Back to Journals » Clinical and Experimental Gastroenterology » Volume 16
Personalized Treatment for Crohn’s Disease: Current Approaches and Future Directions
Authors Clinton JW , Cross RK
Received 30 June 2023
Accepted for publication 4 December 2023
Published 14 December 2023 Volume 2023:16 Pages 249—276
DOI https://doi.org/10.2147/CEG.S360248
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Vipul Yagnik
Joseph William Clinton, Raymond Keith Cross
Department of Medicine, Division of Gastroenterology and Hepatology, University of Maryland School of Medicine, Baltimore, MD, USA
Correspondence: Joseph William Clinton, Department of Medicine, Division of Gastroenterology and Hepatology, University of Maryland School of Medicine, 22 South Greene Street, Baltimore, MD, 21201, USA, Tel +1 703 955 2907, Fax +1 410 328 8318, Email [email protected]
Abstract: Crohn’s disease is a complex, relapsing and remitting inflammatory disorder of the gastrointestinal tract with a variable disease course. While the treatment options for Crohn’s disease have dramatically increased over the past two decades, predicting individual patient response to treatment remains a challenge. As a result, patients often cycle through multiple different therapies before finding an effective treatment which can lead to disease complications, increased costs, and decreased quality of life. Recently, there has been increased emphasis on personalized medicine in Crohn’s disease to identify individual patients who require early advanced therapy to prevent complications of their disease. In this review, we summarize our current approach to management of Crohn’s disease by identifying risk factors for severe or disabling disease and tailoring individual treatments to patient-specific goals. Lastly, we outline our knowledge gaps in implementing personalized Crohn’s disease treatment and describe the future directions in precision medicine.
Keywords: inflammatory bowel disease, precision medicine, personalized medicine, biomarkers, biologic therapy, patient stratification
Introduction
Crohn’s disease (CD) is a type of inflammatory bowel disease (IBD) that is characterized by chronic relapsing inflammation that can affect any segment of the gastrointestinal (GI) tract.1 The global incidence and prevalence of CD has been increasing with improved understanding of the clinical presentation, diagnosis, and natural history of the disease.2 The landscape of treatment options for CD has dramatically progressed over the past two decades and has stimulated a significant increase in research and interest in the field. Despite these advances, the disease course of CD varies widely among patients and predicting individual responses to treatment remains a challenge. Therefore, there has been growing momentum towards a personalized approach to managing CD patients in hopes to tailor treatment towards specific patients’ disease course and manifestations. This review aims to summarize current treatment approaches in CD as well as future directions in CD care that will help achieve the goal of personalized treatment.
Risk Stratification and Predicting Disease Prognosis
The disease course of CD differs among individual patients. Approximately 20% of patients may have prolonged remission after initial presentation.3,4 In contrast, many patients experience a chronic, progressive form of CD and some studies report a 50% risk of developing intestinal complications within 20 years of diagnosis which frequently require surgical intervention.5,6 Furthermore, patients with CD have significantly higher risk of overall mortality and colorectal cancer in patients with colonic involvement compared to the population.7–10 A large meta-analysis demonstrated an all-cause standardized mortality ratio of 1.38 (95% confidence interval, 1.06–1.35) for patients with CD.7 Lastly, CD patients frequently have a significant decrease in quality of life both physically, psychologically, and socioeconomically because of their disease.11–15 Therefore, it is critical to risk stratify individual patients based on their specific disease characteristics to better personalize their treatment and improve outcomes. Predicting disease outcomes in CD is complex with many different variables factoring into overall prognosis. This section will focus on what variables predict severe disease to risk stratify patients and determine which patients would benefit from early advanced therapies (Box 1).16
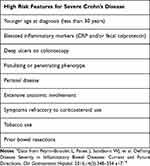 |
Box 1 Risk factors for severe Crohn’s diseasea |
Assessing Current Disease Activity
One of the first steps towards assessing overall disease severity in CD is evaluating the current disease activity. Historically, this has been evaluated with a combination of symptom assessment, biochemical assessment, and endoscopic/radiologic evaluation. Disease activity combined with assessment of an individual patient’s disease characteristics helps to prognosticate overall disease severity and predict outcomes.17
Assessment of current disease activity is done with a combination of subjective symptom scoring measures as well as objective assessment of inflammation including laboratory markers of inflammation and endoscopic evaluation. Two commonly used symptom-based scoring systems are the CD Activity Index (CDAI) and the Harvey-Bradshaw Index (HBI).18 The HBI has been shown to correlate with the CDAI and both are useful tools to help stratify patients into four main categories of disease activity based on symptoms including clinical remission, mild disease, moderate disease, and severe-fulminant disease (Table 1).19
 |
Table 1 Harvey-Bradshaw Indexa |
Next, laboratory markers of inflammation provide useful information for the assessment of current CD inflammatory burden. Two commonly used markers are C-reactive protein (CRP) and fecal calprotectin (FCP). CRP is produced by the liver in response to an inflammatory process, both acute and chronic.20 Several cytokines that are associated with active CD including IL-6, IL-1β, and TNF-α stimulate the production of CRP. However, these cytokines are also produced during other causes of inflammation, such as infection and other inflammatory diseases, and thus CRP is not specific to CD.21 Several studies have demonstrated a correlation between CRP and endoscopic and histologic activity in patients with CD.22,23 Interestingly, Oh et al showed that elevated CRP is associated with CD-related hospitalization and subsequent CD-related intestinal resection even in patients in clinical remission.24 In contrast, some studies have shown a weaker correlation between CRP and disease activity. For example, Denis et al evaluated 28 patients with CDAI > 150 (suggesting at least mild symptoms) but normal CRP levels and found that 92.9% of patients had endoscopic lesions, though most lesions were mild.25 Therefore, clinicians may need to incorporate CRP with other markers of inflammation to obtain the most accurate assessment of CD activity. FCP is another marker of inflammation; however, it differs from CRP in that it is specific to inflammation in the GI tract. Calprotectin is a calcium and zinc bound protein primarily found in neutrophils and its presence in feces is a result of neutrophil migration to the GI tract due to an inflammatory process.26 Several studies have demonstrated a correlation of FCP levels with clinical activity scores and endoscopic activity.27–30 Furthermore, multiple studies demonstrate a stronger correlation of FCP to endoscopic scores of CD activity compared to CRP or other biomarkers of inflammation.31,32 However, some studies suggested that the sensitivity of FCP for detecting endoscopic ulcerations in ileal disease was lower than colonic and ileocolonic disease.28,32,33 A more recent study by Buisson et al demonstrated that FCP can be reliably used to detect endoscopic ulcerations in ileal disease if the FCP threshold is set lower at 200 µg/g, compared to 250 µg/g with ileocolonic or colonic disease.34 The study showed no significant difference in area under curve, sensitivity, and specificity at these thresholds. Therefore, FCP levels are a valuable tool for clinicians to use as an objective marker of GI inflammation and help differentiate patients’ subjective symptoms from true CD activity, though physicians should be aware of the threshold differences of FCP levels based on location of disease.
Other biomarkers have been investigated as markers of CD activity though generally are less specific than CRP and FCP. Erythrocyte sedimentation rate (ESR) is a serum laboratory test that is a non-specific measure of inflammation. ESR is elevated during inflammation because pro-sedimentary factors, primarily fibrinogen, cause red blood cells to stick together and settle faster.35 Alper et al demonstrated a correlation between elevated ESR levels and clinical and endoscopic disease activity.36 However, elevated ESR can be seen in other causes of inflammation, anemia, kidney disease, and certain malignancies so it needs to be combined with other assessments of disease activity for accurate interpretation. Platelet count is another marker of inflammation that has been shown to correlate with disease activity in IBD.37 Further, platelet activation factors including platelet factor 4 and β-thromboglobulin have been shown to correlate with CD activity even in patients with normal CRP.38 The main disadvantage of platelet count in assessing CD activity is its lack of specificity for GI inflammation. Overall, there is no single biomarker that will reliably predict disease activity for each individual patient. Therefore, clinicians should identify the biomarker of inflammation that correlates best with symptoms and endoscopic activity and track that biomarker to guide response to treatment and identify early recurrence of inflammation.
Lastly, endoscopic evaluation should be performed to further assess current disease activity. Two commonly used scoring systems for endoscopic evaluation of CD include the CD Endoscopic Index of Severity (CDEIS) and the Simple Endoscopic Score for CD (SES-CD).39,40 The advantage of these scoring systems is that they provide excellent intra-observer agreement and are reproducible. The main disadvantage is the time required to complete the scoring systems and thus these are more often used in clinical trials than routinely in clinical practice. The SES-CD was derived from the CDEIS in order to simplify the scoring system for endoscopists; both are validated tools and correlate well with clinical parameters (Table 2).41 The resulting scores stratify patients into inactive, mild, moderate, and severe endoscopic disease activity.
 |
Table 2 Simple Endoscopic Score for CD (SES-CD)a |
Other Factors That Influence Disease Severity
In addition to symptoms and inflammatory burden, there are several other disease characteristics that are predictive of overall disease severity (prognosis) and can help guide treatment. These include demographic variables such as age of diagnosis, disease extent including involvement of the rectum, disease phenotype including perianal involvement, and history of intestinal surgeries. To help categorize CD patients by disease severity, several classification systems have been developed. The first was the Rome Classification which was proposed by the International Working Party in 1991 and included disease extent, anatomical location, behavior, and operative history.43 This was revised in 1998 in Vienna at the World Congress of Gastroenterology by including age of onset as well as disease location and behavior.44 This classification was further updated in 2005 to what is known as the Montreal Classification and includes a pediatric age group subset and is the most commonly used classification today (Table 3).45 The Montreal Classification also includes perianal disease and upper GI involvement as potential modifiers of disease behavior and location rather than separate phenotypes.
 |
Table 3 Montreal Classification for CDa |
Disease Behavior
CD behavior has historically been split into three separate phenotypes; inflammatory (also referred to as nonstricturing, nonpenetrating), stricturing, and penetrating. Penetrating disease is considered the most aggressive and severe form of CD and characterized by presence of intestinal fistulas and/or intraabdominal abscess due to transmural inflammation. Fistulas can develop between the bowel and any adjacent organ including other areas of bowel, bladder, and vagina. Furthermore, if the sinus tract is not complete, intraabdominal abscesses can form. This subtype of CD is associated with disabling disease and the need for more frequent surgical intervention.46–50 The stricturing phenotype of CD is characterized by inflammation leading to intestinal fibrosis and luminal narrowing. Once these fibrostenotic changes occur, medical therapies are less likely to reverse this process and surgery is often necessary. Both the stricturing and penetrating phenotypes are considered more aggressive than the inflammatory phenotype as they have repeatedly been demonstrated to be associated with need for initial surgery, re-operation, and need for corticosteroid use.4,51–57 However, chronic untreated inflammation can lead to development of these complications and thus early recognition and treatment of patients with high inflammatory burden may decrease the likelihood of developing these complications.
