4-period replicated crossover design (RTRT – TRTR) is recommended in bioequivalence studies of highly variable drugs.18 The replicated crossover study design has the advantage of reducing the sample size compared to the conventional crossover design. For instance, 62 subjects were needed to obtain a similar power for the conventional 2×2 crossover study design with the intra-subject CV (%) of the Cmax for omega-3-acid ethyl esters (43.1%).16 Considering these highly variable characteristics of the active compounds, a 2×2×4 replicate crossover design was applied to evaluate the bioequivalence between the FDC formulation of atorvastatin and omega-3-acid ethyl esters and the corresponding loose combination. The 4-way replicated crossover design was successfully applied in this study with a relatively small sample size compared to the sample size required for the 2-way crossover design (44 vs 62 subjects, respectively). The reduction in sample size contributed to the efficient recruitment of participants. As the overall study time of replicated crossover design is longer than the conventional 2-way crossover design, it may lead to a higher dropout rate of subjects. Those designs’ pros and cons must be thoroughly considered before the initiation of comparative pharmacokinetic studies.
In this clinical study, the intra-subject CVs (%) for Cmax and AUClast of DHA were 30.8% and 37.5%, respectively, showing high variability. The intra-subject CVs (%) for the Cmax of atorvastatin and the Cmax and AUClast of EPA were approximately 25%, showing a variability close to 30%. According to the MFDS and European Medicines Association, the reference scaled bioequivalence approach can be used in the bioequivalence assessment of highly variable drugs. If the observed reference within-subject standard deviation of the Cmax of DHA were applied, the bioequivalence acceptance range of the Cmax of DHA could have been scaled to 0.7956–1.2570. However, as the GMRs and 90% CIs of the Cmax of DHA were within the conventional bioequivalence acceptance range (0.8–1.25), the scaled bioequivalence approach was not used.
Absorption of omega-3-acid ethyl esters requires stimulation of pancreatic lipase by a high-fat meal.19 In a randomized, open-label study to compare the pharmacokinetics of two different omega-3 fatty acid formulations, the bioavailability of both formulations (omega-3 carboxylic acids and ethyl esters) dramatically increased when they were consumed with a high-fat meal.11 To fully compare the pharmacokinetic characteristics of the FDC and loose combination, a high-fat meal (≥900 kcal and ≥35% lipid content) was given before administration of the study drug. In addition, omega-3 fatty acid consumption from food was controlled by excluding fish-containing meals during the study period.
According to FDA draft guidance on omega-3-acid ethyl esters, baseline measurement should be calculated from an average of three or more samples collected between 24 and 0 hours (inclusive) prior to dosing.20 In this study, six PK blood samples for baseline adjustment were collected at −15, −14, −13, −12, −11, and 0 h predose. Although it was reported that there is a circadian variation in lipid metabolism due to high tissue uptake of fatty acids during sleep,21 the extent of oscillation was not substantial. Additionally, compared to previous PK studies of omega-3 fatty acids,16,22,23 we intensively assessed baseline PK samples, and the baseline fluctuation observed from this study was marginal.
Given that this study was conducted among healthy Korean male participants, potential variations in the PK of endogenous DHA and EPA based on gender, ethnicity, and renal or hepatic impairment remain plausible. However, we suggest that these differences are minimized due to the implementation of the extensive baseline adjustments. Furthermore, considering the study’s findings demonstrating comparable PK and safety between the FDC and the loose combination, these outcomes are anticipated to remain consistent across diverse patient populations.
DHA is an endogenous compound. In the administration of omega-3 fatty acids, findings from a prior clinical trial and published data showed a reduction in plasma DHA concentration for 12 hours after peak levels, followed by a subsequent increase.24,25 An isotope analysis study after EPA supplementation in humans demonstrated a substantial conversion of EPA to DHA, without retroconversion of DHA to EPA.26 In addition, an animal study to investigate the plasma and brain synthesis pathways of DHA also proposed that free plasma EPA entering the liver is desaturated to DHA and secreted into the plasma.27 The plasma DHA level observed after 12 hours is likely to be the concentration of DHA synthesized from EPA rather than the concentration administered as a study drug. Therefore, we planned to collect blood samples for DHA until 12 hours postdose to observe the exact elimination phase.
