Back to Journals » Infection and Drug Resistance » Volume 9
Pharmacist-managed dose adjustment feedback using therapeutic drug monitoring of vancomycin was useful for patients with methicillin-resistant Staphylococcus aureus infections: a single institution experience
Authors Hirano R , Sakamoto Y, Kitazawa J, Yamamoto S, Tachibana N
Received 29 March 2016
Accepted for publication 21 June 2016
Published 14 October 2016 Volume 2016:9 Pages 243—252
DOI https://doi.org/10.2147/IDR.S109485
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Suresh Antony
Ryuichi Hirano,1 Yuichi Sakamoto,2 Junichi Kitazawa,2 Shoji Yamamoto,1 Naoki Tachibana2
1Department of Pharmacy, 2Laboratory Medicine and Blood Transfusion, Aomori Prefectural Central Hospital, Aomori-shi, Japan
Background: Vancomycin (VCM) requires dose adjustment based on therapeutic drug monitoring. At Aomori Prefectural Central Hospital, physicians carried out VCM therapeutic drug monitoring based on their experience, because pharmacists did not participate in the dose adjustment. We evaluated the impact of an Antimicrobial Stewardship Program (ASP) on attaining target VCM trough concentrations and pharmacokinetics (PK)/pharmacodynamics (PD) parameters in patients with methicillin-resistant Staphylococcus aureus (MRSA) infections.
Materials and methods: The ASP was introduced in April 2012. We implemented a prospective audit of prescribed VCM dosages and provided feedback based on measured VCM trough concentrations. In a retrospective pre- and postcomparison study from April 2007 to December 2011 (preimplementation) and from April 2012 to December 2014 (postimplementation), 79 patients were treated for MRSA infection with VCM, and trough concentrations were monitored (pre, n=28; post, n=51). In 65 patients (pre, n=15; post, n=50), 24-hour area under the concentration–time curve (AUC 0–24 h)/minimum inhibitory concentration (MIC) ratios were calculated.
Results: Pharmacist feedback, which included recommendations for changing dose or using alternative anti-MRSA antibiotics, was highly accepted during postimplementation (88%, 29/33). The number of patients with serum VCM concentrations within the therapeutic range (10–20 μg/mL) was significantly higher during postimplementation (84%, 43/51) than during preimplementation (39%, 11/28) (P<0.01). The percentage of patients who attained target PK/PD parameters (AUC 0–24 h/MIC >400) was significantly higher during postimplementation (84%, 42/50) than during preimplementation (53%, 8/15; P=0.013). There were no significant differences in nephrotoxicity or mortality rate.
Conclusion: Our ASP increased the percentage of patients that attained optimal VCM trough concentrations and PK/PD parameters, which contributed to the appropriate use of VCM in patients with MRSA infections.
Keywords: antimicrobial stewardship, prospective audit and feedback, therapeutic drug monitoring, vancomycin
Introduction
Vancomycin (VCM), a glycopeptide antibiotic, has antibiotic activity against Gram-positive bacteria, including methicillin-resistant Staphylococcus aureus (MRSA).1 Acute kidney injury (AKI), which is correlated with high serum VCM concentrations, is a major known side effect of VCM.2 The subtherapeutic range of VCM trough concentrations (<10 μg/mL) is associated with the emergence of heteroresistant vancomycin-intermediate Staphylococcus aureus (hetero VISA), as well as treatment failure in patients with MRSA infections.3,4 Dose adjustment, based on therapeutic drug monitoring (TDM), is required to attain a serum VCM concentration within the therapeutic range.2 Previous studies have demonstrated that VCM 24-hour area under the concentration–time curve (AUC 0–24 h)/minimum inhibitory concentration (MIC) is correlated with treatment outcomes of patients treated with VCM.2,5 Inappropriate pharmacokinetics (PK)/pharmacodynamics (PD) parameters are a potential risk factor for VCM treatment failure in patients with MRSA infections.5–7
Practice guidelines for VCM TDM have been published by the Japanese Society of Chemotherapy and the Japanese Society of Therapeutic Drug Monitoring in Japan.2 In contrast to the 2009 consensus guidelines published by the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists, the guidelines in Japan recommend a wide VCM trough concentration range (10–20 μg/mL).2,8
In our hospital, physicians carried out VCM TDM based on their experience, because pharmacists did not begin to participate in the dose adjustment of VCM until March 2012. Therefore, different target ranges of VCM trough concentrations were observed, which was a crucial problem in VCM monitoring. To resolve this problem, in April 2012, pharmacists put into practice a prospective audit of the initial VCM dosage and provided recommendations on dose adjustments, based on TDM for individual patients with MRSA infections.