Presence of perianal disease is considered a modifier to either of the three above listed phenotypes rather than its own distinct phenotype. However, the presence of perianal disease has been associated with more disabling disease, more complications, and need for greater immunosuppression treatment.49,58–60 Furthermore, Tarrant et al. Observed 715 CD patients in New Zealand and found that presence of perianal disease is a strong predictor of change in CD behavior from inflammatory to stricturing or penetrating phenotypes.61 Therefore, patients with perianal disease should be considered high risk for severe disease and thus treated accordingly.
Disease Location and Extent
CD can affect any part of the GI tract though it is most often seen in the colon and terminal ileum. Approximately 50% of CD patients will have involvement in the terminal ileum, including 30% who have isolated small bowel involvement.62 Disease location has historically been categorized into three subtypes including ileal disease, colonic disease, and ileocolonic disease. This is important as multiple population-based studies have shown an association between presence of small bowel disease and need for surgery.51–53,63 This is likely due to a higher propensity to develop strictures and penetrating complications in small bowel disease.4,5,61,64–66 While colonic involvement in CD is associated with less need for surgery, it increases the risk of colorectal cancer.8–10
Like perianal disease, upper GI involvement of CD is considered a modifier of disease location rather than a separate entity. Some studies suggest upper GI involvement correlates with more severe forms of CD and is associated with increased hospitalizations, need for surgery, and increased risk of progression to stricturing and penetrating phenotypes.5,67,68 However, other studies have not demonstrated these findings and thus the correlation of upper GI involvement and severe CD remains controversial.69–71
Demographic Variables
Younger age at diagnosis has consistently been shown to be a negative prognostic predictor in CD. For example, Polito et al showed that diagnosis of CD prior to age 20 was associated with greater small bowel involvement, more stricturing disease, and a higher frequency of surgery.72 Beaugerie et al similarly showed in a study of 1123 patients that age of diagnosis prior to age 40 was an independent risk factor for development of disabling disease in the next five years.73 Similar findings have been described in many population-based studies across the world.4,58,74,75
Other demographic variables have not shown as strong of correlation to worse disease outcomes. For example, female sex has been evaluated as a potential risk factor for more severe disease with mostly mixed results. A few studies have suggested higher rates of extra-intestinal manifestations (EIMs) in female sex,76–78 though overall rates of disabling disease does not appear to significantly differ between sexes.52 Some older studies suggested a link between racial and ethnic minorities and more severe disease manifestations, such as high operation rates and higher prevalence of EIMs,79,80 though more recent studies have not detected a difference in disease severity, behavior, or EIMs by race.81,82 Similarly, a US-based study showed an association between lower socioeconomic status and worse outcomes in CD.83 In contrast, a French population-based study showed no difference in disease severity based on socioeconomics which may be explained by easier access to healthcare in France.84 Taken together, these data would suggest racial and ethnic minorities and lower socioeconomic status may be linked to worse outcomes likely due to reduced access to healthcare rather than inherently more severe disease.
Tobacco use has repeatedly been shown to be a modifiable risk factor that is predictor of poor outcomes in CD.52,85–88 CD patients should be encouraged to quit smoking and educated about their risks for more severe disease.
Steroid Use
Corticosteroids are frequently used to induce remission in CD but are not an effective maintenance medication. However, the need for steroid treatment is associated with an increased risk for developing disabling disease as well as increased risk of progression to stricturing or penetrating phenotypes.58,68,73,89 Therefore, most patients who require steroids for induction tend to have more severe disease and will require biologic therapies to facilitate withdrawal of steroids and for maintenance of remission.
Personalized Treatment in Crohn’s Disease – Current Approaches
After careful assessment of a patient’s disease activity and overall disease severity, it is now appropriate to determine the best course of treatment. Historically, CD treatment strategies were guided by symptoms alone.90–92 However, there is growing evidence that treatment strategies based on symptom control alone often do not change the natural course of the disease, as underlying inflammation may persist even in the absence of symptoms.93,94 Therefore, current approaches to CD treatment focus on a “treat to target” strategy, which was first introduced in 2015 in the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) program developed by the International Organization for the Study of Inflammatory Bowel Diseases (IOIBD).95 This was later updated in 2021 in STRIDE-II and established evidence-based guidelines for clinically useful treatment targets in CD in order improve long term outcomes and prevent disease-related complications.96 These targets focus on disease remission, which includes symptomatic, biochemical, and endoscopic remission. Even with dramatic advances in available medical therapies for CD, a large proportion of patients enrolled in clinical trials did not achieve biochemical or endoscopic remission. Furthermore, while the focus of this review is on medical therapy of CD, many complications that develop because of the disease, such as strictures and fistulas, will not respond to medical therapy alone and require surgical intervention. Therefore, consultation with surgeons remains an important aspect of patient care. Lastly, given the diversity in clinical presentation and disease course as well as multitude of treatment options available, it is unlikely that clinical trials will produce guidelines on how to treat every clinical scenario in CD. However, clinicians should aim to individualize treatment as much as possible by factoring in treatments that have the best chance for clinical response, while also incorporating patient specific factors such as cost of therapy, patient adherence, safety, and patient preferences.
Available Medical Therapies in Crohn’s Disease
Corticosteroids
Corticosteroids are commonly used as the initial medical therapy for patients with moderate to severe CD who require rapid symptom relief. However, their use is limited to short-term induction because of their association with many side effects including but not limited to increased risk of infection, osteoporosis, weight gain, and venous thromboembolism.97 Corticosteroids have also not been shown to be effective as a maintenance therapy in CD.98 There is growing evidence that even short-term corticosteroid use is associated with adverse events in IBD including sepsis, venous thromboembolism, and fractures.99 Therefore, corticosteroid use should be limited to a short course, ideally less than twelve weeks, and serve as a bridge to maintenance therapy with an advanced therapy. The most frequently prescribed corticosteroid medications in the outpatient setting in CD are budesonide and prednisone. Budesonide is recommended as first line therapy in patients with mild to moderate ileal or proximal colonic disease as it is formulated to undergo pH-dependent release in the distal ileum, followed by absorption and rapid hepatic metabolism to compounds with minimal or no systemic glucocorticoid activity.100 Budesonide has been shown to be as effective at inducing remission in mild to moderate disease as prednisone and prednisolone, but associated with fewer adverse events.101–103 In contrast, prednisone can be used in patients who do not respond to budesonide or patients with more extensive disease.
5-Aminosalicylates (5-ASA)
The 5-ASA class of medications has been widely employed in the treatment of IBD due to their anti-inflammatory effects. Their use in ulcerative colitis (UC) is well established as an effective treatment for induction and maintenance of remission.104,105 However, data supporting its use in CD is controversial. Early meta-analyses of various clinical trials evaluating the efficacy of mesalamine in maintenance of medically induced remission in CD suggested a modest benefit with mesalamine use.106–108 Another meta-analysis from 2010 demonstrated that sulfasalazine was more likely to induce remission compared to placebo, though this effect was only seen in patients with isolated colitis.109 However, a more recent Cochrane systematic review from 2016 found no evidence to suggest that oral 5-ASA preparations are superior to placebo for the maintenance of medically induced remission.110 Lastly, the American College of Gastroenterology CD guidelines recommend against the use of 5-ASAs based on lack of evidence supporting their efficacy.111 Therefore, we suggest against routine use of 5-ASAs in the treatment of CD, though sulfasalazine may be considered in mild cases of colonic CD.
Immunomodulators
The class of medications termed immunomodulators, including azathioprine, 6-mercaptopurine, and methotrexate, have been used to treat patients with IBD since the late 1960’s because of their immunosuppressive effects. Immunomodulators are not effective agents in inducing symptomatic remission in CD because of their slow onset of action, often taking up to three months to take effect.105 Many studies have demonstrated efficacy of immunomodulators as monotherapy in maintenance of remission compared to placebo.112–115 However, the side effect profile of immunomodulators including allergic reactions, pancreatitis, myelosuppression, nausea, infections, hepatotoxicity, pulmonary toxicity (methotrexate only) and malignancy (including nonmelanoma skin cancer and lymphoma) make them a less attractive option compared to advanced therapies.
Combination therapy with an immunomodulator and anti-TNF medication is an alternative approach and generally preferred in those patients who do not have risk factors precluding their use. When used for induction, combination therapy is used for three main purposes: to treat inflammation via multiple mechanisms which leads to synergistic effect, to reduce immunogenicity against biologic therapy, and to improve the pharmacokinetics of biologic therapy. In the SONIC trial of 508 patients with moderate to severe CD who were biologic and immunomodulator treatment naïve, patients who received combination infliximab (IFX) and azathioprine were more likely to achieve steroid-free remission and mucosal healing at 26 weeks compared to both azathioprine or IFX monotherapy.116 Infection rates were similar in patients receiving combination versus monotherapy. Interestingly, a post-hoc analysis of the SONIC trial examined the trough concentrations of IFX in both combination and infliximab monotherapy and found that rates of clinical remission and immunogenicity were not significantly different in the quartile of patients with highest serum IFX concentrations.117 This would suggest that the benefit from combination therapy may primarily be from enhanced pharmacokinetics, and immunogenicity may be avoided with higher serum drug concentrations. The downside to this approach would be the need for proactive drug monitoring and thus higher cost and potential issues with insurance approval for higher doses of the anti-TNF.
There have not been large randomized controlled trials assessing combination adalimumab (ADA) or certolizumab with immunomodulators. However, one systematic review and meta-analysis suggested mild superiority of combination ADA and immunomodulator therapy to ADA monotherapy for induction of remission.118 Another more recent meta-analysis failed to demonstrate superiority of ADA combination therapy compared to monotherapy; however, lower rates of immunogenicity was noted in the combination group.119 Two trials also demonstrated similar reduction in immunogenicity in patients treated with combination IFX and immunomodulator compared to IFX monotherapy and noted no significant difference between use of azathioprine or methotrexate as the immunomodulator.120,121
The benefits of continuing combination treatment for maintenance therapy are less clear and the decision must be individualized. In a randomized controlled trial of 80 patients who achieved remission with combination IFX and an immunomodulator, the authors found similar disease relapse rates between combination and IFX monotherapy-treated patients at 104 weeks.122 However, they did note lower CRP values and higher IFX trough levels in the combination therapy group. Similarly, a meta-analysis of four studies examining the efficacy of ADA monotherapy compared to the combination of ADA and immunomodulator found similar rates of relapse at one year for patients who achieved remission after induction with combination therapy.118 The decision to continue or withdrawal concurrent immunomodulator is nuanced and based on a number of factors including comorbid conditions that make continuing combination therapy higher risk for adverse events, therapeutic drug levels on combination therapy, prior history of immunogenicity, and patient preference. Before withdrawing concurrent immunomodulator therapy, we verify that patients are in biologic and endoscopic remission, assess therapeutic drug levels verifying that the patient is in a therapeutic range, and generally verify receipt of combination therapy for 6–24 months.
