Back to Journals » Medical Devices: Evidence and Research » Volume 10
Perspective on the Rezūm® System: a minimally invasive treatment strategy for benign prostatic hyperplasia using convective radiofrequency water vapor thermal therapy
Authors Woo HH , Gonzalez RR
Received 22 February 2017
Accepted for publication 21 March 2017
Published 27 April 2017 Volume 2017:10 Pages 71—80
DOI https://doi.org/10.2147/MDER.S135378
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Henry H Woo,1 Ricardo R Gonzalez2
1Sydney Adventist Hospital Clinical School, The University of Sydney, Wahroonga, NSW, Australia; 2Houston Metro Urology, Houston, TX, USA
Abstract: Convective radiofrequency (RF) water vapor thermal therapy is a minimally invasive office or outpatient procedure for the treatment of bothersome moderate-to-severe lower urinary tract symptoms associated with benign prostatic hyperplasia (BPH). It provides an option for patients seeking rapid and durable relief of urinary symptoms, improved quality of life, and preservation of sexual function as an alternative to long-term use of drugs and avoidance of the potential side effects of pharmaceuticals or invasive BPH surgery. The procedure is also applicable for the treatment of the median lobe or elevated bladder neck from central zone hyperplasia. This perspective presents a comprehensive overview of the Rezūm® System convective RF thermal therapy device, the principles upon which it is based, the operative procedure, and the clinical evidence accrued to this point in time.
Keywords: prostate, prostatic hyperplasia, lower urinary tract symptoms, thermal therapy, minimally invasive procedure
Introduction
The broad use of medical therapy to manage bothersome lower urinary tract symptoms (LUTS) due to benign prostatic hyperplasia (BPH) has allowed patients to defer surgical therapies. Patients may tolerate side effects of medical therapy in an attempt to avoid complications of surgical therapy. Adherence to medical therapy for symptomatic BPH may be associated with better clinical outcomes; however, several studies indicate that adherence rates are low and may vary according to the class of drug(s) used,1–3 whether it be mono- or combination therapy. Lack of drug compliance is also attributable to several factors, including drug side effects and inadequate improvements in symptoms and quality of life that are lower than perceived expectations.2–4 Although patients may be prepared to wait longer for symptom relief in order to have a reduced risk of surgery,5,6 pharmaceutical agents are costly, particularly for extended periods of time. Measuring bother due to LUTS and impact on the patients’ quality of life with the BPH should be imperative and central to treatment decisions.7
The advent of minimally invasive treatments for LUTS/BPH now allows the urologist to tailor therapy in a continuum of medical management to more invasive surgical procedure approaches. Although minimally invasive procedures such as transurethral needle ablation (TUNA) of the prostate and transurethral microwave thermotherapy (TUMT) are alternatives to surgical intervention, their use is declining.8 A new approach to thermal therapy using convective radiofrequency (RF) water vapor energy has emerged to treat men with moderate-to-severe LUTS, providing early and durable relief of bothersome symptoms and with minimal transient perioperative side effects. This perspective provides an explanation and validation of the technology and clinical evidence supportive of convective RF thermal therapy for LUTS associated with BPH.
Rezūm® System – convective RF water vapor thermal therapy
Procedure
The convective RF thermal therapy procedure utilizes the Rezūm System (NxThera Inc., Maple Grove, MN, USA): it contains an RF power supply generator and single-use transurethral delivery device that incorporates a standard 4 mm 30° rod lens allowing the procedure to be performed under direct cystoscopic visualization (Figure 1). Water vapor thermal energy is created by applying RF current against an inductive coil heater in the handle of the delivery device. The handheld control delivers water vapor providing a consistent energy dose of 208 cal into the prostate tissue through a retractable 18-gauge polyetheretherketone needle; saline flush irrigation enhances visualization and cools the urethral surface.
  | Figure 1 Rezūm® System delivery device and vapor needle. Note: The vapor needle resides within the insulated lumen of the delivery device until it is deployed into the prostate tissue. |
The needle has 12 small emitter holes spaced around its tip at 120° intervals to allow circumferential dispersion of water vapor into the prostate tissue. The patient is placed in the lithotomy position and the delivery device inserted into the urethra. The total penetrating length of the vapor needle is fixed at 10.25 mm. The needle tip is visually positioned and inserted beginning ~1 cm distal to the bladder neck. The transition and central prostate adenoma is precisely targeted. Any intravesical prostatic protrusions of either lateral or median lobes are injected starting 1 cm from the proximal edge of the protrusion. Median lobe treatment entails angulation of the needle perpendicularly into the tissue. The RF water vapor (0.5 mL) is convectively delivered in 9-second injections and dispersed circumferentially to create a 1.5–2.0 cm lesion, remaining confined within the prostate zones. The needle is retracted after each treatment and repositioned in 1 cm increments distal from the previous site to the end of the prostatic tissue just proximal to the verumontanum. The objective of the treatment is to create contiguous, overlapping lesions running parallel to the natural slope of the urethra. The total number of treatments in each lobe of the prostate is determined by the length of the hypertrophied prostatic tissue and can be customized to the configuration of the gland including the median lobe.