Recently, Antimicrobial Stewardship Programs (ASPs) were proposed with the aim of preventing multidrug-resistant bacteria from developing and ensuring the appropriate use of antibiotics.9 However, few studies have attempted to optimize therapeutic monitoring. In particular, whether a pharmacist-managed ASP achieves optimal VCM trough concentrations and PK/PD parameters, in patients with MRSA infections, has not been thoroughly investigated. The main purpose of this study was to evaluate the impact of the ASP on attaining target VCM trough concentrations and PK/PD parameters in patients with MRSA infections.
Materials and methods
Study design and patients
We conducted a retrospective pre- and postcomparison study, from April 2007 to December 2011 (preimplementation group) and from April 2012 to December 2014 (postimplementation group), in Aomori Prefectural Central Hospital, a general hospital with 695 beds in Aomori City, Japan. Of 817 (pre, n=456; post, n=361) patients treated with intravenous VCM, medical records of serum VCM concentrations for 431 patients (pre, n=182; post, n=249) were used for retrospective analysis (Figure 1). Patients treated with VCM for less than 3 days, patients under 18 years, patients with concomitant use of nephrotoxic agents (eg, aminoglycoside antibiotics, cyclosporine, and tacrolimus), patients who did not have steady-state VCM trough concentration samples, patients without isolation of positive cultured MRSA, patients requiring hemodialysis, and patients without symptoms of infection were excluded from the study. Diagnosis of MRSA infection was limited to patients with a positive culture for MRSA from clinical specimens (eg, sputum and pus) and symptoms of infection (eg, fever over 37°C and high concentrations of inflammatory markers). MRSA cultures of typically sterile specimens (eg, spinal fluid and blood) were positive in all patients.10 Seventy-nine patients with MRSA infections were included in this study (pre, n=28; post, n=51). We evaluated VCM trough concentrations at steady state, clinical outcomes, and the percentage of patients who had undergone VCM dose adjustment and patients who had changed to different anti-MRSA antibiotics, based on pharmacist recommendations. The VCM trough concentrations at steady state were defined as those determined after the fifth dose or on day 3 after the initiation of therapy according to the guidelines for TDM.2
  | Figure 1 Study design. Abbreviations: VCM, vancomycin; MRSA, methicillin-resistant Staphylococcus aureus; PK, pharmacokinetics; PD, pharmacodynamics. |
Days from the initiation of VCM therapy to attaining target trough concentration were examined based on the records of TDM. The maintenance dose of VCM was adopted in the evaluation of dosage.2 MIC values for VCM, between 0.5 and 2 μg/mL, were determined by the broth microdilution method, in 66 MRSA isolates (pre, n=15; post, n=51). The VCM AUC 0–24 h was calculated based on the Bayesian method, using Japanese population PK parameters and measured VCM trough concentrations at steady state in 65 patients (pre, n=15; post, n=50). Then, the VCM AUC 0–24 h/MIC ratio was evaluated.11 The study protocol was approved by the Ethics Committee of Aomori Prefectural Central Hospital, and patient anonymity was assured. Patient consent was not required due to the retrospective nature of this study.
Microbial analysis and laboratory data
Susceptibility tests of MRSA isolates for antibiotics were performed according to the clinical and laboratory standards institute recommended method using a MicroScan Walkaway 96Plus® (Beckman Coulter, Brea, CA, USA).12 Serum VCM concentrations were determined by fluorescence polarization immunoassay using a TDX FLX analyzer® (Abbott Laboratories, Lake Bluff, IL, USA), from April 2007 to March 2010. The analysis method was changed to a chemiluminescent immunoassay using an Architect i1000SR analyzer® (Abbott Laboratories) due to discontinuation of regents for the TDX FLX analyzer®.
