Back to Journals » Hepatic Medicine: Evidence and Research » Volume 14
Perspectives on Sarcopenia as a Predictor for Outcomes in Pediatric Patients with Chronic Liver Disease
Authors Chen C , Ayers M, Squires JH, Squires JE
Received 27 July 2022
Accepted for publication 19 October 2022
Published 26 October 2022 Volume 2022:14 Pages 173—183
DOI https://doi.org/10.2147/HMER.S348888
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Gerry Lake-Bakaar
Connie Chen,1 Mary Ayers,1 Judy H Squires,2 James E Squires1
1Division of Gastroenterology, Hepatology and Nutrition, UPMC Children’s Hospital of Pittsburgh, Pittsburgh, PA, USA; 2Department of Radiology, UPMC Children’s Hospital of Pittsburgh, Pittsburgh, PA, USA
Correspondence: James E Squires, Division of Gastroenterology, Hepatology and Nutrition, Children’s Hospital of Pittsburgh, Pittsburgh, PA, 15224, Tel +1 412-692-5180, Fax +1 412-692-7355, Email [email protected]
Abstract: Sarcopenia, a pathologic deficiency of muscle mass and function, has emerged as an important secondary feature of many chronic disease states. For adults with end stage liver disease, there are multiple mechanisms which contribute to sarcopenia and its presence has proven to be an important predictor of morbidity and mortality. In children, there are only a limited number of reports which investigate the role of sarcopenia in liver disease. These studies, which are discussed and summarized in this review, report small, single-center analyses with dissimilar study cohorts and varying clinical definitions. Still, children meeting the study entry criteria have sarcopenia with a reported prevalence of 24– 70%. When assessed, sarcopenia appears to be associated with more severe disease but is independent of the Pediatric End-Stage Liver Disease (PELD) score and does not correlate with age, gender, or traditional anthropometric measures such as weight, height, weight-for-height, or body mass index (BMI). While individual studies may identify sarcopenia as a statistically significant risk factor for certain post-transplant outcomes such as longer ICU stay, longer duration of intubation, repeat operation, development of serious infection, longer hospital stay, death, or long-term growth failure, such associations are not consistently replicated across studies. Finally, although various methods of muscle mass quantification are utilized, the most reported is the total psoas muscle surface area (tPMSA) on computed tomography. This method, along with others such as skeletal muscle area and skeletal muscle index, have had normative values recently defined and these collective efforts should enable researchers a common basis of comparison when delineating sarcopenia, and its impact, across various study populations in future investigations – including in children with liver disease.
Keywords: children, cirrhosis, muscle mass, end-stage liver disease, liver transplant
Background
Defining Sarcopenia
The medical term “sarcopenia” is a neologism with an actively evolving definition. Derived from the Greek words sarx (flesh) and penia (loss), it was initially proposed in 1988 to describe the dramatic decline in muscle mass and function observed in the elderly. At that time, the clinical relevance was unknown.1 In 2010, the European Working Group on Sarcopenia in Older People (EWGSOP) redefined sarcopenia as the presence of low muscle mass accompanied by low muscle function (either strength or physical performance)2 and in 2019 revised their definition, indicating low muscle strength as the primary parameter with the addition of either low muscle quantity or quality.3 In this report, a detailed algorithm was provided for identifying sarcopenia with specific limits for various diagnostic measures. Nonetheless, in clinical practice there remains inconsistency in the tools and criteria used to define sarcopenia.4 This reality is exacerbated in the pediatric population, who are largely unable to undergo performance-based testing and may have difficulty remaining motionless for long imaging studies. Furthermore, age-, ethnicity-, and gender-specific reference values are infrequently established for children.5 Here, we look to provide a broad overview of sarcopenia, focusing on the emerging literature describing its prevalence and impact in children with chronic liver disease.