Principles of water vapor thermal energy therapy for ablation
The principles of RF-generated water vapor thermal energy are based on the thermodynamic properties of water and the use of convective versus conductive heat transfer to ablate tissue. Examples of conductive heat transfer technologies include TUNA using RF and TUMT using microwaves to generate thermal energy. The Rezūm System transurethral thermal therapy utilizes RF to generate wet thermal energy in the form of water vapor (steam). As the vapor (103°C) convectively disperses through the tissue interstices, its phase shifts from vapor back to liquid immediately upon injection into the tissue, releasing and delivering ~208 cal (1 cal = 4.2 J) of thermal energy in 9 seconds. The target tissue temperature reaches ~70°C resulting in irreversible and instantaneous cell death, creating a roughly spherical ablative lesion. This convective heat transfer of vapor physically moving through the tissue involves virtually no discernible thermal gradient. Because vapor has mass and is physically less dense than the compartmental barriers of the prostate (surgical capsule, true capsule, urethra, bladder neck, and external sphincter), it travels through the interstices between cells, remaining within the injected targeted prostate zone until it encounters boundaries (e.g., tissue planes between prostate zones). This has been metaphorically likened to “the way wind blows against a sail without penetrating”.9 No thermal effects have been shown to occur outside of the prostate or in the peripheral zone when the transition zone (TZ) is targeted. In addition, because vapor is wet thermal energy, there is no charring, desiccation, or carbonization of the treated tissue.
In comparison, tissue ablation involving conductive heat transfer (TUNA or TUMT) induces molecular agitation within a tissue mass after direct contact between two surfaces at different temperatures; this causes a thermal gradient. Consequently, higher temperatures and longer periods of heating are required to achieve a therapeutic temperature in the target tissue via conduction versus convection. With conductive ablation therapies, heat transfer transgresses natural tissue boundaries within the prostate. The Rezūm System technology achieves rapid and efficient delivery of thermal energy requiring fewer joules per gram of tissue, ~1/6–1/23, to produce cell necrosis in prostate tissue compared to TUNA and TUMT, respectively (NxThera, Data on File).
Patient selection
Criteria characterizing subjects qualified and treated in the pilot and pivotal trials of the RF thermal therapy discussed in the clinical evidence section include men aged ≥50 years with moderate-to-severe clinical LUTS/BPH, a prostate volume of 20–120 cm3 (pilot trial) and 30–80 cm3 (pivotal trial), a urinary symptom score (International Prostate Symptom Score [IPSS] or American Urological Association [AUA] Symptom Index [AUA-SI]) of ≥13, maximum urinary flow rate (Qmax) of ≤15 mL/s, and a measured postvoiding residual (PVR) urine <250 mL. There were no morphological contraindications to use of the Rezūm procedure; even in patients with an intravesical median lobe. Application of the Rezūm thermal therapy for patients with larger prostates ≥80–≤150 cm3 is currently under investigation in the US as is the application of this technology for the treatment of localized prostate cancer.
Patients can expect to have the Rezūm thermal therapy procedure performed in an office or ambulatory outpatient treatment setting with management of discomfort/pain and anxiety based on the preference and discretion of the urologist. In some centers, conscious sedation is required to be performed in an ambulatory surgery or hospital. Based on the experience from the pivotal clinical trial, it was reported that most patients (68%) received oral agents only (anxiolytic and pain medications) before treatment or either conscious sedation (17%) or prostate block (15%). Safety assessments reported in the clinical evidence section as new experience with the commercial use of the procedure indicate that minimal transient perioperative side effects are anticipated and are those that can occur after routine rigid cystoscopy. These include dysuria, hematuria, and short-lived irritative symptoms. Some patients might expect the need to use a catheter for an average of 3 days after the procedure. While temporary postoperative symptoms may occur, this is balanced with the rapid and significant relief of LUTS beginning as early as 2 weeks after treatment.