Implementation of ASP by pharmacists
From April 2012, our ASP underwent a prospective audit, for the initial prescribed dosage of VCM, appropriate dose recommendations, and alternative anti-MRSA agents based on the results of steady-state VCM trough concentrations and clinical characteristics, by infectious diseases pharmacists. When patients receiving routine VCM therapy were identified, the estimated trough concentrations of the initial maintenance dose were calculated according to Japanese VCM population PK parameters using renal function, age, body weight, and sex.11 Patients who received VCM therapy were identified by auditing records of VCM prescriptions. The concentration estimates were reported to medical physicians on the second day of therapy and a proposed dose adjustment was recommended by pharmacists based on measured VCM trough concentrations. Then, infectious diseases pharmacists offered dose recommendations to physicians, based on VCM steady-state trough concentrations. The target range of VCM trough concentrations was 10–20 μg/mL, according to Japanese VCM TDM practice guidelines published in 2012.2 We recommended decreasing the VCM dose if maximum trough concentration exceeded 20 μg/mL; in these cases, the dosage of VCM was determined based on the renal function and body weight. In cases that the maximum trough concentration was less than 10 μg/mL, it was recommended that physicians increase the dose of VCM. We considered 3 g/d as the upper limit of dose in cases of increasing the dosage. We recommended the continuation of the initial VCM dosage within target range. We also recommended alternative anti-MRSA antibiotics in case we find patients with MRSA of MIC 2 μg/mL for VCM, patients with severe renal function, patients with unstable hemodynamics, or patients with lack of efficacy despite therapeutic range of VCM.
Clinical outcome analysis
VCM-induced AKI was evaluated based on risk injury failure loss end-stage renal disease criteria.13 The efficacy of VCM therapy was evaluated by a 30-day mortality rate, after the initiation of VCM, as previously described.14–16 The length of hospital stay was calculated from the initiation of VCM therapy to hospital discharge.
Calculation of PK parameters
According to previous reports, VCM PK parameters (eg, VCM clearance and volume of distribution) were calculated based on Bayesian forecasting methods, using individual VCM trough concentrations at steady state, creatinine clearance, and the Japanese VCM population PK parameters.11,17 A two-compartment model was applied for estimation of individual VCM PK parameters.11 Calculation of individual VCM AUC 0–24 h was performed based on daily VCM dosage and VCM clearance predicted by Bayesian forecasting methods. Creatinine clearance was calculated using the Cockcroft–Gault equation. Among patients who changed VCM dosage based on TDM, AUC 0–24 h and trough concentration after the change of dosage were adopted for evaluation. The target VCM PK/PD parameter for calculated AUC 0–24 h/MIC was greater than that given in the guidelines.2,8
Statistical analysis
Results are expressed as mean ± standard deviation or n (%). Continuous data were analyzed using the Student’s t-test, and categorical data were analyzed using the χ2 test. Variations in the trough concentration were evaluated based on the coefficient of variation and F-test. The 30-day mortality rate, length of hospital stay, and percentage of patients without therapeutic range of VCM trough concentration were examined by Kaplan–Meier plots and log-rank test. Receiver operating characteristic analysis was performed to predict the cutoff VCM trough concentration associated with AKI. P<0.05 was considered significant. All statistical analyses were performed using Excel-Toukei 2012 (Social Survey Research Information Co, Ltd, Tokyo, Japan).
Results
Patient characteristics
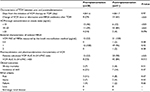
Of 817 (pre, n=456; post, n=361) patients who were treated with intravenous VCM, the percentage of patients whose serum VCM concentrations were measured was significantly higher during postimplementation (69%, 249/361) than during preimplementation (40%, 182/456), according to the χ2 test (P<0.01). There were 79 patients (preimplementation, n=28; postimplementation, n=51) examined in this study (55 males, 24 females; mean age, 68.2±15.8 years old; age range, 22–93 years old). Patient demographics, dose and duration of VCM therapy, laboratory data, and site of infection are listed in Table 1. No significant difference was observed in the duration of VCM therapy and dosage between pre- and postimplementation groups according to the Student’s t-test. The F-test revealed that postimplementation patients had a significantly lower variation of mean VCM trough concentration than preimplementation patients (P<0.01).