Mechanisms of Sarcopenia in Chronic Liver Disease
In recent years, substantial advances have been made in the understanding of the epidemiology and pathophysiology of sarcopenia. Indeed, it is now recognized to be a common secondary feature of many disease states, including adult chronic liver disease (CLD) of various etiologies.6 Many mechanisms are thought to contribute to the pathogenesis of sarcopenia in CLD (Figure 1) which have been studied extensively at the basic science/preclinical level as well as in clinical settings mostly with adult subjects.
 |
Figure 1 Mechanisms of sarcopenia. Abbreviations: BCAA, branched chain amino acids; GH, growth hormone; IGF-1, insulin-like growth factor-1. |
Under normal physiological circumstances, skeletal muscle is maintained as a balance between protein synthesis and breakdown. Protein synthesis is largely controlled by mammalian target of rapamycin (mTOR) in muscle cells. The mTOR pathway is activated by exercise, branched chain amino acids (BCAAs), and hormones such as testosterone, insulin, and insulin-like growth factor 1 (IGF-1) through activation of protein kinase B. Conversely, inhibition of muscle cell protein synthesis and autophagy are induced by factors such as myostatin, impaired mitochondrial function, insulin resistance, and reactive oxygen species.7
Several complications of CLD have been shown to disrupt skeletal muscle homeostasis and contribute to the development of sarcopenia. For example, in cirrhosis, reduced hepatic glucose stores incite a glucose starvation response, inducing skeletal muscle proteolysis for gluconeogenesis. This results in reduced BCAAs which are essential for preservation of muscle mass, leading to muscle degradation. Additionally, impaired ureagenesis and portosystemic shunting in patients with CLD result in hyperammonemia which can increase sarcopenia by various mechanisms including increased expression of myostatin,8 direct inhibition of mTOR,9 ammonia-mediated mitochondrial dysfunction, and the generation of reactive oxygen species.10
Also in cirrhotic liver disease, increased serum aromatase activity and systemic inflammation results in reduced serum testosterone levels which contribute to sarcopenia, particularly in males. Otherwise, liver cirrhosis can result in changes to the intestinal microbiota leading to increased gut translocation, impaired short-chain fatty acid production, and dysregulation of glutathione, a key antioxidant. Such changes will contribute to chronic inflammation and oxidative stress which compounds sarcopenia.11
Malnutrition has also been identified an important contributing factor. In CLD patients, sub-optimal nutritional intake results from low appetite and/or dyspepsia related to ascites, organomegaly, or medications, despite increased metabolic demand.5,12 Additionally, cholestasis results in intestinal malabsorption of fats and fat-soluble vitamins including Vitamin D, which is implicated in skeletal muscle homeostasis.7
Ironically, sarcopenia is now increasingly recognized in obese patients with non-alcoholic fatty liver disease.13 While the pathogenesis of sarcopenia and NAFLD is complex, insulin resistance is suspected to significantly contribute. In non-disease states, insulin acts as an anabolic hormone to stimulate amino acid uptake and protein synthesis as well as glucose uptake, glycogen synthesis, triglyceride uptake, and fatty acid synthesis.14 In insulin resistance, these pathways are dysregulated and are thought to lead to fat accumulation within muscle tissue and a chronic subclinical inflammatory state which may promote muscle degradation and autophagy. Furthermore, the downregulation of the insulin receptor pathway results in decreased phosphorylation of protein kinase B and, consequently, decreased activation of several pathways for muscle protein synthesis.15 Other factors thought to contribute to the development of sarcopenia in NAFLD include 1.) decreased levels of IGF-1, a hormone produced by hepatocytes and myocytes which acts to stimulate muscle protein synthesis, 2.) physical inactivity and lower energy expenditure which promote fat deposition and sarcopenia, and 3.) the pro-inflammatory milieu of NAFLD which results in a state of chronic, low-grade inflammation resulting in muscle catabolism via IL-6 and TNF-alpha activity.7
Sarcopenia in Adult Liver Disease
In the adult CLD population, sarcopenia has been found to be a predictor of important adverse post-transplant outcomes such as survival,16–21 severe infections,16,18,20,22,23 intensive care unit (ICU) length of stay (LOS),16,19 duration of intubation,16,19 total hospital LOS,19,22 and postoperative complications.16,21 For adults awaiting transplantation, sarcopenia is associated with increased healthcare-related costs24 and incidence of hepatic encephalopathy.25 Additionally, sarcopenia has been associated with greater mortality while awaiting transplant independent of the modified end-stage liver disease (MELD) scores,16,26 and a modified MELD score model which factored in presence of sarcopenia demonstrated improved prediction of mortality.21
Of note, most sarcopenia studies in adults with chronic liver disease identify sarcopenia by measures of muscle mass alone.16 Since these studies have demonstrated significant associations, the American Association for the Study of Liver Diseases (AASLD) published a practice guideline in 2021 for evaluation and management of sarcopenia in patients with cirrhosis, with an operational definition of sarcopenia as the phenotypic manifestation of loss of muscle mass.27 In adults, methods of measuring muscle mass have included anthropometrics, bioelectric impedance analysis (BIA), psoas ultrasound, Dual energy X-ray absorptiometry (DEXA), magnetic resonance imaging (MRI), and computed tomography (CT). The current gold standard for assessment of muscle mass in cirrhosis is by CT. Notably, the use of CT specifically for detecting sarcopenia is generally considered impractical. However, as abdominal CTs are often required for other indications, these images can also be used to estimate skeletal muscle mass. Typically, muscle mass is reported with the skeletal muscle index (SMI) calculated as the cross-sectional muscle area at L3 indexed to height.27
Pediatric Chronic Liver Disease and Sarcopenia
Measuring sarcopenia in pediatric CLD remains an uncommon standard practice. However, several recently published studies demonstrate a high prevalence of sarcopenia, as well as an association between sarcopenia and outcomes, particularly post-transplant outcomes. The findings of these studies are summarized in Table 1.