Conclusion
The FDC of atorvastatin and omega-3-acid ethyl esters showed comparable PK characteristics to the corresponding loose combination, offering a convenient therapeutic option for the treatment of combined hyperlipidemia.
Data Sharing Statement
The data supporting the published results of this study will be shared upon a reasonable request made to the corresponding author or sponsor.
Acknowledgment
Juyoung Khwarg received a scholarship from the BK21FOUR education program.
Funding
This study was sponsored by Korea United Pharm. Inc., Seoul, Republic of Korea.
Disclosure
Won-Ho Kang, Youn Woong Choi, Dae Chul Ha, RaeHoon Jung, Min-Gu Han, Won Tae Jung, Kyu-Yeol Nam, and YeSeul Kim are employees of Korea United Pharm. Inc. Hye Jung Lee, Kyu Yeon Kim, Ki-Sun Jeong, and Chongho Won are employees of Caleb Multilab, Inc. The other authors do not have any conflicts of interest for this study.
References
1. Nikos Pappan AR. Dyslipidemia. Treasure Island (FL): StatPearls Publishing; 2022.
2. Ference BA, Ginsberg HN, Graham I, et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J. 2017;38(32):2459–2472. doi:10.1093/eurheartj/ehx144
3. Cholesterol Treatment Trialists, C. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170 000 participants in 26 randomised trials. Lancet. 2010;376(9753):1670–1681. doi:10.1016/S0140-6736(10)61350-5
4. Baigent C, Keech A, Kearney PM, et al. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90 056 participants in 14 randomised trials of statins. Lancet. 2005;366(9493):1267–1278. doi:10.1016/S0140-6736(05)67394-1
5. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. J Am Coll Cardiol. 2019;73(24):e285–e350. doi:10.1016/j.jacc.2018.11.003
6. Jellinger PS, Handelsman Y, Rosenblit PD, et al. American Association of clinical endocrinologists and American college of endocrinology guidelines for management of dyslipidemia and prevention of cardiovascular disease. Endocr Pract. 2017;23:1–87. doi:10.4158/EP171764.APPGL
7. Mach F, Baigent C, Catapano AL, et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk: the task force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS). Eur Heart J. 2019;41(1):111–188.
8. Harris WS, Miller M, Tighe AP, et al. Omega-3 fatty acids and coronary heart disease risk: clinical and mechanistic perspectives. Atherosclerosis. 2008;197(1):12–24. doi:10.1016/j.atherosclerosis.2007.11.008
9. Shearer GC, Savinova OV, Harris WS. Fish oil -- how does it reduce plasma triglycerides? Biochim Biophys Acta. 2012;1821(5):843–851. doi:10.1016/j.bbalip.2011.10.011
10. Oscarsson J, Hurt-Camejo E. Omega-3 fatty acids eicosapentaenoic acid and docosahexaenoic acid and their mechanisms of action on apolipoprotein B-containing lipoproteins in humans: a review. Lipids Health Dis. 2017;16(1):149. doi:10.1186/s12944-017-0541-3
11. Davidson MH, Stein EA, Bays HE, et al. Efficacy and tolerability of adding prescription omega-3 fatty acids 4 g/d to simvastatin 40 mg/d in hypertriglyceridemic patients: an 8-week, randomized, double-blind, placebo-controlled study. Clin Ther. 2007;29(7):1354–1367. doi:10.1016/j.clinthera.2007.07.018
12. Yokoyama M, Origasa H, Matsuzaki M, et al. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis. Lancet. 2007;369(9567):1090–1098. doi:10.1016/S0140-6736(07)60527-3
13. Kim JH, Sunwoo J, Song JH, et al. Pharmacokinetic interaction between atorvastatin and omega-3 fatty acid in healthy volunteers. Pharmaceuticals. 2022;15(8):962. doi:10.3390/ph15080962