Biologic Therapies
TNF Inhibitors
TNF inhibitors or anti-TNF medications were the first biologic therapy approved for treatment of CD, starting with IFX in 1998. They remain one of the most frequently used class of therapies in CD because of their efficacy and abundance of clinical data collected over the last three decades since their introduction. The three medications in this class approved for CD are IFX, ADA, and certolizumab. IFX was first evaluated in the short term by Targan et al who demonstrated a clinical response in 41% of patients treated with a single dose of infliximab at 5 mg/kg compared to 12% of patients treated with placebo.123 A follow up study termed the ACCENT 1 trial by Hanauer et al demonstrated that patients who had clinical response to an initial dose of IFX were more likely to remain in remission at week 30, week 54, and to discontinue steroids if they were kept on maintenance IFX dosing every 8 weeks compared to placebo.124 Similarly, ADA was FDA approved for the treatment of CD in 2007 following the CHARM trial which demonstrated that patients treated with ADA were more likely to achieve remission at 26 weeks (47% vs 17%) and maintain remission at 56 weeks (41% vs 12%) compared to placebo-treated patients.125 Lastly, certolizumab was FDA approved for treatment of CD in 2008 following a trial by Sandborn et al which showed improved clinical response rate at weeks 6 and 26 compared to placebo.126 However, no significant difference was seen in rates of remission compared to placebo.
Efficacy in Fistulizing Disease
Treatment of perianal and fistulizing CD is complex and frequently requires a combination of medical and surgical therapy. TNF inhibitors, particularly IFX, have the strongest supporting evidence of all biologic therapies in the medical treatment of perianal and fistulizing CD. In a study of 94 CD patients with either abdominal or perianal draining fistulas, Present et al showed that treatment with IFX at either 5 mg/kg or 10 mg/kg achieved a 50% or more reduction in number of draining fistulas in two consecutive follow up visits at a significantly higher rate compared to placebo (68%, 56%, vs 26% respectively).127 They also noted significantly more patients in the IFX groups had closure of all fistulas compared to placebo (55%, 38%, vs 13% respectively). Following this, the ACCENT II trial by Sands et al evaluated 306 CD patients with either abdominal or perianal fistulas that were treated with 5 mg/kg infliximab at 0, 2, and 6 weeks and then continued every 8-week maintenance dosing infliximab or placebo. The authors found that in patients who responded to initial induction therapy, those treated with IFX maintenance therapy were significantly more likely to have complete absence of draining fistulas compared to placebo (36% vs 19%).128 A post hoc analysis of the ACCENT II trial evaluated women with rectovaginal fistulas at baseline and found that among responders to IFX induction, 72.2% of rectovaginal fistulas were no longer draining at week 14.129 Further, the duration of fistula closure was longer for patients on IFX maintenance treatment compared to placebo (46 weeks vs 33 weeks median). There are no large prospective randomized trials that evaluated ADA or certolizumab for fistula closure though some evidence suggests they may be effective for fistula healing. A small open label trial showed a significant decrease in the mean number of draining fistulas per day in patients treated with ADA compared to placebo.130 In the subset of patients from the CHARM trial with draining fistulas at baseline, patients treated with ADA had a higher rate of fistula response and closure compared to placebo, though this was not statistically significant.125 Post hoc analysis of two ADA maintenance trials did not find a statistical difference in fistula closure in patients treated with ADA compared to placebo.131,132 Similar findings were seen in post hoc analysis of certolizumab trials which suggested possible benefit for fistula closure, though no direct studies have assessed this.132,133
Comparing TNF Inhibitors
There are no head-to-head randomized controlled trials that compare the efficacy of the different TNF inhibitors in the treatment of CD. However, there have been several meta-analyses published which suggest that there are no significant differences in the efficacy of IFX and ADA, but that certolizumab may be less likely to induce remission compared to the other two agents.134–136 Therefore, certolizumab is used as a second- or third-line agent in the class. IFX has historically been administered as an intravenous (IV) infusion whereas ADA is administered as a subcutaneous injection, which some patients may prefer as a more practical route of administration. However, a SC form of IFX has been developed and a recent phase III trial compared its efficacy to placebo in maintenance of moderate to severe CD.137 The LIBERTY-CD trial included 343 patients with moderate to severe CD who underwent IFX IV induction and had clinical response and then were randomized to receive IFX SC 120 mg every two weeks or placebo. At week 54, the rate of clinical remission was greater in the IFX SC compared to placebo (62.3% vs 32.1% respectively, P < 0.0001) as well as endoscopic response rate (51.1% vs 17.9% respectively, P < 0.0001). Celltrion SC formulation of IFX, marketed as Zymfentra, was recently FDA approved for the use in maintenance of moderate to severe CD.138 This may be another draw for IFX in the treatment of CD as many patients prefer SC administration for practical purposes.
It is not recommended to effectively switch between TNF inhibitors for non-medical reasons as this may worsen outcomes. This was evaluated in the SWITCH trial which examined patients previously controlled with IFX who were then randomized to continue IFX or switch to ADA. The authors found that those that switched to ADA were significantly more likely to develop loss of tolerance and loss of efficacy than those that continued IFX.139 However, switching to a second TNF inhibitor after failure or intolerance to a first TNF inhibitor is a potential option for patients. In a randomized controlled trial of 325 patients with CD previously treated with IFX and with either loss of response or intolerance to the medication, 21% of patients who were treated with ADA compared to 7% of patients treated with placebo achieved clinical remission at week 4 (p < 0.001).132 These results may be improved with knowledge of therapeutic drug levels. Patients with loss of response with significant antidrug antibodies are likely to respond to a switch within class whereas those with therapeutic drug levels are likely best served by changing to a therapy with a different mechanism of action.
Safety Profile
TNF inhibitors are well tolerated, and most common adverse effects are minor and do not require drug discontinuation. However, serious adverse events have been reported; therefore, patients should be counseled on these potential adverse effects before initiating the medication. The main adverse effects associated with TNF inhibitor use include but are not limited to increased risk of infection (particularly reactivation of tuberculosis and Hepatitis B), melanoma skin cancer, lymphoma, demyelinating disorders, lupus-like reactions, and skin reactions.140–146 Prior to starting TNF inhibitors, all patients should be screened for exposure to tuberculosis and hepatitis B. The risks of infection and lymphoma are accentuated in patients on a TNF inhibitor and immunomodulator.147 Once patients initiate therapy, they should receive all age-appropriate vaccinations. Additionally, they should receive vaccines to prevent pneumococcus and shingles regardless of age. We similarly recommend vaccinations in other advanced therapies. They also should undergo regular dermatologic evaluation and gynecology care for women.
Medications Targeting Leukocyte Trafficking
Vedolizumab
Vedolizumab (VDZ) is a humanized anti-α4β7 integrin monoclonal antibody which blocks the interaction between α4β7 integrin and MAdCAM-1, which is mainly expressed on the GI tract endothelial cells.148 This is thought to prevent leukocyte trafficking specifically in the GI tract. VDZ has been shown to be effective for achieving increased rates of clinical response, clinical remission, and steroid-free remission compared to placebo. In a large randomized controlled trial by Sandborn et al of 368 patients, VDZ-treated patients had higher rates of clinical remission at week 6 compared to placebo (14.5% vs 6.2%, P = 0.02), and those who had response to induction had higher likelihood of remaining in clinical remission at week 52 if treated with every 8 or 4 weeks maintenance dosing of VDZ compared to placebo (39.0%, 36%, and 21.6% respectively, p < 0.001 and P = 0.004).149 Another study by Sands et al evaluated patients who previously failed TNF inhibitor therapy and compared treatment with VDZ to placebo.150 There was no significant difference in rates of clinical remission at week 6 between VDZ and placebo-treated patients; however, there was a significant difference at week 10 (26.6% vs 12.1%, p = 0.001), suggesting a longer onset of action of VDZ compared to TNF inhibitors. Two other large meta analyses found similar findings of VDZ efficacy in induction and maintenance of clinical outcomes although efficacy seems to be greatest in TNF inhibitor naïve versus TNF inhibitor exposed patients.151,152 VDZ has been shown to be effective as monotherapy because of low rates of immunogenicity in clinical trials. VDZ has historically been administered as an IV infusion, however a recent phase III clinical trial examined a new SC formulation.153 The VISIBLE 2 trial evaluated 410 patients with moderate to severe CD who were clinical responders to VDZ IV induction therapy and randomized them to receive VDZ 108 mg SC every two weeks or placebo. The authors found that at week 52, 48.0% of patients receiving vedolizumab SC were in clinical remission compared to 34.3% receiving placebo (p = 0.008). Vedolizumab SC is currently undergoing reviewal by the FDA for approval for maintenance therapy for moderate to severe CD.154 This is exciting as many patients prefer SC injection as a more practical route of administration.
Safety Profile
VDZ has a very favorable safety profile compared to other biologics for management of CD. Long-term safety data suggested a mildly increased rate of infections compared to placebo (0.85 vs 0.70 infections per patient year).155 Common adverse reactions include headaches, arthralgias, and nasopharyngitis. There have been four cases of serious liver injury attributed to VDZ in long-term safety trials. Rates of malignancy were low and similar to placebo (0.4% vs 0.3%). Injection site reaction was the only new safety event observed for VDZ SC.153
Natalizumab
Natalizumab is an anti-α4 integrin antibody which interferes with leukocyte trafficking in the GI tract and brain.156 Natalizumab has been shown to be effective at inducing clinical response and clinical remission at rates that are comparable to VDZ as shown in two large meta-analyses.151,157 It was also shown to be equally efficacious for both anti-TNF naïve and anti-TNF exposed patients. However, the main drawback with use of natalizumab in CD is the risk of progressive multifocal leukoencephalopathy (PML), caused by the John Cunningham (JC) virus. A large safety study by Bloomgren et al demonstrated that the rate of PML among patients who were positive for anti-JC virus antibodies, had taken immunosuppressants before the initiation of natalizumab therapy, and had received 25 to 48 months of natalizumab treatment was as high as 1.1 cases per 100 patients.158 Therefore, natalizumab is primarily used in medically refractory patients or those with concurrent multiple sclerosis; patients must be screened for JC virus antibodies prior to initiation of therapy and every 6 months thereafter.