 |
Table 1 Summary of Existing Data for Sarcopenia in Pediatric Liver Disease |
Nearly all existing studies investigate muscle mass alone, with the majority quantifying muscle mass by measurement of total psoas muscle surface area (tPMSA) on cross-sectional imaging, CT being the most common modality (Figure 2). Measurement of tPMSA occurred at several lumbar levels but most often at L3/4 and L4/5. DEXA and BIA were utilized for muscle measurement in the remaining studies. As such, there has been variation in the way that sarcopenia has been defined. Many of the earlier studies chose to compare outcomes to a measure of muscle mass as a continuous variable; such measures include tPMSA, skeletal muscle mass, psoas muscle index (PMI = tPMSA / height), or skeletal muscle index.12,28–31 Reference values for tPMSA for children ages 1–16 years were published in 2020 alongside the creation of a convenient online tool.32 Since then, most studies have shifted toward defining sarcopenia by tPMSA z-score below –2 (or equivalently <2 standard deviation [SD] below mean, below 5th percentile) based on criteria utilized by the same group. Three of these studies,33–35 utilized reference values for pediatric tPMSA in children aged 1–16 published in 2020 by Lurz et al.32 One study which involved children younger than 12 months used imaging from matched controls to create normative values.36 Some research groups have chosen to delineate sarcopenia with the assistance of normative pediatric values established for skeletal muscle mass by DEXA37 or muscle to fat ratio by BIA.38 One study did utilize both measures of muscle mass (determined via DEXA analysis) and function (via grip strength) to define sarcopenia.39
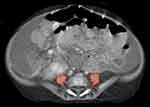 |
Figure 2 Axial contrast-enhanced CT of the abdomen and pelvis in a 6-month-old male with biliary atresia demonstrates sarcopenia, with diminutive psoas muscles. |
Sarcopenia in Chronic Liver Disease Prior to Transplant
Nine studies were identified which investigated sarcopenia in pediatric patients with end-stage liver disease (ESLD) requiring transplantation, all of which were retrospective. Of these studies, eight investigations measured muscle mass from tPMSA on cross-sectional imaging obtained prior to transplantation. Four of these were interested in the relationship between sarcopenia and post-transplant outcomes and defined sarcopenia as tPMSA z-score below −2. In these studies, the prevalence of sarcopenia in the pre-transplant population was reported to be 24–56%.33,35,36,40 The remaining four studies were descriptive, cross-sectional reports in pre-transplant patients which did not define sarcopenia but evaluated tPMSA or PMI as continuous variables in comparison to other patient characteristics.12,28,29,31
Three studies compared tPMSA in ESLD pre-transplant patients to age- and gender-matched controls and found that tPMSA was significantly lower in the ESLD group.28,29,31 While some studies noted a significant correlation in CLD patients between sarcopenia measures and baseline anthropometric data (height, weight, body mass index [BMI], mid upper arm circumference [MUAC], triceps skin fold [TSF])12,31,33 others did not find this relationship.29,35–37,39 One study reported that pre-transplant CLD patients with sarcopenia were more likely to receive nutritional support (odds ratio 12.83).33 Multiple studies have also reported a significantly lower baseline albumin level in sarcopenic patients28,36,37 though this was also not replicated in other studies.33,34 Jitwongwai et al reported a lower PMI in patients who died while awaiting transplantation (353 vs 417 mm2/m2); this difference was statistically significant in univariate analysis but not in multivariate analysis.40 No studies have found a significant correlation between sarcopenia and Pediatric End-Stage Liver Disease (PELD) scores.29,31,33,35,36,40
Sarcopenia and Post-Liver Transplant Outcomes
Of studies that have examined pediatric liver transplant outcomes associated with sarcopenia, there has been notable variation in study population characteristics (see Table 2) as well as findings (see Table 3). In a study of biliary atresia patients, Takeda et al reported significantly increased median operative room (OR) time (511 vs 476 minutes) and intraoperative blood loss in sarcopenic patients (109 vs 76.4 g/kg);36 these measures were not investigated elsewhere. While some studies found a significant correlation between sarcopenia and overall hospital LOS,37,40 this was an inconsistent finding.31,33,36 With the exception of Jitwongwai et al,40 a statistically significant increase in ICU LOS was found in multiple studies.33,35,37 However, the clinical significance of this finding varied greatly; while Woolfson et al reported a median ICU LOS of 3.5 days in the sarcopenia group vs 2.0 days in the non-sarcopenia group33 Mager et al reported approximately 20 vs 7.5 days in the ICU.37 Notably, the significant findings in Verhagen et al regarding ICU and hospital LOS were weak (with correlation coefficient of R = −0.3 for both outcomes) and only seen in patients below 1 year of age.35