The AUA guidelines have established that IPSS improvement of ≥3 points represents a meaningful clinical response for the patient.10 Clinical evidence after thermal therapy indicates that the patient may expect that IPSS decreases to exceed this minimum AUA response criterion. A moderate symptom improvement (≥5-point decrease in IPSS) was shown to be sustained in 84% of men, and a marked reduction (≥8-point decrease in IPSS) in 74% of patients throughout 2 years in a pivotal trial.11 This level of improvement is superior to that achieved with modern oral pharmaceutical agents and, most importantly, are achieved within the first month after treatment. Consequently, this minimally invasive procedure warrants consideration as a first-line therapeutic alternative to medical therapy and to standard surgical intervention for those seeking treatment for bothersome moderate-to-severe LUTS due to BPH.
Selection of convective water vapor thermal therapy presents no anatomical or technical challenges for patient selection. An advantage of the Rezūm System procedure is that hypertrophy in all prostate zones can be treated including intravesical prostatic protrusions, both of the lateral and median lobes, and any elevated central zone (CZ) at the bladder neck.
Prostate functional anatomy and planning Rezūm thermal therapy
Prior to commencing with a Rezūm procedure, the prostate anatomic features and location of the adenomatous enlargements are evaluated (Figure 2). Pretreatment flexible cystoscopy is performed to determine the number of vapor injections and placement of injections to target the bulk of the tissue. This takes into consideration the length of the prostate, enlargement of lateral lobes, and hypertrophy of the CZ (elevated bladder neck) and includes retroflexion for a clear view of the bladder neck and presence of a median lobe and intravesical protrusion. The patient’s pain tolerance and propensity for bleeding can be assessed. Transurethral ultrasound (TRUS) is also recommended to determine prostate size and to determine the prostatic urethra/bladder neck angle. A steep prostatic urethral angle (PUA) may be indicative of a large median lobe or hypertrophied CZ.
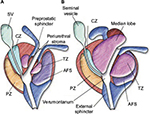  | Figure 2 Prostate zonal anatomy. Notes: (A) Young male with minimal TZ hypertrophy. The preprostatic sphincter and periejaculatory duct zone (CZ) are defined. (B) Older male with TZ hypertrophy, which effaces the preprostatic sphincter and compresses the periejaculatory duct zone. Reprinted from Int J Radiat Oncol Biol Phys, 63(2), McLaughlin PW, Troyer S, Berri S, et al, Functional anatomy of the prostate: implications for treatment planning, 479–491, Copyright (2005), with permission from Elsevier.13 Abbreviations: CZ, central zone; SV, seminal vesicle; TZ, transition zone; AFS, anterior fibromuscular stroma; PZ, peripheral zone. |
When the Rezūm delivery device is inserted, the optical “field of view” is 0.5 cm and can be used to measure the distance from the bladder neck to the proximal verumontanum (seminal colliculus). The estimated number of vapor injections per lobe is one to two when the distance is <2 cm, two to three injections for a distance of 2–3 cm, and three to four injections for a distance of >3 cm. When present, the median lobe treatment typically involves one to three injections. These treatment numbers are based on each water vapor injection creating a 1.5–2 cm ablative lesion.
Both the TZ and the CZ of the prostate can be treated with the Rezūm procedure. It is worthy of repetition that when the TZ is targeted for treatment, the tissue planes between the prostate zones act as a barrier; no thermal effects occur in the peripheral zone (PZ) or beyond the prostate capsule. The normal TZ comprises 5% of the prostate glandular tissue and consists of two small lobules of glandular tissue that surround the proximal prostatic urethra just superior to the verumontanum. The TZ is a common site for BPH. The CZ is located at the base of the prostate between the peripheral zone and the TZ and accounts for ~25% of the glandular tissue. The TZ and CZ are designated the internal or central gland, as they are barely distinguishable from one another. Due to the occurrence of BPH with increasing age, the morphology of the central gland changes from CZ to TZ. When the TZ is enlarged and the nodules advance, the PZ and CZ undergo compression and may be displaced to the base of prostate and overlap the TZ. A recent study indicates that the PZ may also contribute to benign growth, although not as much as the TZ; in 65% of 510 men (average age =62 years) with noncancerous prostates, the PZ and CZ volumes were >30 cm3.12 Based on the origin of hyperplasia, the term median lobe hyperplasia may be used to denote hyperplasia of the periurethral glands. Median lobe hypertrophy represents a unique form of prostate enlargement. It is often associated with obstruction caused by a ball valve mechanism in which the median lobe closes over the urethral opening during urination.13 Little attention has been paid to understanding the prevalence of PZ/CZ or median zone hypertrophy and contribution to LUTS. In Rezūm clinical trials, 28% of all 253 patients presenting for treatment had a median lobe.11,14 Information on the prevalence of a median lobe in benign hypertrophic prostates was not found but will likely vary as does the individual expressions of LUTS in patients with BPH.