Medications Targeting IL-12/23
Ustekinumab
Ustekinumab (UST) is a monoclonal antibody to the p40 subunit of IL-12 and IL-23, which are pro-inflammatory cytokines with receptors on T cells, natural killer cells, and antigen-presenting cells.159 UST at a dose of 130 mg or 6 mg/kg was shown to be more effective than placebo in achieving clinical response at week 6 after induction both in patients who were previously treated with TNF inhibitors (34.3%, 33.7%, and 21.5%, respectively, p ≤ 0.003 for both doses) and those who failed other conventional therapy (51.7%, 55.5%, and 28.7%, respectively, p < 0.001 for both doses).160 Patients who responded to UST induction were randomized to receive UST maintenance therapy at every 8- or 12-week dosing or placebo. UST-treated patients were significantly more likely to be in remission at week 44 compared to placebo (53.1% and 48.8%, vs 35.9% respectively, p = 0.005 and p = 0.04). Therefore, UST can be used as first line treatment or considered as a second line agent in patients who previously failed other therapies including TNF inhibitors. Clinical trials also noted overall low rates of immunogenicity to UST (<3%) and thus UST is frequently used as monotherapy rather than combined with an immunomodulator.
Safety Profile
UST has a very favorable safety profile. The main adverse events seen in clinical trials of UST include hypersensitivity reactions (<1%), nonmelanoma skin cancer (0.2%, similar to placebo), other malignancies (0.2%), and serious infections (2.3%, similar to placebo).148 A long-term safety study of patients with psoriasis treated with UST termed the PSOLAR registry found low rates of malignancy (0.68/100PY), major cardiac events (0.33/100PY), serious infections (1.60/100PY), and overall mortality (0.46/100PY).161 Notably, unadjusted rates of serious infection for IFX (2.91/100PY) and other biologics (1.91/100PY) were numerically higher compared with UST (0.93/100PY).161
Risankizumab
Risankizumab is a monoclonal antibody that binds to the p19 subunit of IL-23 leading to decreased inflammation.162 The ADVANCE and MOTIVATE trials were two phase 3 randomized controlled trials that evaluated risankizumab for induction therapy in CD finding that risankizumab at a doses of 600 and 1200 mg were significantly more likely to induce remission compared to placebo (45%, 42%, vs 25% respectively in ADVANCE, 42%, 40%, vs 20% respectively in MOTIVATE).163 The ADVANCE trial included both patients who were biologic naïve and biologic exposed while MOTIVATE only included patients who were biologic exposed. Following this, the FORTIFY maintenance trial demonstrated that patients who responded to induction and were treated with risankizumab maintenance therapy at 360 mg were more likely to be in clinical remission (52% vs 41%) and endoscopic response (47% vs 22%) at week 52 compared to placebo.164 Therefore, risankizumab is a viable option for treatment of patients with moderate to severe CD both in biologic naïve and biologic exposed patients.
Safety Profile
Risankizumab was well tolerated in clinical trials with low overall rates of adverse events. The FORTIFY trial reported overall low rates of serious infections (3–4%), malignancy (1%), injection site reactions (5–6%), major cardiac events (1%), and hepatic events (3–4%).164 Long-term safety data on patients with psoriasis treated with risankizumab similarly showed low rates of serious adverse events (7.8 per 100 PY), serious infections (1.2 per 100 PY), nonmelanoma skin cancer (NMSC) (0.7 per 100 PY), malignant tumors excluding NMSC (0.5 per 100 PY), and major cardiac events (0.3 per 100 PY).165 Because of a case of drug-induced liver injury in the ADVANCE and MOTIVATE trials, monitoring of hepatic function tests during induction is recommended.
Small Molecules
JAK Inhibitors
Upadacitinib
In May 2023 following two phase 3 induction trials U-EXCEL and U-EXCEED and one maintenance trial U-ENDURE, the FDA approved upadacitinib for treatment of adults with moderate to severe CD.166 Upadacitinib is an oral Janus kinase (JAK) inhibitor that down-regulates multiple proinflammatory cytokines, including interleukin IL-2, IL-4, IL-6, IL-7, IL-9, IL-15, IL-21, and interferon gamma, that are relevant to the pathogenesis of CD.167 The induction trials included 526 patients in U-EXCEL and 495 patients in U-EXCEED who were randomized in a 2:1 ratio to receive 45 mg upadacitinib or placebo once daily for 12 weeks.166 The authors found a statistically significant difference in rates of CDAI clinical remission (49.5% vs 29.1%, in U-EXCEL and 38.9% vs 21.1%, in U-EXCEED, p < 0.001 for both comparisons) and endoscopic response (45.5% vs 13.1% in U-EXCEL and 34.6% vs 3.5% in U-EXCEED, p < 0.001 for both comparisons) for patients treated with upadacitinib compared to placebo. The maintenance trial U-ENDURE included patients who had clinical response to upadacitinib induction therapy and were randomized in a 1:1:1 ratio to receive 15 mg upadacitinib, 30 mg upadacitinib, or placebo once daily for 52 weeks. The authors found a statistically significant difference in rates of CDAI clinical remission (37.3% vs 47.6% vs 15.1%, p < 0.001 for both comparisons), and endoscopic response (27.6% vs 40.1% vs 7.3%, p < 0.001 for both comparisons) for 15 mg upadacitinib and 30 mg upadacitinib compared to placebo. Of note, in U-EXCEL 45.4% had previous failure to biologic therapy and in U-ENDURE 75.1% had previous failure to biologic therapy. These results suggest that upadacitinib is effective treatment option for the management of adult patients with moderate to severe CD both in biologic naïve and exposed patients. Furthermore, the practical benefits and ease of administration of an oral medication is attractive to many patients.
Safety Profile
In long-term safety data studies of upadacitinib in the treatment of rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, and atopic dermatitis, the main adverse events included but were not limited to increased risk of herpes zoster infections, serious infections, non-melanoma skin cancer, and elevations in creatine phosphokinase levels.168 In the previously mentioned CD phase 3 trials, similar findings of increased risk of herpes zoster infections, serious infections, and elevated creatine kinase were seen.168 There was also a dose-dependent effect with upadacitinib therapy for herpes zoster infection, hepatic disorder, and neutropenia. Lastly, there were four cases of gastrointestinal perforation with 45 mg upadacitinib treatment during the induction trials as well as one gastrointestinal perforation in all three groups in the maintenance trial. However, it is unclear if this adverse event was treatment related or an inherent risk of disease progression in CD. Lastly, there was a large post marketing trial which compared patients treated with another JAK inhibitor tofacitinib and patients treated with TNF inhibitors for rheumatoid arthritis and were at least 50 years of age and had at least one cardiovascular risk factor.169 The authors found increased rates of major adverse cardiovascular events (defined as cardiovascular death, myocardial infarction, and stroke), thrombosis (including deep venous thrombosis, pulmonary embolism, and arterial thrombosis), and malignancies (excluding NMSC, lymphomas, and lung cancer in current or past smokers). This risk was not seen in a long-term safety study of UC patients treated with tofacitinib.170 Extrapolating data from rheumatologic disease to IBD is difficult as they have different pathogenesis and the demographics of the patient population are quite different with RA patients being older and with a greater number of comorbid conditions including cardiovascular risk factors. Regardless, treatment with any JAK inhibitor should be used with caution in older patients and patients with cardiovascular risk factors. Overall, upadacitinib was well tolerated in the CD trials however more long-term data is needed to identify rare or long latency events.
Comparing Advanced Therapies
Despite the rapidly increasing number of drug therapies available for treatment of CD, primary nonresponse or loss of response remains common. Additionally, no diagnostic tests exist to guide clinicians as to which treatment is “right” for a given patient. Ideally, there would be clear comparative data of available biologic therapies in CD to help guide clinicians. However, head-to-head trials are sparse and much of our knowledge comes from meta-analyses and expert opinion.
The SEAVUE trial by Sands et al is one of the few head-to-head randomized controlled trials of biologic therapies in CD comparing the efficacy of UST and ADA in 386 patients who had failed conventional therapy and were biologic naïve.171 Patients were randomized to ADA or UST induction followed by maintenance therapy with a primary endpoint of clinical remission at week 52 (CDAI score <150). There was no significant difference in the proportion of patients in clinical remission between UST and ADA-treated patients (65% vs 61%, p = 0.42). ADA-treated patients had high rates of anti-drug antibodies compared to UST-treated patients (74% vs 2%); however, the presence of anti-drug antibodies was not associated with response to treatment. Another head-to-head randomized controlled phase 3 trial titled SEQUENCE recently released preliminary data comparing UST to risankizumab.172 The study included patients with moderate to severe CD who had previously failed anti-TNF therapy and found that rates of clinical remission at week 24 for patients treated with risankizumab was non-inferior to UST (clinical remission rates 59% vs 40% respectively). Furthermore, patients treated with Risankizumab met superiority compared to those treated with UST in regard to endoscopic remission rates at week 48 (32% vs 16% respectively, p < 0.0001). Full results from the SEQUENCE trial are expected to be presented at future medical meetings and later submitted for publication.
A large systematic review and network meta-analysis of phase 2 and phase 3 randomized controlled trials was published in 2021 by Singh et al.173 The authors found that for biologic naïve patients, IFX monotherapy (odds ratio [OR] 4.53), combination IFX and azathioprine (OR 7.49), ADA (OR 3.01), and UST (OR 2.63) were significantly more likely to induce remission compared to certolizumab. Combination therapy with IFX and azathioprine was also significantly more likely to induce remission compared to VDZ (OR 3.76). For biologic exposed patients, ADA after loss of response to IFX (OR 2.82), and risankizumab (OR 2.10) were significantly more likely to induce remission compared to VDZ. Therefore, the authors suggest that IFX in combination with azathioprine and ADA have strongest evidence for first line therapy and ADA or risankizumab are preferred therapies in anti-TNF exposed patients.