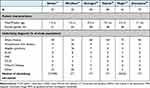 |
Table 2 Comparison of Transplant Study Populations |
 |
Table 3 Impact of Sarcopenia on Transplant Outcomes |
In the perioperative period, some studies show an association between sarcopenia and longer duration of intubation/ventilator dependence36,37 though others do not.31,33 While Takeda et al found a correlation between sarcopenia and increased vascular post-transplant complications in biliary atresia patients (specifically portal vein stenosis, with an incidence of 19.0% in the sarcopenia group vs 5.9% of the non-sarcopenia group),36 this was not seen in other, more heterogeneous transplant cohorts.31,33,35 Similarly, Jitwongwai et al found that re-operation occurred in 38.9% of the low muscle mass group vs 15.4% of the high muscle mass group,40 a finding which was not otherwise replicated.31,35 No studies found an increased risk for post-transplant biliary complications31,33,35,36 or rejection33,35–37 in sarcopenic patients. An increased risk of post-transplant infection was identified (28.6% vs 10.3%) in sarcopenic patients in one study which included only transplant recipients with a primary diagnosis of biliary atresia (BA),36 but this effect was not seen elsewhere31,33 including within one study which evaluated BA patients as a distinct subgroup.35
Boster et al reported a severe and statistically significant correlation between sarcopenia and mortality. In this study, sarcopenia was undefined, and outcomes were compared to tPMSA measured on CT as a continuous variable. Survival analysis revealed a 60% increased risk of death for every 100 mm2 decrease in tPMSA for all patients (either awaiting or status post transplantation). In multivariate regression analysis, they determined 4.9 times higher risk for death with every decrease in tPMSA by one standard deviation. Interestingly, this same study did not find any significant correlation to any other post-transplant outcome including duration of intubation, hepatic artery thrombosis, portal vein thrombosis, biliary complications, return to OR, serious infections, hospital LOS, or readmission rates.31 This has been a somewhat isolated finding in the existing literature. Other studies found no significant correlation between sarcopenia and death.35,36,40 One study reported zero patient fatalities during the study period, precluding comparison.33
Lastly, Mager et al reported longitudinal data from a cohort of post-transplant patients who underwent annual DEXA scans for evaluation of body composition. This was the only study which evaluated sarcopenia in transplant patients without cross-sectional imaging. Sarcopenia was defined as skeletal muscle mass Z-score < –2. Children with sarcopenia had significantly lower weight and height velocities after liver transplant. Lower weight and height z-scores persisted up to 12 years post-transplant; the average weight and height z-scores were consistently less than 0 in the sarcopenia group while those of the non-sarcopenia group were consistently greater than 0. Additionally, the sarcopenic patients were seen to have more long-term hospital utilization, particularly in the total LOS for readmissions (approximately 50 days vs 10 days). Mortality outcomes were not reported.37
Disease-Specific Sarcopenia
NAFLD
A small but growing body of literature has reported on the relationship between sarcopenia and pediatric NAFLD. Muscle mass in pediatric NAFLD was first investigated by Pacifico et al who reported on 234 obese/overweight youths and demonstrated that those with lower muscle mass were at greater risk of NAFLD and that in a subset of patients with biopsy proven NAFLD, there was an associated risk between reduced muscle mass and the presence of inflammation histologically.41 Subsequently, Yodoshi et al compared the PMI to the NAFLD activity score (NAS), a measure of histologically observed disease severity that incorporates steatosis grade, lobular inflammation, and liver cell injury, reporting a significant negative association between PMI and higher NAS score.30 More recently, the same group published similar results utilizing BIA to quantify muscle mass in association with NAS score and again noted muscle mass indices had significant negative associations with the NAS score and degree of histologic steatosis.42 In accordance with these findings, Kwon and Jeong also published a study utilizing BIA and found NAFLD was significantly associated with both sarcopenia prevalence and lower muscle to fat ratio (MFR).38 Collectively, these studies support the relationship between NAFLD and sarcopenia. However, what is also clear is that future efforts will be needed to standardize evaluation methods so that the true prevalence can be appreciated, and ultimately discover what if any clinical implications there are to the presence of sarcopenia in pediatric NAFLD.