Structural differences in the prostate may affect the incidence and severity of LUTS as well as responses to therapy. PUA may be one method to assess the presence of bladder outlet obstruction (BOO) in men with LUTS and/or BPH. The PUA determined with TRUS is the angle formed by two rays of the proximal and distal prostatic urethra on the midsagittal plane image. PUA is associated with urinary symptom severity and Qmax in men with LUTS and a small prostate volume.15 The correlation between PUA ≥35° and incidence of BOO was found to be significant in 260 men with LUTS/BPH.16 In consideration of the uniqueness of individual patients and variations in prostate enlargement in the etiology of LUTS, the ability to treat all prostate zones without restrictions with the Rezūm System provides an opportunity for early therapeutic intervention for relief of LUTS/BPH.
Clinical evidence for convective RF water vapor thermal therapy
The convective water vapor energy technology is a natural, yet powerful new platform technology that utilizes stored thermal energy of water vapor (steam) created with RF power to result in instantaneous cell death in targeted tissue. The principles of convective RF thermal therapy and characteristic ablation of prostatic adenomas have been demonstrated in a series of well-planned investigations including histologic, radiographic, and clinical trial studies reported in peer-reviewed journals (Table 1). Based on this collective evidence, the Rezūm System received approval for a CE marking for a medical device in 2013 and US Food and Drug Administration clearance in 2015.
  | Table 1 Scientific studies of Rezūm® System convective radiofrequency water vapor thermal therapy published in peer-reviewed journals |
First-in-man (FIM) acute clinical trial and validation of thermal ablation technology
The FIM study of patients with enlarged prostates and LUTS was the earliest characterization of thermal ablation evaluating the histologic effects of the thermal therapy-treated prostates of patients who were previously scheduled for an open prostate adenectomy as reported by Dixon et al.9 Extirpated adenomas were grossly examined after whole mount sectioning and staining with triphenyl-tetrazolium chloride (TTC) to discern metabolically active (TTC stained) and irreversibly damaged tissue. Hematoxylin and eosin (H&E) staining of selected areas of the whole mounts identified viable and necrotic tissue and thermal boundaries. TTC vital staining showed defects associated with the water vapor injection sites and no thermal effects outside of the prostate. H&E staining revealed that the thermal therapy treatment resulted in distinct demarcations between nonviable treated tissue and untreated prostate.
FIM optimization and pilot study with magnetic resonance imaging (MRI) assessments
This initial validation study in the Dominican Republic was followed by subsequent ethics committee approval for a total 65-patient pilot study for LUTS/BPH, adding two additional study centers, Sweden and Czech Republic, and to include serial gadolinium-enhanced MRIs performed after the thermal therapy in 59 of the 65 patients at each scheduled follow-up visit through 6 months. MRI scans in these pilot studies were conducted at the three centers, and data were analyzed by an independent urologist (Biomedical Imaging Resource; Mayo Clinic, Rochester, MN, USA). Three-dimensional (3D) volume renderings allowed visualization of thermal lesions in relation to prostate zonal anatomy and lesion resolution after the water vapor thermal therapy.9,17 The pilot study thermal treatment procedure evolved with slight modifications to optimize water vapor delivery, dosimetry, and endoscopic technique based on characterization of the ablative lesions with MRI, time course to resolution, and changes in prostate volume. As reported by Mynderse et al,17 MRI and 3D renderings confirmed that the water vapor thermal lesions were limited and remained within the targeted treatment of the TZ; no compromised integrity of the bladder, rectum, or striated urinary sphincter was observed. The volume of thermal lesions showed almost complete resolution at 3 and 6 months after treatments commensurate with reduction of the TZ and whole prostate volumes.