14. Woo JS, Hong SJ, Cha DH, et al. Comparison of the efficacy and safety of atorvastatin 40 mg/ω-3 fatty acids 4 g fixed-dose combination and atorvastatin 40 mg monotherapy in hypertriglyceridemic patients who poorly respond to atorvastatin 40 mg monotherapy: an 8-week, multicenter, randomized, double-blind Phase III Study. Clin Ther. 2021;43(8):1419–1430. doi:10.1016/j.clinthera.2021.07.001
15. Chung I, Oh J, Lee S, et al. A post hoc analysis of intra-subject coefficients of variation in pharmacokinetic measures to calculate optimal sample sizes for bioequivalence studies. Transl Clin Pharmacol. 2018;26(1):6–9. doi:10.12793/tcp.2018.26.1.6
16. Davidson MH, Johnson J, Rooney MW, et al. A novel omega-3 free fatty acid formulation has dramatically improved bioavailability during a low-fat diet compared with omega-3-acid ethyl esters: the ECLIPSE (Epanova(®) compared to Lovaza(®) in a pharmacokinetic single-dose evaluation) study. J Clin Lipidol. 2012;6(6):573–584. doi:10.1016/j.jacl.2012.01.002
17. Whitfield LR, Stern RH, Sedman AJ, et al. Effect of food on the pharmacodynamics and pharmacokinetics of atorvastatin, an inhibitor of HMG-CoA reductase. Eur J Drug Metab Pharmacokinet. 2000;25(2):97–101. doi:10.1007/BF03190074
18. Guidance for Industry. Bioequivalence Studies with Pharmacokinetic End-Points for Drugs Submitted Under an Anda. U.S. Food and Drug Administration; 2021.
19. Lawson LD, Hughes BG. Absorption of eicosapentaenoic acid and docosahexaenoic acid from fish oil triacylglycerols or fish oil ethyl esters co-ingested with a high-fat meal. Biochem Biophys Res Commun. 1988;156(2):960–963. doi:10.1016/S0006-291X(88)80937-9
20. U.S. Food and Drug Administration. Draft Guidance on Omega-3-Acid Ethyl Esters. U.S. Food and Drug Administration; 2020.
21. Jackson PA, Husberg C, Hustvedt S-O, et al. Diurnal rhythm of plasma EPA and DHA in healthy adults. Prostaglandins Leukot Essent Fatty Acids. 2020;154:102054. doi:10.1016/j.plefa.2020.102054
22. Lapointe J-F, Harvey L, Aziz S, et al. A single-dose, comparative bioavailability study of a formulation containing OM3 as phospholipid and free fatty acid to an ethyl ester formulation in the fasting and fed states. Clin Ther. 2019;41(3):426–444. doi:10.1016/j.clinthera.2019.01.017
23. Jing S, Zhang Z, Chen X, et al. Pharmacokinetics of single and multiple doses of omega-3 carboxylic acids in healthy Chinese subjects: a Phase I, open-label study. Clin Pharmacol Drug Dev. 2020;9(8):985–994. doi:10.1002/cpdd.800
24. Chevalier L, Plourde M. Comparison of pharmacokinetics of omega-3 fatty acid supplements in monoacylglycerol or ethyl ester in humans: a randomized controlled trial. Eur J Clin Nutr. 2021;75(4):680–688. doi:10.1038/s41430-020-00767-4
25. Chevalier L, Vachon A, Plourde M. Pharmacokinetics of supplemental omega-3 fatty acids esterified in monoglycerides, ethyl esters, or triglycerides in adults in a randomized crossover trial. J Nutr. 2021;151(5):1111–1118. doi:10.1093/jn/nxaa458
26. Metherel AH, Irfan M, Klingel SL, et al. Compound-specific isotope analysis reveals no retroconversion of DHA to EPA but substantial conversion of EPA to DHA following supplementation: a randomized control trial. Am J Clin Nutr. 2019;110(4):823–831. doi:10.1093/ajcn/nqz097
27. Metherel AH, Rezaei K, Lacombe RJS, et al. Plasma unesterified eicosapentaenoic acid is converted to docosahexaenoic acid (DHA) in the liver and supplies the brain with DHA in the presence or absence of dietary DHA. Biochim Biophys Acta Mol Cell Biol Lipids. 2021;1866(8):158942. doi:10.1016/j.bbalip.2021.158942
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.