Biliary Atresia
Biliary atresia (BA) is one of the most common indications for pediatric liver transplant. In infants with BA, assessing nutritional status is notoriously difficult as standard anthropometrics are difficult to interpret in the context of hepatosplenomegaly, ascites, and increased body fat stores secondary to disease processes. Grutters et al showed that CT-measured tPMSA (indexed by patient length) had no correlation with MUAC in a study of 80 patients with BA under 2 years.12
Takeda et al published a study on sarcopenia and post-transplant outcomes in biliary atresia patients. Sarcopenia was defined as tPMSA z-score < –2 and was measured from CT imaging. In this study (n = 89), 24% of patients met criteria for sarcopenia. In terms of baseline characteristics, the sarcopenic patients had significantly lower portal vein diameters (3.5 vs 4.6 mm), increased incidence of portal vein hypoplasia (62% vs 28%), and higher incidence of retrograde portal vein flow (52.4 vs 25%). There was no significant difference in age at liver transplantation, gender, total parenteral nutrition (TPN) support, PELD score, or anthropometrics at liver transplantation. The sarcopenia group saw significant differences in longer operating time (511 vs 476 minutes), higher degree of blood loss (108.8 vs 76.4 g/kg), higher incidence of portal vein stenosis (23.8% vs 4.4%), higher incidence of bloodstream infection (28.6 vs 10.3%), and longer duration of ventilator use (2.0 vs 1.0 days). There was no association found with risks of biliary duct stenosis, rejection, or cytomegalovirus (CMV) infection. Duration of hospital stay was not significantly different in the sarcopenia group. Patients with sarcopenia had lower rates of one-year patient survival (90.5% vs 98.5%) and one-year graft survival (85.7% vs 97.1%) which were not statistically significant (p = 0.07 and 0.05 respectively). In multivariate analysis, presence of sarcopenia increased the odds of bloodstream infection with odds ratio = 3.44 (p = 0.04). After transplantation, tPMSA increased in both sarcopenic and non-sarcopenic patients regardless of age at transplant. Sarcopenic patients who underwent liver transplant ≥6mo of age had a greater increase in tPMSA than patients transplanted <6mo of age.36
Autoimmune Liver Disease
To date, only one study has been published focusing on sarcopenia in pediatric autoimmune liver disease. In this study by Amevor et al, included patients under 25 years old; had a diagnosis of autoimmune hepatitis (AIH), primary sclerosing cholangitis (PSC), or autoimmune sclerosing cholangitis (ASC); and had abdominal MRI imaging. Sarcopenia was defined as tPMSA < 5th percentile as measured at L3/4 (based on criteria published by Lurz et al32). Measures of visceral and subcutaneous fat were also collected from MRI. Additional data was collected regarding demographics, anthropometrics, biochemical labs, and parent-reported quality of life (QoL). The final study population (n = 52) had median age 16 years with a preponderance for male gender (57%) and Caucasian race (95%). Forty-eight percent of patients met criteria for sarcopenia; based on BMI alone, only 3% would have fallen into the malnutrition category. The population with sarcopenia were significantly more likely to have greater visceral fat area (3156 vs 2084 mm2, p = 0.005) and lower parent-reported general health scores (50 vs 75 out of maximum score 100, p = 0.045). There was no significant difference found when compared against gender, age, underlying diagnosis, presence of IBD, history of corticosteroid exposure, other labs, or other QoL outcomes.34
Conclusions
The emerging literature on sarcopenia highlights multiple investigative opportunities – particularly in children with chronic liver disease. At the forefront will be how the field looks to define sarcopenia and what instruments should be utilized to measure and quantify its prevalence. To date, studies have shown sarcopenia to be prevalent in children with various liver diseases; however, its utility as an outcome predictor is limited and the published results are disparate. Nonetheless, strides have been made toward optimizing the capacity to prognosticate the impact of sarcopenia on pediatric liver disease. The use of tPMSA on CT imaging at L3/4 and L4/5 seems promising as a measure of sarcopenia, and more recent efforts to define pediatric-specific norms for skeletal muscle area and skeletal muscle index will compliment and advance the field.43 These published pediatric reference values will enable more standardized comparisons and a shift away from single-center experiences, increasing the potential for meaningful multi-center collaborations, and drive future prospective research endeavors.