  | Table 1 Patient characteristics Note: Data are mean ± SD or n (%). Abbreviations: VCM, vancomycin; CV, coefficient of variation; SD, standard deviation. |
Comparison of clinical and laboratory parameters in patients with pre- and postimplementation
Comparisons of VCM trough concentration at steady state, clinical outcomes, and PK/PD parameters between pre- and postimplementation of ASP are summarized in Table 2. The percentage of patients whose VCM dose was changed or who received alternative anti-MRSA antibiotics after first TDM was significantly higher during postimplementation (65%, 33/51) than during preimplementation (29%, 8/28). The number of patients with VCM trough concentration in the subtherapeutic range (<10 μg/mL) was significantly lower during postimplementation (12%, 6/51) than during preimplementation (46%, 13/28). The number of patients with VCM trough concentrations in the therapeutic range (10–20 μg/mL) was significantly higher during postimplementation (84%, 43/51) than during preimplementation (39%, 11/28). The number of patients with VCM trough concentration in the supratherapeutic range (>20 μg/mL) was lower during postimplementation (4%, 2/51) than during preimplementation (14%, 4/28); however, the χ2 analysis showed that this difference was not significant (P=0.096). The percentage of patients who attained the target PK/PD parameter value (VCM AUC 0–24 h/MIC >400) was significantly higher during postimplementation (84%, 42/50) than during preimplementation (53%, 8/15; P=0.013).
The mortality rate within 30 days after the initiation of VCM therapy was similar during postimplementation (4%, 2/51) and preimplementation (7%, 2/28). The incidence of VCM-induced AKI during postimplementation (14%, 7/51) was similar to that during preimplementation (21%, 6/28).
Pharmacist recommendations and percent acceptance by physicians in postimplementation
Pharmacist recommendations and percent acceptance during postimplementation are listed in Table 3. Increasing the dose of VCM was recommended for ten patients. Decreasing the dose of VCM was recommended for 18 patients. In five patients with 2 μg/mL of MRSA MIC value for VCM, VCM-induced AKI, unstable renal function, and lack of efficacy of VCM therapy in two patients with meningitis or pneumonia, the use of alternative anti-MRSA antibiotics was recommended for the following reasons. Pharmacists recommended that physicians use alternative anti-MRSA agents and change the dose of VCM for the 33 patients. Physicians made the recommended changes for 29 patients (88%). Percent acceptance for each recommendation is as follows: increasing of VCM dosage, 70% (7/10); decreasing of VCM dosage, 94% (17/18); and alternative anti-MRSA antibiotics, 100% (5/5).
  | Table 3 Pharmacist recommendations and percent acceptance by physicians in postimplementation Abbreviations: VCM, vancomycin; MRSA, methicillin-resistant Staphylococcus aureus. |
Comparison of VCM trough concentration, AKI, and mortality rate
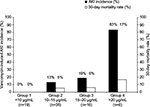
According to trough concentrations, patients were divided into four groups. Group 1 was VCM trough concentration <10 μg/mL (n=19), Group 2 was 10–15 μg/mL (n=38), Group 3 was 15–20 μg/mL (n=16), and Group 4 was >20 μg/mL (n=6). The AKI incidence and 30-day mortality rate of each group were compared (Figure 2). The incidence of AKI and the 30-day mortality rate of each group were as follows: Group 1: 0% (0/19), 0% (0/19); Group 2: 13% (5/38), 5% (2/38); Group 3: 19% (3/16), 6% (1/16); and Group 4: 83% (5/6), 17% (1/6), respectively. There was a significant difference in the incidence of AKI in Group 1 vs Group 3 (P=0.048), Group 1 vs Group 4 (P<0.01), Group 2 vs Group 4 (P<0.01), and Group 3 vs Group 4 (P<0.01). No significant difference was observed in the 30-day mortality rate among the groups. The area under the receiver operating characteristic curve was 0.831 for VCM-induced AKI. The cutoff VCM trough concentration for AKI was 14.5 μg/mL (sensitivity, 74%; specificity, 77%).
Kaplan–Meier plots of sequential parameters among patients during pre- and postimplementation
Comparisons of sequential parameters using Kaplan–Meier plots between pre- and postimplementation of ASP are shown in Figure 3. The percentage of patients without target range of VCM trough concentration was significantly lower during post implementation than during preimplementation according to log-rank test (P<0.01). No significant difference was observed in the 30-day mortality rate or length of stay between pre- and postimplementation. We divided the patients into two groups according to days from the initiation of VCM therapy to attaining target range to evaluate whether the days to a therapeutic range of concentration are related to the clinical outcomes. Group A included patients attaining therapeutic range within 3 days of therapy (n=53), and Group B included patients attaining therapeutic range after more than 4 days of therapy (n=26). Group B included patients with subtherapeutic range of trough concentration. The cutoff value of days was determined by the median days from the initiation of VCM therapy to attaining target range among the total number of patients. The 30-day mortality rate and the length of hospital stay of each group were as follows: Group A: 6% (3/53) and 53.7±61.8 days, and Group B: 4% (1/26) and 50.4±51.1 days, respectively. No significant difference was observed in the 30-day mortality rate or length of hospitalization among the groups according to log-rank test.