Two-year outcomes of pilot trial
Dixon et al14,18 reported the 1- and 2-year clinical outcomes of the multicenter pilot study involving 65 men (mean age: 66.6 ± 7.7 years; mean prostate volume: 48.6 ± 20.5 cm3) with moderate (32%) to severe (68%) LUTS (mean IPSS: 21.6 ± 5.5; mean Qmax: 7.9 ± 3.2 mL/s). The mean number of water vapor injections to lateral lobes was 4.6 (range: 2–9). Median lobe treatment in 22% of men involved 1.8 water vapor injections (range: 1–3). Clinically and statistically significant improvements in urinary symptoms (−6.5 point IPSS reduction from baseline), flow rate (2.0-point increase), and quality-of-life measures were evident as early as 1 month after treatment. The treatment responses were optimal at 3–12 months (−12.6-point IPSS reduction from 21.6 at baseline to 9.2; a 4.6-point Qmax increase from 7.9 at baseline to 12 mL/s), each p < 0.001; these responses remained consistent and significant over 24 months of follow-up. Both storage and voiding components of the IPSS showed significant improvements. There were no procedural restrictions to the treatment of median lobes; outcomes were similar in patients with or without an identified median lobe. Transient mild-to-moderate adverse events were reported within the first month after treatment; most were typical of those following endoscopic instrumentation and resolved within a few days to 4 weeks. No clinically significant changes in sexual function were reported in this study; no de novo erectile dysfunction (ED) occurred.
Randomized controlled multicenter trial
A registration-directed pivotal, randomized controlled trial (Rezūm II RCT) of the convective RF water vapor thermal therapy was conducted to evaluate the effectiveness and safety of the Rezūm System for the treatment of men with moderate-to-severe LUTS associated with BPH.19 The study compared thermal therapy with a control/sham procedure of rigid cystoscopy and simulated active treatment sounds. The primary end point at 3 months was mean IPSS change in the treatment arm with a superiority margin of 25% over the change in the control arm. Study participants and study personnel administering outcome questionnaires were double blinded until the 3-month follow-up. The trial was conducted in 15 centers in the US in men aged ≥50 years with a prostate volume 30–80 cm3, IPSS >13 and maximum flow rate ≤15 mL/s. Qualified participants were randomized 2:1, resulting in 136 receiving active treatment and 61 the control sham procedure.
McVary et al19 reported that the primary effectiveness end point for the RCT of thermal therapy was achieved at 3 months with IPSS reduced by 50% (−11.2 points) versus a 20% (−4.3 points) reduction for the controls (p < 0.0001). When expressed as a qualitative responder (mild to marked) outcome, a ≥8-point improved IPSS, defined as a marked response,20,21 was recorded in 74% of treatment group patients compared with 31% of the control group at 3 months and was sustained at this level at 1 year. When expressed quantitatively, urinary symptoms were significantly reduced from baseline as early as 2 weeks after treatment by 11 IPSS points (−50%), at 3 months and sustained through 12 months (−53%). Notable are subjects with severe symptoms (IPSS ≥19) with mean decreases of −14.2 ± 7.3 points at 6 months and 13.5 ± 6.9 at 12 months. Other outcomes for the treatment group included Qmax and quality-of-life measures (IPSS-QoL and BPHII), significantly improved in comparison to controls with effects sustained at 12 months. The thermal treatment procedures were performed in an office-based or ambulatory surgery center with minimal pain management, based on the investigators clinical judgment and standard of practice. Catheterization in the perioperative period was at the investigators’ discretion and not dictated by the technology or protocol. The default to a conservative approach in the clinical trial resulted in 68% of catheterizations as precautionary and 32% (39 of 122) due to an unsuccessful voiding trial before discharge.
Evaluation of sexual function in pivotal trial
McVary et al22 reported on the preservation of sexual function in the Rezūm II RCT using validate questionnaires, IIEF-EF for erectile function, and MSHQ-EjD for ejaculatory function. The prevalence of LUTS and ED with advancing age and concurrence of both conditions mandates the need to examine how treatment for LUTS affects sexual health in men with BPH.23 At baseline, 52% of patients in the active treatment group entered the trial with a history of ED, mean age of 5.2 ± 5.7 years and ~16% had moderate and ~33% had severe ED (based on the IIEF-EF scores). After the convective RF thermal therapy, there was no evidence of treatment- or device-related de novo ED. The IIEF-EF and MSHQ-EjD scores were not different in the active and control groups at 3 months and remained unchanged from baseline at 12 months (p < 0.001).