Abbreviations
ASC, autoimmune sclerosing cholangitis; BA, biliary atresia; BCAA, branched chain amino acid; BIA, bioelectrical impedance analysis; BMI, body mass index; CFLD, cystic fibrosis-associated liver disease; CLD, chronic liver disease; CT, computed tomography; DEXA, dual energy X-ray absorptiometry; ESLD, end-stage liver disease; IBD, inflammatory bowel disease; LOS, length of stay; MELD, Model for End-Stage Liver Disease score; MFR, muscle to body fat ratio; MRI, magnetic resonance imaging; MUAC, mid-upper arm circumference; NAFLD, non-alcoholic fatty liver disease; NAS, NAFLD Activity Score; PELD, Pediatric End-Stage Liver Disease score; PMI, psoas muscle index = tPMSA / height; QoL, quality of life; SMM, skeletal muscle mass; SMI, skeletal muscle mass index; TCA, tricarboxylic acid; tPMSA, total psoas muscle surface area; TSF, triceps skinfold.
Disclosure
Dr Connie Chen reports grants from Cystic Fibrosis Foundation, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Rosenberg IH. Sarcopenia: origins and clinical relevance. J Nutr. 1997;127(5):990S–991S. doi:10.1093/jn/127.5.990S
2. Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on sarcopenia in older people. Age Ageing. 2010;39(4):412–423. doi:10.1093/ageing/afq034
3. Cruz-Jentoft AJ, Bahat G, Bauer J, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48(1):16–31. doi:10.1093/ageing/afy169
4. Meza-Valderrama D, Marco E, Dávalos-Yerovi V, et al. Sarcopenia, malnutrition, and cachexia: adapting definitions and terminology of nutritional disorders in older people with cancer. Nutrients. 2021;13:3. doi:10.3390/nu13030761
5. Merli M. Pediatric sarcopenia: exploring a new concept in children with chronic liver disease. J Pediatr. 2020;96(4):406–408. doi:10.1016/j.jped.2019.08.001
6. Cespiati A, Meroni M, Lombardi R, Oberti G, Dongiovanni P, Fracanzani AL. Impact of sarcopenia and myosteatosis in non-cirrhotic stages of liver diseases: similarities and differences across aetiologies and possible therapeutic strategies. Biomedicines. 2022;10(1). doi:10.3390/biomedicines10010182
7. Yang YJ, Kim DJ. An overview of the molecular mechanisms contributing to musculoskeletal disorders in chronic liver disease: osteoporosis, sarcopenia, and osteoporotic sarcopenia. Int J Mol Sci. 2021;22:5. doi:10.3390/ijms22052604
8. Dasarathy S. Myostatin and beyond in cirrhosis: all roads lead to sarcopenia. J Cachexia Sarcopenia Muscle. 2017;8(6):864–869. doi:10.1002/jcsm.12262
9. Zietz B, Lock G, Plach B, et al. Dysfunction of the hypothalamic-pituitary-glandular axes and relation to child-Pugh classification in male patients with alcoholic and virus-related cirrhosis. Eur J Gastroenterol Hepatol. 2003;15(5):495–501. doi:10.1097/01.meg.0000059115.41030.e0
10. Kosenko E, Venediktova N, Kaminsky Y, Montoliu C, Felipo V. Sources of oxygen radicals in brain in acute ammonia intoxication in vivo. Brain Res. 2003;981(1–2):193–200. doi:10.1016/s0006-8993(03)03035-x
11. Ebadi M, Bhanji RA, Mazurak VC, Montano-Loza AJ. Sarcopenia in cirrhosis: from pathogenesis to interventions. J Gastroenterol. 2019;54(10):845–859. doi:10.1007/s00535-019-01605-6
12. Grutters LA, Pennings JP, Bruggink JLM, et al. Body composition of infants with biliary atresia: anthropometric measurements and computed tomography-based body metrics. J Pediatr Gastroenterol Nutr. 2020;71(4):440–445. doi:10.1097/MPG.0000000000002859