Predicting and Monitoring Response to Therapy
With the push towards personalized medicine in CD, it is important to identify patients most likely to respond to individual treatments, including biologic therapies. However, there are few validated clinical tools for predicting response to therapy that are widely available at this time for use by clinicians. Currently, clinicians rely of therapeutic drug monitoring and specific biomarkers in addition to subjective clinical parameters to help guide adjustments in therapy.
Along with clinical assessment of patients with risk factors for severe or disabling disease, machine learning models are being developed to help clinicians predict which patients are at risk for severe disease and require early intervention. Waljee et al developed a random forest model using data from IBD patients at the Veterans Health Administration and were able to accurately predict the risk of hospitalization and/or corticosteroid use for IBD within 6 months.174 The authors noted that the five leading risk factors for hospitalization and/or steroid use were age, mean serum albumin, immunosuppressive medication use, and mean and highest platelet counts. Models such as this would be helpful to simplify the process of identifying high-risk CD patients for clinicians so that they could be initiated on early therapy to prevent complications.
For thiopurine based immunomodulators, including azathioprine and 6-mercaptopurine, measurement of thiopurine methyltransferase (TPMT) polymorphisms is an effective tool to improve overall clinical response and prevent adverse effects of therapy, particularly bone marrow suppression.175 Azathioprine and 6-mercaptopurine are metabolized by TPMT and hypoxanthine phosphoribosyl transferase (HPRT) to 6-thioguanine nucleotides (6-TGn) and 6-methyl mercaptopurine (6-MMP).176 The metabolite 6-TGn functions as a purine antagonist inducing lymphocytotoxicity and immunosuppression. Genetic polymorphisms in TPMT activity have been observed with either low or intermediate activity of the enzyme which leads to preferential metabolism of 6-mercaptopurine to 6-TGn.176,177 This can lead to potentially toxic levels of 6-TGn and resulting bone marrow suppression.178–180 Therefore, patients with intermediate or low TPMT activity are recommended to undergo a low-dose treatment strategy with azathioprine or 6-mercaptopurine and several studies have demonstrated that this strategy is effective and safe.181,182 TPMT activity measurement is recommended for all patients prior to initiation of azathioprine or 6-mercaptopurine to help predict clinical response and adjust dosing to prevent potentially toxic side effects.183 Furthermore, therapeutic drug monitoring of thiopurine based immunomodulators is currently performed by measuring the metabolites 6-TGn and 6-MMP. However, these tests are expensive and poor predictors of clinical response as shown by a large meta-analysis which estimated a sensitivity and specificity of 62% and 72% respectively.184–186 Therefore, Waljee et al developed a machine learning algorithm (MLA) that incorporated laboratory values and patient age in a population of IBD patients on thiopurine therapy and more accurately predicted clinical responders compared to measurement of 6-TGn levels.187 Waljee et al subsequently created further MLAs that predicted clinical response on more objective measures rather than subjective patient responses and found that the MLAs continued to more accurately predict clinical remission compared to 6-TGn levels and those patients in MLA predicted remission had less adverse clinical events including new steroid prescriptions, hospitalizations, and abdominal surgeries.188 These MLA are not currently widely available however the authors noted that a trial of three MLAs used at the University of Michigan, which replaced thiopurine metabolite measurement, reduced total expenditures by $75,000. This is promising that easily accessible and practical MLAs will be developed for widespread clinical use.
Similarly, multiple tools are employed to monitor response to biologic therapies. Therapeutic drug monitoring (TDM) is a clinical tool that involves measurement of serum drug levels and anti-drug antibodies.189 Historically, TDM was used in a reactive manner when there was suspected loss of response of a biologic therapy. Clinicians measure serum drug levels and anti-drug antibodies to determine the presence and mechanism of loss of response to help guide whether therapy should be dose optimized, switched to another therapy within the same class, or switched to another class of biologic therapy. This strategy is effective as demonstrated in the PANTS trial which showed that low serum drug levels was associated with primary non-response in CD patients treated with IFX or ADA. Similarly, multiple studies have shown that anti-drug antibodies are associated with treatment failure due to increased clearance of the drug.190,191 More recently, proactive TDM has been explored as a personalized treatment strategy to identify patients at risk of treatment failure prior to them showing clinically apparent effects. Several studies have supported this approach citing association of proactive TDM with several important clinical outcomes including need to discontinue drug therapy, steroid-free clinical remission, need for surgery, need for hospitalization, and mucosal healing.192–196 In contrast, several large clinical trials demonstrated that proactive TDM compared to reactive were similarly efficacious.197–199 Therefore, most guidelines do not make strong recommendations on the use of proactive TDM as more understanding of which patients would benefit from this approach is needed.183 Along with TDM, other biomarkers of inflammation such as FCP are useful in monitoring response to therapy and tailoring therapy adjustments to specific patients. The CALM trial demonstrated that combining concentrations of FCP and CRP with assessment of clinical symptoms was superior to assessment of clinical symptoms alone. This was likely due to rapid optimization of therapy and thus higher proportion of patients with improved clinical outcomes including mucosal healing, deep remission, biological remission, and steroid-free remission.200 As discussed previously, no single biomarker will reliably predict disease for every patient and therefore a combination of multiple biomarkers is needed to best monitor response to therapy.
Predicting individual response to biologic therapy is a challenging endeavor but one of great research interest. Dulai et al published a validated scoring system for predicting response to VDZ in which they collected data from the GEMINI 2 trial of CD patients treated with VDZ for 26 weeks and validated the scoring system with data from the VICTORY cohort.201 The authors identified lack of previous anti-TNF treatment (+3 points), absence of prior bowel surgery (+2 points), absence of fistulizing disease (+2 points), baseline level of albumin (+0.4 points per g/L), and baseline CRP (reduction of 0.5 points for values between 3.0 and 10.0 mg/L and 3.0 points for values >10.0 mg/L) as factors associated with remission. Scores of 13 or greater identified patients achieving clinical remission with sensitivity of 92%, corticosteroid-free remission with sensitivity 94%, and mucosal healing with sensitivity of 100%. Though it was not designed for CD, Waljee et al also developed a clinical prediction tool for predicting corticosteroid-free endoscopic remission with vedolizumab in ulcerative colitis using random forest models.202 The models used data up to week 6 of VDZ therapy from phase 3 clinical trials of VDZ for induction and maintenance of UC and were able to accurately predict corticosteroid-free endoscopic remission (area under receiver operating curve (AUROC) 0.73 (95% CI: 0.65–0.82). The authors noted the five strongest predictors of corticosteroid-free endoscopic remission at week 52 were FCP at week 6, the slope of VDZ level, the slope of FCP, albumin at week 6, and VDZ level at week 6. Waljee et al developed similar machine learning models to predict Crohn’s disease remission beyond week 42 in patients treated with UST using phase 3 clinical trial data.203 Using demographic and laboratory data through week 8 of UST therapy, the models accurately predicted Crohn’s disease remission (defined as CRP level lower than 5 mg/dL as a proxy for biological remission) with AUROC 0.78 (95% CI, 0.69–0.87).
Various biomarkers have been investigated to help predict response to therapy, though few have proven reliable. However, Caviglia et al published a study that investigated multiple biomarkers including serum zonulin, a biomarker of intestinal permeability; soluble CD163, a macrophage activation marker; and a panel of serum cytokines in IBD patients treated with biologic therapy compared to control patients diagnosed with irritable bowel syndrome.204 Out of all investigated biomarkers, the authors found that reduction in levels of IL-6 from baseline to 10 weeks of biologic therapy was associated with a 4.7-fold higher probability of achieving a clinical response at 12 months of biologic therapy compared to patients with no IL-6 reduction. IL-6 is a promising biomarker that may help predict response to therapy, though it is not specific to CD and is produced in other causes of inflammation. Lastly, there has been a push towards evaluating genetic markers that may predict response to biologic therapies. However, they have generally performed poorly in predicting response to specific therapies. For example, a systematic review by Bek et al found no genetic markers currently available which are adequately predictive of anti-TNF response.205
Pragmatically, applying individualized prediction tools for each therapy is cumbersome and unlikely to be widely adopted. However, some existing tools, such as CDPATH, can help patients make more informed decisions about their management.206 The CDPATH tool uses blood tests to help predict the potential risk of developing serious Crohn’s disease-related complications within three years. The tool is used primarily for prognostic purposes, however the information provided is helpful for both patients and clinicians to personalize their treatment plan based on predicted risks of severe disease. Development of clinical prediction tools that are practical, accessible, and can accurately predict response to available therapies is needed and currently not available.
Extraintestinal Manifestations
Extraintestinal manifestations (EIMs) are a diverse collection of conditions that occur outside the GI tract and frequently affect patients with CD. Trikudanathan, Venkatesh, and Navaneethan detail many of EIMs seen in CD in their review article.207 The authors note that some of these EIMs are directly related to GI inflammation including some forms of inflammatory arthritis, oral aphthous ulcers, erythema nodosum, and episcleritis. Another group of EIMs appear to follow an independent course from the underlying GI disease including polyarticular arthritis, ankylosing spondylitis, pyoderma gangrenosum, primary sclerosing cholangitis, and uveitis. Regardless of the correlation between EIMs and intestinal inflammation, the presence of concurrent EIM can have a detrimental effect on quality of life and increase the complexity of treatment. Personalizing biologic therapy in patients with concurrent EIM is even more critical (Table 4). For example, anti-TNF may be preferred in patients with peripheral or axial arthritis whereas risankizumab or UST may be preferred in patients with concurrent psoriasis.
 |
Table 4 Biologic Therapy for Management of EIMs |
TNF Inhibitors
TNF inhibitors demonstrated efficacy for cutaneous, musculoskeletal, and ocular EIMs in several studies. A large systematic review noted that patients with pyoderma gangrenosum treated with TNF inhibitors had complete response in 21–25% of patients in interventional and 92–100% patients in non-interventional studies.208 There were comparable results seen in erythema nodosum and stomatitis. TNF inhibitors led to reduction of arthralgias (47.1% to 26.8% in one open label trial) and arthritis (8.7% to 2.1% and 58% to 12.5% in two open label trials). TNF inhibitors also improved symptoms in ocular manifestations in most patients. Furthermore, a pooled analysis of IFX-treated EIMs (musculoskeletal, ocular, and cutaneous manifestations) in the Swiss IBD cohort study showed clinical improvement in 74% of patients.209 This same group found clinical improvement in ADA-treated EIMs in 70% of patients.