A unique additional analysis was conducted in the cohort of sexually active men in this study (90 of 136) to understand the impact of pretreatment erectile function on changes in IIEF-EF scores after treatment.22 Applying the criterion of minimal clinically important difference (MCID) developed for use in drug studies of ED by Rosen et al,24 the authors evaluated the amount of change in the IIEF-EF needed to be clinically relevant to patients relative to baseline ED severity. To illustrate this concept, an MCID would require a minimal increase of 2 in the IIEF-EF score for men with mild ED, an increase of 5 for moderate ED, and an increase of 7 for severe ED. This first application of the MCID concept for a device as reported for this study indicated that after thermal therapy, 32% of patients achieved MCIDs in erectile function at 3 months, and 27% at 1 year including in those with moderate-to-severe ED. These responder changes would have gone unappreciated with only the IIEF-EF questionnaire scores. Further analysis compared outcomes for obese (BMI >30) and nonobese subjects (BMI ≤29) for sexual function. Obese subjects were more likely to have severe ED but experienced similar rates of improvement in urinary symptoms (decreased IPSS), MCIDs for erectile function and improved bother in ejaculatory bother (MSHQ-EjD bother) scores as did nonobese subjects.25
Preservation of sexual function remained substantiated among the 188 subjects in the crossover RCT 2-year follow-up reported by Roehrborn et al.11 No de novo ED was reported. Perceived minimal decreases in ejaculatory volume occurred in nine men (4.8% of 188) and anejaculation in five (2.7%). Contingent factors are associated with observations in these 14 men; 71% (10/14) had a previous history of ED and 36% (5/14) ED at study entry; the median lobe was treated in half. Anejaculation was not investigated with laboratory testing to establish whether a true physiologic retrograde ejaculation was occurring, and subjects’ self-reports of changes were not substantiated in the MSHQ-EjD (ejaculatory function) questionnaire responses. Although these ejaculatory function changes were infrequent and possibly secondary to decreased resistance in the bladder neck after treatment of the median lobe, clinicians should adequately inform patients about this possibility.
Two-year outcomes of RCT
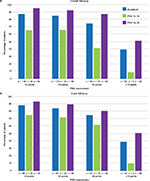
The 2-year follow-up of the Rezūm II RCT was reported by Roehrborn et al.11 Notable is the study’s high retention rate of 89.6% subjects at 1 year and 80.7% at 2 years and the sustainability of responder outcomes as shown in Figure 3. IPSS remained significantly improved by 51% (−11.2-point score reduction) at 24 months compared with baseline; responses closely replicate the urinary symptom changes observed in the pilot study. Subjects with either moderate (IPSS ≤18) or severe (IPSS ≥19) symptoms both achieved improved scores over 2 years of follow-up (Figure 4). Peak flow rate and quality-of-life measures improved by ~50% or more and remained significant and durable over 2 years, p < 0.0001. Relief of urinary symptoms and bother including the ICS male IS and the OAB-q bother and HRQL scores were sustained through 2 years (p < 0.0001). Rezūm convective RF thermal therapy had no negative effect on erectile function. No significant changes in IIEF-EF and ejaculatory function (MSHQ-EjD Function) scores occurred relative to baseline. Bother associated with ejaculation (MSHQ-EjD Bother) remained significantly improved from 12 and 24 months after treatment (p ≤ 0.0118). No late occurring device or procedure-related AEs were reported.
  | Figure 3 IPSS changes throughout 24 months after convective RF thermal therapy in the RCT11 and pilot study14 and 12 months in the crossover study,11 showing similarity and durability of improvements in the three studies. Notes: Values are the means, and errors bars represent the 95% CI. With the exception of the sham/control arm of the RCT, improvements relative to baseline are significant at all time points, p < 0.001. Abbreviations: CI, confidence interval; FIM, first-in-man; IPSS, International Prostate Symptom Score; RF, radiofrequency; RCT, randomized controlled trial. |
Crossover design study of control subjects within the RCT
As reported by Roehrborn et al,11 thermal therapy was evaluated in a crossover design study involving 61 subjects originally randomized to the control/sham procedure arm of the RCT. After unblinding, 53 subjects requalified for treatment with thermal therapy and were followed for 1 year. The authors note the rapid relief of urinary symptoms and reproducibility of the responses to thermal therapy when compared with the pilot trial and RCT (Figure 3). The importance of a crossover study, in which subjects serve as self-controls, is to negate a possible placebo effect with this therapy. Three procedure-related serious adverse events occurred in two subjects; one with a bladder neck contracture and bladder calculus at 6 months posttreatment and the second with urosepsis after follow-up cystoscopy. Other nonserious, anticipated postprocedure events, typically mild to moderate in severity and transient, were dysuria and hematuria.