13. Sinn DH, Kang D, Kang M, et al. Nonalcoholic fatty liver disease and accelerated loss of skeletal muscle mass: a longitudinal cohort study. Hepatology. 2022. doi:10.1002/hep.32578
14. Dimitriadis G, Mitrou P, Lambadiari V, Maratou E, Raptis SA. Insulin effects in muscle and adipose tissue. Diabetes Res Clin Pract. 2011;93(Suppl 1):S52–9. doi:10.1016/S0168-8227(11)70014-6
15. Cleasby ME, Jamieson PM, Atherton PJ. Insulin resistance and sarcopenia: mechanistic links between common co-morbidities. J Endocrinol. 2016;229(2):R67–81. doi:10.1530/JOE-15-0533
16. Ooi PH, Hager A, Mazurak VC, et al. Sarcopenia in chronic liver disease: impact on outcomes. Liver Transpl. 2019;25(9):1422–1438. doi:10.1002/lt.25591
17. Kaido T, Ogawa K, Fujimoto Y, et al. Impact of sarcopenia on survival in patients undergoing living donor liver transplantation. Am J Transplant. 2013;13(6):1549–1556. doi:10.1111/ajt.12221
18. Krell RW, Kaul DR, Martin AR, et al. Association between sarcopenia and the risk of serious infection among adults undergoing liver transplantation. Liver Transpl. 2013;19(12):1396–1402. doi:10.1002/lt.23752
19. DiMartini A, Cruz RJ, Dew MA, et al. Muscle mass predicts outcomes following liver transplantation. Liver Transpl. 2013;19(11):1172–1180. doi:10.1002/lt.23724
20. Masuda T, Shirabe K, Ikegami T, et al. Sarcopenia is a prognostic factor in living donor liver transplantation. Liver Transpl. 2014;20(4):401–407. doi:10.1002/lt.23811
21. Valero V, Amini N, Spolverato G, et al. Sarcopenia adversely impacts postoperative complications following resection or transplantation in patients with primary liver tumors. J Gastrointest Surg. 2015;19(2):272–281. doi:10.1007/s11605-014-2680-4
22. Montano-Loza AJ, Meza-Junco J, Baracos VE, et al. Severe muscle depletion predicts postoperative length of stay but is not associated with survival after liver transplantation. Liver Transpl. 2014;20(6):640–648. doi:10.1002/lt.23863
23. Jeon JY, Wang H-J, Ock SY, et al. Newly developed sarcopenia as a prognostic factor for survival in patients who underwent liver transplantation. PLoS One. 2015;10(11):e0143966. doi:10.1371/journal.pone.0143966
24. van Vugt JLA, Levolger S, de Bruin RWF, van Rosmalen J, Metselaar HJ, IJzermans JNM. Systematic review and meta-analysis of the impact of computed tomography-assessed skeletal muscle mass on outcome in patients awaiting or undergoing liver transplantation. Am J Transplant. 2016;16(8):2277–2292. doi:10.1111/ajt.13732
25. Bhanji RA, Moctezuma-Velazquez C, Duarte-Rojo A, et al. Myosteatosis and sarcopenia are associated with hepatic encephalopathy in patients with cirrhosis. Hepatol Int. 2018;12(4):377–386. doi:10.1007/s12072-018-9875-9
26. Durand F, Buyse S, Francoz C, et al. Prognostic value of muscle atrophy in cirrhosis using psoas muscle thickness on computed tomography. J Hepatol. 2014;60(6):1151–1157. doi:10.1016/j.jhep.2014.02.026
27. Lai JC, Tandon P, Bernal W, et al. Malnutrition, frailty, and sarcopenia in patients with cirrhosis: 2021 practice guidance by the American association for the study of liver diseases. Hepatology. 2021;74(3):1611–1644. doi:10.1002/hep.32049
28. Mangus RS, Bush WJ, Miller C, Kubal CA. Severe sarcopenia and increased fat stores in pediatric patients with liver, kidney, or intestine failure. J Pediatr Gastroenterol Nutr. 2017;65(5):579–583. doi:10.1097/MPG.0000000000001651