Discussion
A prospective audit and feedback is one of the ASPs recommended by the Infectious Diseases Society of America.9 One of the advantages of this intervention is that it enables real-time education of physicians without infringing upon physician autonomy to prescribe medications.18 Some studies have demonstrated that implementation of a pharmacist-led VCM dosing protocol achieved a higher percentage of patients with VCM trough concentrations within the therapeutic range.19,20 However, there are few hospitals implementing such pharmacist-managed protocol therapy, because pharmacists are not permitted to write prescriptions and perform clinical laboratory tests in Japan. Therefore, we considered a prospective audit and feedback as a realistic approach to improve VCM therapeutic monitoring work, and carried out this program.
In our study, a significant difference was observed in patients with trough concentrations within the subtherapeutic range (<10 μg/mL) between pre- and postimplementation. In approximately 50% of the patients, the trough concentrations were within the subtherapeutic range during preimplementation, although a consensus review of guidelines for VCM TDM was published in 2009.8 The subtherapeutic range of VCM trough concentrations is associated with the emergence of hetero VISA.3 VCM therapy has not been recommended for patients with hetero-VISA due to lack of clinical efficacy.21 The number of patients with VCM trough concentrations within the therapeutic range (10–20 μg/mL) was significantly higher during postimplementation (84%, 43/51) than during preimplementation (39%, 11/28). There was a significantly higher percentage of patients attaining the target PK/PD parameter value (AUC 0–24 h/MIC >400) during postimplementation (84%, 42/50) than during preimplementation (53%, 8/15). A significant difference was observed in trough concentrations between pre- and postimplementation based on the results of the F-test. These favorable effects on laboratory parameters were responsible for our ASP and high acceptance rate of recommendations by physicians during post implementation. No significant difference was observed in the incidence of AKI between pre- and postimplementation. A similar finding that a pharmacist-managed VCM protocol attained a higher rate of VCM trough concentrations within the therapeutic range, without increasing nephrotoxicity, has been reported.19 Pharmacist recommendations to decrease the VCM dose had a high percent acceptance (94%, 17/18) during postimplementation. In addition, as a result of these recommendations, the percentage of patients with a VCM trough concentration in the supratherapeutic range (>20 μg/mL) decreased; there was the associated benefit of an increase in the number of patients who attained a therapeutic range of trough concentration without an increase in AKI.
We divided all patients into four groups and compared the AKI incidence rate and 30-day mortality rate of each group. There was a significant difference in the incidence of AKI between patients with >20 μg/mL and the other groups. We identified the cutoff VCM trough concentration that was associated with AKI: 14.5 μg/mL (sensitivity, 74%; specificity, 77%). This cutoff value compared well with a previous study.22 High trough concentration (>15–20 μg/mL) was associated with an increase in VCM-induced AKI.23 On the other hand, this range was recommended in guidelines to attain efficacy in patients with complicated MRSA infections (eg, bacteremia and meningitis).2,8 Therefore, close attention should be paid to nephrotoxicity in patients who maintain trough concentrations within this range.
Limitations
The limitations of this study were as follows. There was no significant difference in the 30-day mortality rate and length of hospital stay, although a large number of patients could attain VCM trough concentrations within the therapeutic range (10–20 μg/mL) during postimplementation. A previous study found that the efficacy of VCM therapy was improved by changing the target range of VCM trough concentrations from 5–20 μg/mL to 15–20 μg/mL in patients with MRSA infections.24 Our results differed from previous findings. In addition to VCM trough concentration, other factors (eg, higher acute physiology and chronic health evaluation II score, MRSA strains that had 2 μg/mL MIC value for VCM) have also been reported as risk factors for VCM treatment failure in patients with MRSA infection.25,26 Among the 66 patients isolated MRSA with MIC values for VCM between 0.5–2 μg/mL, only one patient (2%, 1/66) had 2 μg/mL MIC value. In our hospital, approximately 96% of patients (64/66) had 1 μg/mL MIC value for VCM, which is higher than that of previous reports.26 A significant difference was observed in some clinical parameters (eg, sex, patients with skin and soft tissue infection, and bacteremia infection) between pre- and postimplementation because a matched case–control study could not be performed due to lack of cases. This is a retrospective pre- and postcomparison study at a single institution. In addition to being a single-institution study, the sample size is small, because VCM serum concentration monitoring was performed in few patients in the preimplementation group. Therefore, meaningful statistical analyses could not be performed due to the insufficient number of patients. Further research, accounting for these factors, is required to evaluate the impact of ASP on the efficacy of VCM therapy.