Outcomes related to treatment of subjects with a median lobe
The ability to treat the median lobe had an appreciable impact on outcomes. Of the total 188 subjects treated with convective RF thermal therapy in the RCT and crossover studies, a median lobe or elevated bladder neck from CZ hyperplasia was identified in 70/188 (37%) subjects, 58 of these subjects received treatment inclusive of the median lobe at investigators’ discretion.11 Improvements in IPSS and urinary flow in patients with or without a treated median lobe were similar and significant in both cohorts throughout 24 months as compared to baseline. After ablation of the median lobe, these 58 subjects had greater decreases in PVR throughout follow-up (general estimation equation p = 0.04, adjusted for baseline PVR). At 24 months, the median PVR decrease was 46% (median 84.3 at baseline) compared with the 15% PVR reduction in subjects without a median lobe (median 66 at baseline).
Of 188 subjects, 8 had a secondary treatment, which is a 4.2% retreatment rate over a 24-month period; one subject underwent open prostatectomy before unblinding, three had a secondary Rezūm procedure, and four underwent TURP. Six of these eight interventions were related to subsequent removal of the median lobe that was not initially treated.
Initial observations postdevice clearance
An interesting initial observation has been documented in postapproval utilization of the Rezūm System thermal therapy for the treatment of patients with LUTS/BPH in urinary retention.26 Thirty men, mean prostate size = 64 cm3, mean PVR = 538 mL/s, who were either dependent on an indwelling catheter or clean intermittent catheterization were treated with thermal therapy; 28 of 30 had a median lobe treated. Within 1 month after thermal therapy (mean 6.4 vapor injections to TZs and mean one vapor injection to median lobe per procedure), 77% (23/30) of the patients were catheter independent; IPSS was reduced to 9 and PVR reduced by 84% to 84 mL/s. Long-term evaluations are necessary to assess the durability of this therapy for patients with urinary retention.
Conclusion
Convective RF water vapor thermal therapy for prostate hyperplasia represents a new minimally invasive procedure that results in a significant early onset and enduring relief of BPH symptoms with preservation of erectile function in subjects followed up for 2 years. The procedure can be performed in an office or ambulatory outpatient with minimal transient perioperative side effects. The convective thermal therapy alleviates bothersome LUTS that result from the prostatic enlargement in a broad range of patients – including those with a median lobe – and warrants consideration as a first-line treatment prior to use of pharmaceutical agents. For many patients, it will be an appropriate and low-risk treatment in the continuum between medical management and more invasive surgical approaches. The Rezūm System and procedure provide versatility for application to a variety of prostate gland morphologies.
Acknowledgment
This study was sponsored by NxThera Inc., Maple Grove, MN, USA.
Disclosure
The authors report no conflicts of interest in this work.
References
Koh JS, Cho KJ, Kim HS, Kim JC. Twelve-month medication persistence in men with lower urinary tract symptoms suggestive of benign prostatic hyperplasia. Int J Clin Pract. 2014;68(2):197–202. | ||
Cindolo L, Pirozzi L, Fanizza C, et al. Drug adherence and clinical outcomes for patients under pharmacological therapy for lower urinary tract symptoms related to benign prostatic hyperplasia: population-based cohort study. Eur Urol. 2015;68(3):418–425. | ||
Cindolo L, Pirozzi L, Sountoulides P, et al. Patient’s adherence on pharmacological therapy for benign prostatic hyperplasia (BPH)-associated lower urinary tract symptoms (LUTS) is different: is combination therapy better than monotherapy? BMC Urol. 2015;15:96–102. | ||
Nichol MB, Knight TK, Wu J, Barron R, Penson DF. Evaluating use patterns of and adherence to medications for benign prostatic hyperplasia. J Urol. 2009;181(5):2214–2221. | ||
Kaplan S, Naslund M. Public, patient, and professional attitudes towards the diagnosis and treatment of enlarged prostate: a landmark national US survey. Int J Clin Pract. 2006;60(10):1157–1165. | ||
Emberton M, Marberger M, de la Rosette J. Understanding patient and physician perceptions of benign prostatic hyperplasia in Europe: the Prostate Research on Behaviour and Education (PROBE) Survey. Int J Clin Pract. 2008;62(1):18–26. | ||
Jepsen JV, Bruskewitz RC. Comprehensive patient evaluation for benign prostatic hyperplasia. Urology. 1998;51(4A suppl):13–18. | ||