29. Lurz E, Patel H, Frimpong RG, et al. Sarcopenia in children with end-stage liver disease. J Pediatr Gastroenterol Nutr. 2018;66(2):222–226. doi:10.1097/MPG.0000000000001792
30. Yodoshi T, Orkin S, Arce Clachar A-C, et al. Muscle mass is linked to liver disease severity in pediatric nonalcoholic fatty liver disease. J Pediatr. 2020;223(93–99). doi:10.1016/j.jpeds.2020.04.046
31. Boster JM, Browne LP, Pan Z, Zhou W, Ehrlich PF, Sundaram SS. Higher mortality in pediatric liver transplant candidates with sarcopenia. Liver Transpl. 2021;27(6):808–817. doi:10.1002/lt.26027
32. Lurz E, Patel H, Lebovic G, et al. Paediatric reference values for total psoas muscle area. J Cachexia Sarcopenia Muscle. 2020;11(2):405–414. doi:10.1002/jcsm.12514
33. Woolfson JP, Perez M, Chavhan GB, et al. Sarcopenia in children with end-stage liver disease on the transplant waiting list. Liver Transpl. 2021;27(5):641–651. doi:10.1002/lt.25985
34. Amevor AA, Yodoshi T, Trout AT, et al. Sarcopenia is highly prevalent in children with autoimmune liver diseases and is linked to visceral fat and parent-perceived general health. Liver Int. 2022;42(2):394–401. doi:10.1111/liv.15108
35. Verhagen MV, Levolger S, Hulshoff JB, et al. Utility of preoperative computed tomography-based body metrics in relation to postoperative complications in pediatric liver transplantation recipients. Liver Transpl. 2021;27(12):1779–1787. doi:10.1002/lt.26205
36. Takeda M, Sakamoto S, Uchida H, et al. Impact of sarcopenia in infants with liver transplantation for biliary atresia. Pediatr Transplant. 2021;25(5):e13950. doi:10.1111/petr.13950
37. Mager DR, Hager A, Ooi PH, Siminoski K, Gilmour SM, Yap JYK. Persistence of sarcopenia after pediatric liver transplantation is associated with poorer growth and recurrent hospital admissions. JPEN J Parenter Enteral Nutr. 2019;43(2):271–280. doi:10.1002/jpen.1414
38. Kwon Y, Jeong SJ. Relative skeletal muscle mass is an important factor in non-alcoholic fatty liver disease in non-obese children and adolescents. J Clin Med. 2020;9:10. doi:10.3390/jcm9103355
39. Rezende IFB, Conceição-Machado MEP, Souza VS, Santos EMD, Silva LR. Sarcopenia in children and adolescents with chronic liver disease. J Pediatr. 2020;96(4):439–446. doi:10.1016/j.jped.2019.02.005
40. Jitwongwai S, Lertudomphonwanit C, Junhasavasdikul T, et al. Low psoas muscle index as an unfavorable factor in children with end-stage liver disease undergoing liver transplantation. Pediatr Transplant. 2021;25(5):e13996. doi:10.1111/petr.13996
41. Pacifico L, Perla FM, Andreoli G, Grieco R, Pierimarchi P, Chiesa C. Nonalcoholic fatty liver disease is associated with low skeletal muscle mass in overweight/obese youths. Front Pediatr. 2020;8:158. doi:10.3389/fped.2020.00158
42. Yodoshi T, Orkin S, Romantic E, et al. Impedance-based measures of muscle mass can be used to predict severity of hepatic steatosis in pediatric nonalcoholic fatty liver disease. Nutrition. 2021;91:111447. doi:10.1016/j.nut.2021.111447
43. Somasundaram E, Castiglione JA, Brady SL, Trout AT. Defining normal ranges of skeletal muscle area and skeletal muscle index in children on CT using an automated deep learning pipeline: implications for sarcopenia diagnosis. AJR Am J Roentgenol. 2022;219(2):326–336. doi:10.2214/AJR.21.27239
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