The ASP in this study was a real-time audit of prescribed VCM dosage and recommendations based on the results of TDM by infectious diseases pharmacists. We were unable to participate in the initial VCM dosage calculation, which was the most serious limitation of our ASP. A previous study demonstrated that the loading dosage approach allowed for the VCM trough concentration to rapidly approach the therapeutic range, which led to favorable clinical outcomes, although no significant difference was observed in mortality rate concerning early attaining of therapeutic range in this study.27 However, this approach has not yet been accepted in many hospitals.28 The use of a weight-based dosing protocol increased the percentage of patients who could rapidly achieve the optimal VCM PK/PD parameter value.20 Prescription writing by pharmacists is strictly limited in Japan. Pharmacist-managed treatment protocols will be required to address this limitation.
Conclusion
Pharmacist recommendations based on the results of VCM TDM were highly accepted by many physicians in our hospital. Our ASP increased the percentage of patients attaining optimal VCM trough concentration and the PK/PD parameter value, which contributed to the appropriate use of VCM in patients with MRSA infections. Further research is required to evaluate the impact of this ASP on the efficacy of VCM therapy.
Acknowledgments
The authors thank Dr Motoki Ohnishi (Department of General Medicine, Aomori Prefectural Central Hospital), Kota Nakaya, Chika Kudo, and Katsuyoshi Osanai (Department of Pharmacy, Aomori Prefectural Central Hospital) for their comments and help on this study.
Disclosure
The authors report no conflicts of interest in this work.
References
Levine DP. Vancomycin: a history. Clin Infect Dis. 2006;42 (Suppl 1):S5–S12. | ||
Matsumoto K, Takesue Y, Ohmagari N, et al. Practice guidelines for therapeutic drug monitoring of vancomycin: a consensus review of the Japanese Society of Chemotherapy and the Japanese Society of Therapeutic Drug Monitoring. J Infect Chemother. 2013;19(3):365–380. | ||
Charles PG, Ward PB, Johnson PD, Howden BP, Grayson ML. Clinical features associated with bacteremia due to heterogeneous vancomycin-intermediate Staphylococcus aureus. Clin Infect Dis. 2004;38(3):448–451. | ||
Kullar R, Davis SL, Levine DP, Rybak MJ. Impact of vancomycin exposure on outcomes in patients with methicillin-resistant Staphylococcus aureus bacteremia: support for consensus guidelines suggested targets. Clin Infect Dis. 2011;52(8): 975–981. | ||
Moise-Broder PA, Forrest A, Birmingham MC, Schentag JJ. Pharmacodynamics of vancomycin and other antimicrobials in patients with Staphylococcus aureus lower respiratory tract infections. Clin Pharmacokinet. 2004;43(13):925–942. | ||
Ghosh N, Chavada R, Maley M, van Hal SJ. Impact of source of infection and vancomycin AUC0-24/MICBMD targets on treatment failure in patients with methicillin-resistant Staphylococcus aureus bacteraemia. Clin Microbiol Infect. 2014;20(12):1098–1105. | ||
Men P, Li HB, Zhai SD, Zhao RS. Association between the AUC0-24/MIC ratio of vancomycin and its clinical effectiveness: a systematic review and meta-analysis. PLoS One. 2016;11(1):e0146224. | ||
Rybak M, Lomaestro B, Rotschafer JC, et al. Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm. 2009;66(1):82–98. | ||
Dellit TH, Owens RC, McGowan JE Jr, et al. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis. 2007;44(2):159–177. | ||
Huang SS, Platt R. Risk of methicillin-resistant Staphylococcus aureus infection after previous infection or colonization. Clin Infect Dis. 2003;36(3):281–285. | ||
Yasuhara M, Iga T, Zenda H, et al. Population pharmacokinetics of vancomycin in Japanese adult patients. Ther Drug Monit. 1998;20(2):139–148. | ||
Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing: M100-S16. Wayne, PA: Clinical and Laboratory Standards Institute; 2006. | ||
Hoste EA, Clermont G, Kersten A, et al. RIFLE criteria for acute kidney injury are associated with hospital mortality in critically ill patients: a cohort analysis. Crit Care. 2006;10(3):R73. | ||
Hermsen ED, Hanson M, Sankaranarayanan J, Stoner JA, Florescu MC, Rupp ME. Clinical outcomes and nephrotoxicity associated with vancomycin trough concentrations during treatment of deep-seated infections. Expert Opin Drug Saf. 2010;9(1):9–14. | ||
Shoji H, Maeda M, Shirakura T, et al. More accurate measurement of vancomycin minimum inhibitory concentration indicates poor outcomes in methicillin-resistant Staphylococcus aureus bacteraemia. Int J Antimicrob Agents. 2015;46(5):532–537. | ||
Tadros M, Williams V, Coleman BL, et al. Epidemiology and outcome of pneumonia caused by methicillin-resistant Staphylococcus aureus (MRSA) in Canadian hospitals. PloS One. 2013;8(9):e75171. | ||
Suzuki Y, Kawasaki K, Sato Y, et al. Is peak concentration needed in therapeutic drug monitoring of vancomycin? A pharmacokinetic-pharmacodynamic analysis in patients with methicillin-resistant staphylococcus aureus pneumonia. Chemotherapy. 2012;58(4):308–312. | ||
Chung GW, Wu JE, Yeo CL, Chan D, Hsu LY. Antimicrobial stewardship: a review of prospective audit and feedback systems and an objective evaluation of outcomes. Virulence. 2013;4(2):151–157. | ||
Momattin H, Zogheib M, Homoud A, Al-Tawfiq JA. Safety and outcome of pharmacy-led vancomycin dosing and monitoring. Chemotherapy. 2015;61(1):3–7. | ||
Li J, Udy AA, Kirkpatrick CM, Lipman J, Roberts JA. Improving vancomycin prescription in critical illness through a drug use evaluation process: a weight-based dosing intervention study. Int J Antimicrob Agents. 2012;39(1):69–72. | ||
Casapao AM, Leonard SN, Davis SL, et al. Clinical outcomes in patients with heterogeneous vancomycin-intermediate Staphylococcus aureus (hVISA) bloodstream infection. Antimicrob Agents Chemother. 2013;57(9):4252–4259. | ||
Fujii S, Takahashi S, Makino S, et al. Impact of vancomycin or linezolid therapy on development of renal dysfunction and thrombocytopenia in Japanese patients. Chemotherapy. 2013;59(5):319–324. | ||
Van Hal SJ, Paterson DL, Lodise TP. Systematic review and meta-analysis of vancomycin-induced nephrotoxicity associated with dosing schedules that maintain troughs between 15 and 20 milligrams per liter. Antimicrob Agents Chemother. 2013;57(2):734–744. | ||
Kullar R, Davis SL, Taylor TN, Kaye KS, Rybak MJ. Effects of targeting higher vancomycin trough levels on clinical outcomes and costs in a matched patient cohort. Pharmacotherapy. 2012;32(3):195–201. | ||
Stevens V, Lodise TP, Tsuji B, et al. The utility of acute physiology and chronic health evaluation II scores for prediction of mortality among intensive care unit (ICU) and non-ICU patients with methicillin-resistant Staphylococcus aureus bacteremia. Infect Control Hosp Epidemiol. 2012;33(6):558–564. | ||
Takesue Y, Nakajima K, Takahashi Y, et al. Clinical characteristics of vancomycin minimum inhibitory concentration of 2 μg/mL methicillin-resistant Staphylococcus aureus strains isolated from patients with bacteremia. J Infect Chemother. 2011;17(1):52–57. | ||
Cardile AP, Tan C, Lustik MB, et al. Optimization of time to initial vancomycin target trough improves clinical outcomes. Springerplus. 2015;4:364. | ||
Davis SL, Scheetz MH, Bosso JA, Goff DA, Rybak MJ. Adherence to the 2009 consensus guidelines for vancomycin dosing and monitoring practices: a cross-sectional survey of U.S. hospitals. Pharmacotherapy. 2013;33(12):1256–1263. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.