Medications Targeting Leukocyte Trafficking
VDZ is gut selective and thus one may expect it is not effective in treatment of EIMs. However, a study of 294 IBD patients treated with VDZ of which 49 had at least one EIM reported clinical remission rates of 44.7% for arthritis/inflammatory arthralgia (n = 47) and 75% for cutaneous manifestations (n = 4).210 In contrast, a meta-analysis which included three interventional studies, five non-interventional studies and three case series found no significant effect on arthralgias/arthritis or cutaneous manifestations after treatment with VDZ.211 Therefore, VDZ should be prescribed for treatment of intestinal activity rather than for EIMs although it still may be beneficial for EIMs that parallel intestinal activity.
Medications Targeting IL-12/23
There are currently no prospective studies evaluating the efficacy of UST in the treatment of EIMs. However, some retrospective studies have suggested improvement in arthralgia symptoms as well as dermatologic manifestations. One retrospective study of 152 patients with CD of which 46 patients reported arthralgia at baseline found that 82.6% of patients had complete resolution of arthralgia symptoms at week 52.212 A second retrospective study of 70 IBD patients (64 with CD, 6 with UC) who were started on UST for concomitant active psoriasis (45 patients) or psoriatic arthritis (25 patients) found that 60% of patients with psoriatic arthritis achieved clinical remission.213 Among the 45 patients with psoriasis, 84.2% achieved clinical remission. Finally, there have been several case reports and series suggesting clinical improvement or remission in patients with erythema nodosum or pyoderma gangrenosum, though larger studies are needed to draw any significant conclusions regarding UST’s efficacy in these conditions.214–217
The other major drug in the IL-12/23 class risankizumab has not yet been evaluated for efficacy in treatment of EIMs and thus recommendations regarding its use cannot be made. However, risankizumab is FDA approved for treatment of psoriasis and some studies suggest improved efficacy compared to UST.218 Whether similar effects may be seen in other cutaneous EIMs remains unknown.
JAK Inhibitors
As upadacitinib was only recently FDA approved in May 2023, there is sparse data on its efficacy in treating EIMs for CD patients. However, analysis of upadacitinib clinical trials for UC show promising evidence supporting their efficacy in treating peripheral and axial arthropathies.219,220 Data from two phase 3 induction trials U-ACHIEVE and U-ACCOMPLISH and one maintenance trial U-ACHIEVE were analyzed and found that anemia, peripheral arthropathy, and axial arthropathy were the most common EIMs present at the start of the trials. The authors found that there was increased percentage of patients with resolution of peripheral and axial arthropathy at week 8 for patients treated with upadacitinib 45 mg daily compared to placebo (54.7% vs 42.1%, p > 0.05). At week 52, there was a statistically significant increased proportion of patients with resolution of peripheral and axial arthropathy for patients treated with upadacitinib 30 mg daily compared to placebo (66.7% vs 22.2%, p = 0.01) and a non-statistically significant increased proportion of patients treated with upadacitinib 15 mg daily compared to placebo (38.5% vs 22.2%, p > 0.05). Incidence of other EIMs was low (<2%) and so no significant conclusions can be drawn about the efficacy of upadacitinib in their management. Lastly, there have been case reports of patients with rheumatologic disorders including rheumatoid arthritis and spondyloarthritis with associated pyoderma gangrenosum that showed improvement in the skin lesions after treatment with upadacitinib.221,222 However, more data is needed before any significant conclusions can be drawn about its efficacy in treating dermatologic EIMs.
Non-Medical Approaches to Personalized Medicine in IBD
Beyond medical therapy, there has been increased emphasis on non-pharmacologic approaches towards medical treatment in IBD that may improve overall patient care and quality of life. Furthermore, technology has increasingly been used in medical care to better facilitate medical access for patients. An example of this is the TELE-IBD trial which compared patients with IBD who were randomized to a telemedicine intervention group or standard care.223 The telemedicine intervention patients received a series of texts either weekly or every other week which assessed IBD symptoms using modified disease activity scores. The authors found that quality of life scores were increased in all groups but the telemedicine intervention patients had less IBD-related hospitalizations and were more likely to have non-invasive diagnostic tests and electronic encounters compared to controls. Further research is needed but this study highlights that telemedicine may be an alternative approach to IBD care that could tailor to individual patient needs and decrease costs and utilization in the process.
Another non-pharmacologic approach to IBD care is the evolving concept of the IBD medical home.224 The concept of a patient centered medical home was originally designed in primary care as a way to organize and coordinate all aspects of an individual patient’s medical care in a comprehensive care network.225 Several studies have suggested improved patient and provider satisfaction as well as decreased unplanned care, but mixed data on costs savings.226,227 Given the complex nature of IBD and its widespread effects beyond the GI tract including medical, behavioral, and socioeconomic impacts, there is a unique opportunity for value-based care in a patient centered model. Two examples of IBD medical homes are the University of Pittsburgh model and the Illinois Gastroenterology Group’s Project Sonar. The University of Pittsburgh group analyzed data from patients enrolled in their specialty medical home and found that compared to the year prior to enrollment in the program, patients had a significant reduction in emergency department hospitalizations, as well as significant reductions in clinical disease activity scores and improved quality of life scores.228 Project SONAR also reports significant reductions in emergency department visits, hospitalization, and improvement in patient satisfaction.229
The CAPTURE IBD trial explored a similar model to the medical home by incorporating proactive symptoms monitoring with a care coordinator triggered algorithms to create a patient-tailored multicomponent care coordination intervention.230 This trial randomized IBD patients at a single center to a care coordination intervention or usual care. Patients in the care coordination intervention underwent monthly symptom assessment via an assigned care coordinator and then based on the results would trigger tailored algorithms according to the specific symptom domain of concern. Examples of the patient tailored algorithms included expedited appointments, medication adherence counselling, social or behavioral health referrals, or lab evaluation. The authors found that patients in the care coordination intervention had significant improvement in symptom scores compared to patients who underwent usual care, without added IBD-related costs. This concept is promising as it appears to be low cost and does not require major organizational restructuring.
Lastly, another exciting concept for improving IBD care is the Oshi Health application.231 The Oshi Health application is designed for patients with various GI conditions including IBD and allows users to connect with a professional team of providers including a gastroenterologist, nurse practitioner, registered dietician, psychologist, and health coach, all completely virtually through the application. The application also tracks symptoms and wellbeing through weekly surveys, incorporates data from common fitness devices to help patients stick with designed goals, and provides medical and nutritional education pertaining to IBD. The company presented clinical trial data at the IHI Forum in January 2023 of 332 patients with IBD, irritable bowel syndrome, or functional GI disorders and found high rates of patient satisfaction (98%), improved quality of life (89%), symptom improvement (92%), and decreased healthcare utilization including a 64% decrease in avoidable GI-related emergency department visits.232 The study also reported decreases in GI-related healthcare cost ($6724 per patient) and all-cause healthcare costs ($10,292 per patient). The company notes that an additional analysis of an IBD cohort will be published once data collection is complete. A limitation to this concept is that Oshi Health is only available to in-network patients with Aetna insurance though access is expected to become more widely available once more clinical trial data is published.
Proposed Positioning and Treatment Algorithm in Advanced Therapy of Moderate to Severe Crohn’s Disease
As previously stated, it is unlikely that clinical trials will produce guidelines for treating every clinical scenario in CD. In general, clinicians should focus on a “treat to target” strategy with the goals of symptomatic, biologic, and endoscopic remission. A particular emphasis should be placed on endoscopic remission as this appears to improve long-term clinical outcomes. This was evidenced by the CALM study which showed that patients in deep remission (CD endoscopic index of severity scores below 4, with no deep ulcerations or steroid treatment for 8 or more weeks) had significantly less major adverse events including new internal fistulas or abscesses, strictures, perianal fistulas or abscesses, hospitalization, or surgery.233 This section will aim to provide a general treatment algorithm for clinicians with the caveat that every scenario will not be addressed and patient-specific factors such as cost, individual risk factors, and personal preferences need to be incorporated into the treatment decision (Figures 1 and 2).
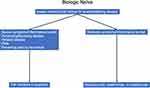 |
Figure 1 Proposed biologic therapy positioning for biologic naïve patients. Abbreviation: EIMs, extraintestinal manifestations. |
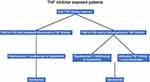 |
Figure 2 Proposed positioning for TNF inhibitor exposed patients. Abbreviations: PNR, primary nonresponse; LOR, loss of response. |
Biologic Naïve Patients
TNF inhibitors, specifically IFX and ADA, have the most robust data supporting their efficacy in CD and are likely appropriate for most patients with moderate to severe disease assuming the safety profile is acceptable for the patient. Additionally, with the advent of biosimilars to both IFX and ADA, these agents may be the most cost-effective treatment in most bio-naïve patients.234 TNF inhibitors, particularly IFX, should be considered first line when treating patients who have more severe symptoms/inflammatory burden and in certain subtypes of CD including those with stricturing or penetrating phenotypes, perianal disease, EIMs, and to prevent post-operative recurrence. Combination therapy with an immunosuppressant should be considered when using IFX in patients whom the safety profile is acceptable; alternatively, optimized monotherapy utilizing TDM can be considered.
Risankizumab, VDZ, and UST are all appropriate initial therapies in biologic naïve patients, particularly in those with more moderate symptoms/inflammatory burden and without EIMs. However, as mentioned previously risankizumab and UST are the treatment of choice in patients with concomitant psoriasis. Assuming equal access to therapy, it is our practice to offer novel biologic therapy to bionaive patients with moderate symptoms/inflammatory burden due to the enhanced safety profile of these drugs, reserving anti-TNF therapy for those with more severe disease and/or in special situations as outlined above.
Upadacitinib was shown to be effective in biologic naïve patients in clinical trials.166 However, it is currently only FDA approved for treatment of adults with moderate to severe CD who have had an inadequate response or intolerance to one or more TNF inhibitors.235 Therefore, upadacitinib is not recommended as first line therapy.
Biologic Exposed Patients
In general, patients who have primary nonresponse or loss of response to a TNF inhibitor are less likely to respond to other biologic therapies than patients who are bionaïve. The reasons for this are multifactorial but include more severe disease, patient pharmacokinetics favoring immunogenicity to treatment, and/or underlying fibrosis that results in complicated disease. The exception to this rule may be risankizumab which appears to be efficacious regardless of prior drug exposure.173
For patients who were previously treated with TNF inhibitors and had subsequent loss of response, switching to a second TNF inhibitor or risankizumab has the strongest supporting evidence.132,163 For patients who do not achieve therapeutic response with a TNF inhibitor despite therapeutic drug levels, selecting an agent from a different class such as risankizumab would be appropriate. Following these medications, UST is likely the next best therapy followed by VDZ. Upadacitinib is another option as a second or third line therapy and clinical trials showed excellent efficacy in biologic exposed patients.166 Furthermore, upadacitinib is an oral tablet which is attractive for many patients. However, comparative data for upadacitinib versus other biologic therapies in CD is lacking and so comments regarding its positioning versus the IL12/23 class cannot be made at this time. Clinicians should have shared decision making with patients regarding the benefits including efficacy in biologic exposed patients and ease of administration, as well as the disadvantages including lack of comparative data in CD and potential safety concerns.