Malaeb BS, Yu X, Marshall McBeab A, Elliott S. National trends in surgical therapy for benign prostatic hyperplasia in the United States (2000-2008). Urology. 2012;79(5):1111–1117. | ||
Dixon CM, Cedano ER, Mynderse LA, et al. Transurethral convection water vapor as a treatment for lower urinary tract symptomology due to benign prostatic hyperplasia using the Rezūm® system: evaluation of acute ablative capabilities in the human prostate. Res Rep Urol. 2015;7:13–18. | ||
American Urological Association. American Urological Association Guideline: Management of Benign Prostatic Hyperplasia (BPH). 2010. Available from: http://www.auanet.org/common/pdf/education/clinical-guidance/Benign-Prostatic-Hyperplasia.pdf. Accessed February 20, 2017. | ||
Roehrborn CG, Gange SN, Gittelman MC, et al. Convective water vapor energy (WAVE) ablation therapy: durable two-year results and prospective blinded crossover study for treatment of lower urinary tract symptoms due to benign prostatic hyperplasia. J Urol. 2017. In press 2017. | ||
Gjengstø P, Halvorsen OJ, Akslen LA, et al. Benign growth of different prostate zones in aging men with slightly elevated PSA in whom prostate cancer has been excluded: a prospective study of 510 men. Urology. 2003;62(3):447–450. | ||
McLaughlin PW, Troyer S, Berri S, et al. Functional anatomy of the prostate: implications for treatment planning. Int J Radiat Oncol Biol Phys. 2005;63(2):479–491. | ||
Dixon CM, Cedano ER, Pacik D, et al. Two-year results after convective water vapor energy treatment of symptomatic benign prostatic hyperplasia. Res Rep Urol. 2016;8:207–216. | ||
Kang DH, Lee JY, Hah YS, Chung DY, Lee DH, Cho KS. Correlation of prostatic urethral angle with the severity of urinary symptom and peak flow rate in men with small prostate volume. PLoS One. 2014;9(8):1–9. | ||
Ku JH, Ko DW, Cho JY, Oh SJ. Correlation between prostatic urethral angle and bladder outlet obstruction index in patients with lower urinary tract symptoms. Urology. 2010;75(6):1467–1471. | ||
Mynderse LA, Hanson D, Robb RA, et al. Rezūm system water vapor treatment for lower urinary tract symptoms/benign prostatic hyperplasia: validation of convective thermal energy transfer and characterization with magnetic resonance imaging and 3-dimensional renderings. Urology. 2015;86(1):122–127. | ||
Dixon CM, Cedano ER, Pacik D, et al. Efficacy and safety of Rezūm system water vapor treatment for lower urinary tract symptoms secondary to benign prostatic hyperplasia. Urology. 2015;86(5):1042–1047. | ||
McVary KT, Gange SN, Gittelman MC, et al. Minimally invasive prostate convective water vapor energy (WAVE) ablation: a multicenter, randomized, controlled study for treatment of lower urinary tract symptoms secondary to benign prostatic hyperplasia. J Urol. 2016;195(5):1529–1538. | ||
Barry MJ, Williford WO, Chang Y, et al. Benign prostatic hyperplasia specific health status measures in clinical research: how much change in the American Urological Association symptom index and the benign prostatic hyperplasia impact index is perceptible to patients? J Urol. 1995;154(5):1770–1774. | ||
Roehrborn CG, Wilson TH, Black LK. Quantifying the contribution of symptom improvement to satisfaction of men with moderate to severe benign prostatic hyperplasia: 4-year data from the CombAT trial. J Urol. 2012;187(5):1732–1738. | ||
McVary KT, Gange SN, Gittelman MC, et al. Erectile and ejaculatory function preserved with convective water vapor energy treatment of LUTS secondary to BPH: randomized controlled study. J Sex Med. 2016;3(6):924–933. | ||
Egan KB, Burnett AL, McVary KT, et al. The co-occurring syndrome—coexisting erectile dysfunction and benign prostatic hyperplasia and their clinical correlates in aging men: results from the National Health and Nutrition Examination Survey. Urology. 2015;86(3):570–580. | ||
Rosen RC, Allen KR, Ni X, Araujo AB. Minimal clinically important differences in the erectile function domain of the International Index of Erectile Function Scale. Eur Urol. 2011;60(5):1010–1016. | ||
Gupta N, Köhler TS, McVary KT, et al. Convective radiofrequency water vapor energy ablation (Rezūm®) effectively treats lower urinary tract symptoms due to benign prostatic enlargement regardless of obesity while preserving erectile and ejaculatory function. J Urol. 2017:197(Suppl 4):e609. | ||
Gupta N, Holland B, Delfino K, et al. Convective radiofrequency water vapor energy prostate ablation (Rezūm®) effectively treats urinary retention. J Urol. 2017:197(Suppl 4):e337. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.