Future Directions
One of the main areas of research in CD management is the development of accurate biomarkers that can reliably predict prognosis and response to therapy. Personalized therapy is well established in other medical fields such as oncology as individual biomarkers are used to help guide treatment and prognosis for many different cancers. An example of this is HER-2 positive breast cancer which is treated with an anti-HER2 receptor monoclonal antibody.236 In contrast, the treatment approach in CD is often reactive in nature and changes in therapy are based on worsening clinical parameters. The fundamental challenge with developing precise and personalized treatment in CD is the complex and multifactorial pathogenesis leading to heterogenous disease phenotypes and clinical course. Therefore, the future of personalized medicine in CD will likely need to incorporate multiple biomarkers derived from different pathophysiologic processes.
Genetics is an area of increasing research interest in IBD to better understand the pathogenesis of IBD and help predict disease course. Multiple genes involved in various pathways of the inflammatory process of IBD have been discovered such as microbial sensing (NOD2, CARD9, and RIPK2), intestinal barrier function (C1orf106 and HNF4A), innate and adaptive immune signaling (NLRP7, IL18RAP, CD28, IFNG, PTPN22, STAT4, IL6ST, IL23R, RORC, and IL17RA), and fibrosis (OSMR and SMAD3).237 Despite these discoveries, few specific gene variants have been found to reliably predict disease course or are readily available to be used in clinical practice. There are some promising targets, however. For example, an observational study of 1240 biologic naïve patients with CD found a significant association with the HLA-DQA1*05 allele and development of immunogenicity to TNF inhibitors. This is a promising potential biomarker as approximately 40% of Europeans carry this allele and may help predict which patients should be treated with combination therapy.238
Another area of inquiry that shows promise is molecular profiling, particularly transcriptomics. For example, two studies showed that increased expression of OSM, OSMR, and TREM1 was associated with failure of TNF inhibitor therapy and thus could be a potential biomarker and therapeutic target in CD.239,240 Similarly, another study identified four genes (PIWIL1, MAATS1, RGS13, and DCHS2) whose expression levels in colon tissue predicted response and endoscopic remission with VDZ treatment.241 While these examples of using molecular profiling in CD are encouraging, further investigation is needed before they can be employed clinically.
Lastly, an area of tremendous research interest is the role of the gut microbiome in CD and its impact on response to therapy. In a study of 85 patients with IBD who were to be treated with VDZ, greater α-diversity and abundance of Roseburia inulinivorans and Burkholderiales species was associated with remission at week 14 in patients with CD.242 Similarly, a study of 232 CD patients treated with UST induction found that higher numbers of Faecalibacterium and Bacteroides species was associated with remission at 6 weeks.243 Though these studies are small, they suggest that the presence of specific bacterial species and diversity of bacteria may predict response to CD therapy. However, more research is needed into understanding the mechanism for why these altered responses occur.
Conclusion
Despite increased number of drugs available for treatment of CD, achieving clinical, biologic, and endoscopic remission remains a challenge. Therefore, the push towards personalized treatment is paramount in IBD although the lack of accurate biomarkers limits the precision of providers. Our approach is to consider a number of factors when initiating treatment including severity of symptoms and inflammatory burden, risk factors for a more severe disease course, comorbid conditions including EIMs, prior response to treatment, patient adherence, patient preferences, and specific scenarios including perianal disease and post operative treatment. We use existing head-to-head trials and network meta-analyses to guide decision making and to help limit the number of options patients must consider. When possible, we try to provide the preferred therapy for patients. We are eager to have precision diagnostics in the future that guide clinicians in selecting the “right therapy, for the right patient, and the right time”.
Disclosure
JWC reports no conflicts of interest in this work. RKC has received income from consulting and participation in advisory boards for AbbVie, Adiso, BMS, Fresenius Kabi, Fzata, Janssen, Magellan Health, Pfizer, Samsung bioepis, Sandoz, Sebela, and Takeda. RKC is a member of the Executive Committee for the IBD Education Group and is Scientific Co-Director of the CorEvitas Registry.
References
1. Actis GC, Pellicano R, Fagoonee S, Ribaldone DG. History of Inflammatory Bowel Diseases. J Clin Med. 2019;8(11):1970. doi:10.3390/jcm8111970
2. Ng SC, Shi HY, Hamidi N, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 2017;390(10114):2769–2778. doi:10.1016/S0140-6736(17)32448-0
3. Farmer RG, Whelan G, Fazio VW. Long-term follow-up of patients with Crohn’s disease. Relationship between the clinical pattern and prognosis. Gastroenterology. 1985;88(6):1818–1825. doi:10.1016/0016-5085(85)90006-X
4. Solberg IC, Vatn MH, Hoie O, et al. Clinical course in Crohn’s disease: results of a Norwegian population-based ten-year follow-up study. Clin Gastroenterol Hepatol. 2007;5(12):1430–1438. doi:10.1016/j.cgh.2007.09.002
5. Thia KT, Sandborn WJ, Harmsen WS, Zinsmeister AR, Loftus EV
6. Frolkis AD, Dykeman J, Negron ME, et al. Risk of surgery for inflammatory bowel diseases has decreased over time: a systematic review and meta-analysis of population-based studies. Gastroenterology. 2013;145(5):996–1006. doi:10.1053/j.gastro.2013.07.041
7. Bewtra M, Kaiser LM, TenHave T, Lewis JD. Crohn’s disease and ulcerative colitis are associated with elevated standardized mortality ratios: a meta-analysis. Inflamm Bowel Dis. 2013;19(3):599–613. doi:10.1097/MIB.0b013e31827f27ae
8. Ekbom A, Helmick C, Zack M, Adami HO. Increased risk of large-bowel cancer in Crohn’s disease with colonic involvement. Lancet. 1990;336(8711):357–359. doi:10.1016/0140-6736(90)91889-I
9. Friedman S, Rubin PH, Bodian C, Goldstein E, Harpaz N, Present DH. Screening and surveillance colonoscopy in chronic Crohn’s colitis. Gastroenterology. 2001;120(4):820–826. doi:10.1053/gast.2001.22449
10. Maykel JA, Hagerman G, Mellgren AF, et al. Crohn’s colitis: the incidence of dysplasia and adenocarcinoma in surgical patients. Dis Colon Rectum. 2006;49(7):950–957. doi:10.1007/s10350-006-0555-9
11. Le Berre C, Ananthakrishnan AN, Danese S, Singh S, Peyrin-Biroulet L. Ulcerative colitis and crohn’s disease have similar burden and goals for treatment. Clin Gastroenterol Hepatol. 2020;18(1):14–23. doi:10.1016/j.cgh.2019.07.005
12. van der Have M, van der Aalst KS, Kaptein AA, et al. Determinants of health-related quality of life in Crohn’s disease: a systematic review and meta-analysis. J Crohns Colitis. 2014;8(2):93–106. doi:10.1016/j.crohns.2013.04.007
13. Floyd DN, Langham S, Severac HC, Levesque BG. The economic and quality-of-life burden of Crohn’s disease in Europe and the United States, 2000 to 2013: a systematic review. Dig Dis Sci. 2015;60(2):299–312. doi:10.1007/s10620-014-3368-z
14. Gao N, Qiao Z, Yan S, Zhu L. Evaluation of health-related quality of life and influencing factors in patients with Crohn disease. J Int Med Res. 2022;50(5):3000605221098868. doi:10.1177/03000605221098868
15. Lundquist LR, Rasmussen B, Waldorff FB, Wehberg S, Kjeldsen J, Haastrup P. Predictors of health-related quality of life in patients with Crohn’s disease receiving biological therapy. Scand J Gastroenterol. 2021;56(12):1434–1441. doi:10.1080/00365521.2021.1974086
16. Peyrin-Biroulet L, Panes J, Sandborn WJ, et al. Defining disease severity in inflammatory bowel diseases: current and future directions. Clin Gastroenterol Hepatol. 2016;14(3):348–354 e17. doi:10.1016/j.cgh.2015.06.001
17. Koliani-Pace JL, Siegel CA. Beyond disease activity to overall disease severity in inflammatory bowel disease. Lancet Gastroenterol Hepatol. 2017;2(9):624–626. doi:10.1016/S2468-1253(17)30212-1
18. Harvey RF, Bradshaw JM. A simple index of Crohn’s-disease activity. Lancet. 1980;1(8167):514. doi:10.1016/s0140-6736(80)92767-1
19. Vermeire S, Schreiber S, Sandborn WJ, Dubois C, Rutgeerts P. Correlation between the Crohn’s disease activity and Harvey-Bradshaw indices in assessing Crohn’s disease severity. Clin Gastroenterol Hepatol. 2010;8(4):357–363. doi:10.1016/j.cgh.2010.01.001
20. Vermeire S, Van Assche G, Rutgeerts P. Laboratory markers in IBD: useful, magic, or unnecessary toys?. Gut. 2006;55(3):426–431. doi:10.1136/gut.2005.069476
21. Iskandar HN, Ciorba MA. Biomarkers in inflammatory bowel disease: current practices and recent advances. Transl Res. 2012;159(4):313–325. doi:10.1016/j.trsl.2012.01.001
22. Solem CA, Loftus EV
23. Jones J, Loftus EV, Panaccione R, et al. Relationships between disease activity and serum and fecal biomarkers in patients with Crohn’s disease. Clin Gastroenterol Hepatol. 2008;6(11):1218–1224. doi:10.1016/j.cgh.2008.06.010
24. Oh K, Oh EH, Baek S, et al. Elevated C-reactive protein level during clinical remission can predict poor outcomes in patients with Crohn’s disease. PLoS One. 2017;12(6):e0179266. doi:10.1371/journal.pone.0179266
25. Denis MA, Reenaers C, Fontaine F, Belaiche J, Louis E. Assessment of endoscopic activity index and biological inflammatory markers in clinically active Crohn’s disease with normal C-reactive protein serum level. Inflamm Bowel Dis. 2007;13(9):1100–1105. doi:10.1002/ibd.20178
26. Pathirana WGW, Chubb SP, Gillett MJ, Vasikaran SD. Faecal calprotectin. Clin Biochem Rev. 2018;39(3):77–90.
27. Lee YW, Lee KM, Lee JM, et al. The usefulness of fecal calprotectin in assessing inflammatory bowel disease activity. Korean J Intern Med. 2019;34(1):72–80. doi:10.3904/kjim.2016.324
28. D’Haens G, Ferrante M, Vermeire S, et al. Fecal calprotectin is a surrogate marker for endoscopic lesions in inflammatory bowel disease. Inflamm Bowel Dis. 2012;18(12):2218–2224. doi:10.1002/ibd.22917
29. Vieira A, Fang CB, Rolim EG, et al. Inflammatory bowel disease activity assessed by fecal calprotectin and lactoferrin: correlation with laboratory parameters, clinical, endoscopic and histological indexes. BMC Res Notes. 2009;2:221. doi:10.1186/1756-0500-2-221
30. Sipponen T, Karkkainen P, Savilahti E, et al. Correlation of faecal calprotectin and lactoferrin with an endoscopic score for Crohn’s disease and histological findings. Aliment Pharmacol Ther. 2008;28(10):1221–1229. doi:10.1111/j.1365-2036.2008.03835.x
31. Lobaton T, Lopez-Garcia A, Rodriguez-Moranta F, Ruiz A, Rodriguez L, Guardiola J. A new rapid test for fecal calprotectin predicts endoscopic remission and postoperative recurrence in Crohn’s disease. J Crohns Colitis. 2013;7(12):e641–51. doi:10.1016/j.crohns.2013.05.005
32. Schoepfer AM, Beglinger C, Straumann A, et al. Fecal calprotectin correlates more closely with the Simple Endoscopic Score for Crohn’s disease (SES-CD) than CRP, blood leukocytes, and the CDAI. Am J Gastroenterol. 2010;105(1):162–169. doi:10.1038/ajg.2009.545
33. Gecse KB, Brandse JF, van Wilpe S, et al. Impact of disease location on fecal calprotectin levels in Crohn’s disease. Scand J Gastroenterol. 2015;50(7):841–847. doi:10.3109/00365521.2015.1008035
34. Buisson A, Mak WY, Andersen MJ, et al. Fecal calprotectin is highly effective to detect endoscopic ulcerations in crohn’s disease regardless of disease location. Inflamm Bowel Dis. 2021;27(7):1008–1016. doi:10.1093/ibd/izaa269
35. Mendoza JL, Abreu MT. Biological markers in inflammatory bowel disease: practical consideration for clinicians. Gastroenterol Clin Biol. 2009;33 Suppl 3:S158–73. doi:10.1016/S0399-8320(09)73151-3
36. Alper A, Zhang L, Pashankar DS. Correlation of erythrocyte sedimentation rate and C-reactive protein with pediatric inflammatory bowel disease activity. J Pediatr Gastroenterol Nutr. 2017;65(2):e25–e27. doi:10.1097/MPG.0000000000001444
37. Nakarai A, Kato J, Hiraoka S, et al. An elevated platelet count increases the risk of relapse in ulcerative colitis patients with mucosal healing. Gut Liver. 2018;12(4):420–425. doi:10.5009/gnl17236
38. Takeyama H, Mizushima T, Iijima H, et al. Platelet activation markers are associated with crohn’s disease activity in patients with low C-reactive protein. Dig Dis Sci. 2015;60(11):3418–3423. doi:10.1007/s10620-015-3745-2
39. Mary JY, Modigliani R. Development and validation of an endoscopic index of the severity for Crohn’s disease: a prospective multicentre study. Groupe d’Etudes Therapeutiques des Affections Inflammatoires du Tube Digestif (GETAID). Gut. 1989;30(7):983–989. doi:10.1136/gut.30.7.983
40. Daperno M, D’Haens G, Van Assche G, et al. Development and validation of a new, simplified endoscopic activity score for Crohn’s disease: the SES-CD. Gastrointest Endosc. 2004;60(4):505–512. doi:10.1016/s0016-5107(04)01878-4
41. Ferrante M, Colombel JF, Sandborn WJ, et al. Validation of endoscopic activity scores in patients with Crohn’s disease based on a post hoc analysis of data from SONIC. Gastroenterology. 2013;145(5):978–986 e5. doi:10.1053/j.gastro.2013.08.010
42. Koutroumpakis E, Katsanos KH. Implementation of the simple endoscopic activity score in crohn’s disease. Saudi J Gastroenterol. 2016;22(3):183–191. doi:10.4103/1319-3767.182455
43. Sachar DB, Andrews HA, Farmer RG, et al. Proposed classification of patient subgroups in Crohn’s disease. Gastroenterol Int. 1992;5(3):141–154.
44. Gasche C, Scholmerich J, Brynskov J, et al. A simple classification of Crohn’s disease: report of the working party for the world congresses of gastroenterology, Vienna 1998. Inflamm Bowel Dis. 2000;6(1):8–15. doi:10.1097/00054725-200002000-00002
45. Silverberg MS, Satsangi J, Ahmad T, et al. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a working party of the 2005 Montreal world congress of gastroenterology. Can J Gastroenterol. 2005;19 Suppl A:5A–36A. doi:10.1155/2005/269076
46. Greenstein AJ, Lachman P, Sachar DB, et al. Perforating and non-perforating indications for repeated operations in Crohn’s disease: evidence for two clinical forms. Gut. 1988;29(5):588–592. doi:10.1136/gut.29.5.588
47. Hamon JF, Carbonnel F, Beaugerie L, et al. Comparaison de l’evolution a long terme des maladies de Crohn perforantes et non perforantes [Comparison of long-term course of perforating and non-perforating Crohn disease]. Gastroenterol Clin Biol. 1998;22(6–7):601–606.
48. Aeberhard P, Berchtold W, Riedtmann HJ, Stadelmann G. Surgical recurrence of perforating and nonperforating Crohn’s disease. A study of 101 surgically treated Patients. Dis Colon Rectum. 1996;39(1):80–87. doi:10.1007/BF02048274
49. Sugita A, Fukushima T, Yamazaki Y, et al. クローン病の術後再発形態:穿孔型と非穿孔型の比較 [Postoperative recurrence form of Crohn’s disease: comparison between perforating and non perforating types]. Nihon Geka Gakkai Zasshi. 1993;94(2):114–118. Japan.
50. Fernandez-Clotet A, Panes J, Ricart E, et al. Predictors of bowel damage in the long-term progression of Crohn’s disease. World J Clin Cases. 2022;10(33):12208–12220. doi:10.12998/wjcc.v10.i33.12208
51. Veloso FT, Ferreira JT, Barros L, Almeida S. Clinical outcome of Crohn’s disease: analysis according to the Vienna classification and clinical activity. Inflamm Bowel Dis. 2001;7(4):306–313. doi:10.1097/00054725-200111000-00005
52. Romberg-Camps MJ, Dagnelie PC, Kester AD, et al. Influence of phenotype at diagnosis and of other potential prognostic factors on the course of inflammatory bowel disease. Am J Gastroenterol. 2009;104(2):371–383. doi:10.1038/ajg.2008.38
53. Oostenbrug LE, van Dullemen HM, te Meerman GJ, Jansen PL, Kleibeuker JH. Clinical outcome of Crohn’s disease according to the Vienna classification: disease location is a useful predictor of disease course. Eur J Gastroenterol Hepatol. 2006;18(3):255–261. doi:10.1097/00042737-200603000-00005
54. Nemetz A, Molnar T, Zagoni T, et al. Phenotypes defined by the ”Vienna Classification” in 100 Hungarian patients with Crohn’s disease. Rev Esp Enferm Dig. 2003;95(8):533–8, 527–33.
55. Hofer B, Bottger T, Hernandez-Richter T, Seifert JK, Junginger T. The impact of clinical types of disease manifestation on the risk of early postoperative recurrence in Crohn’s disease. Hepatogastroenterology. 2001;48(37):152–155.
56. Papi C, Festa V, Leandro G, et al. Long-term outcome of Crohn’s disease following corticosteroid-induced remission. Am J Gastroenterol. 2007;102(4):814–819. doi:10.1111/j.1572-0241.2007.01055.x
57. Fan Y, Zhang L, Omidakhsh N, et al. Patients with stricturing or penetrating Crohn’s disease phenotypes report high disease burden and treatment needs. Inflamm Bowel Dis. 2022. doi:10.1093/ibd/izac162
58. Yang CH, Ding J, Gao Y, Chen X, Yang ZB, Xiao SD. Risk factors that predict the requirement of aggressive therapy among Chinese patients with Crohn’s disease. J Dig Dis. 2011;12(2):99–104. doi:10.1111/j.1751-2980.2011.00484.x
59. Siproudhis L, Mortaji A, Mary JY, Juguet F, Bretagne JF, Gosselin M. Anal lesions: any significant prognosis in Crohn’s disease?. Eur J Gastroenterol Hepatol. 1997;9(3):239–243. doi:10.1097/00042737-199703000-00004
60. Hellers G, Bergstrand O, Ewerth S, Holmstrom B. Occurrence and outcome after primary treatment of anal fistulae in Crohn’s disease. Gut. 1980;21(6):525–527. doi:10.1136/gut.21.6.525
61. Tarrant KM, Barclay ML, Frampton CM, Gearry RB. Perianal disease predicts changes in Crohn’s disease phenotype-results of a population-based study of inflammatory bowel disease phenotype. Am J Gastroenterol. 2008;103(12):3082–3093. doi:10.1111/j.1572-0241.2008.02212.x
62. Cheifetz AS. Management of active Crohn disease. JAMA. 2013;309(20):2150–2158. doi:10.1001/jama.2013.4466
63. Borley NR, Mortensen NJ, Chaudry MA, et al. Recurrence after abdominal surgery for Crohn’s disease: relationship to disease site and surgical procedure. Dis Colon Rectum. 2002;45(3):377–383. doi:10.1007/s10350-004-6186-0
64. Smith BR, Arnott ID, Drummond HE, Nimmo ER, Satsangi J. Disease location, anti-Saccharomyces cerevisiae antibody, and NOD2/CARD15 genotype influence the progression of disease behavior in Crohn’s disease. Inflamm Bowel Dis. 2004;10(5):521–528. doi:10.1097/00054725-200409000-00005
65. Louis E, Michel V, Hugot JP, et al. Early development of stricturing or penetrating pattern in Crohn’s disease is influenced by disease location, number of flares, and smoking but not by NOD2/CARD15 genotype. Gut. 2003;52(4):552–557. doi:10.1136/gut.52.4.552
66. Aldhous MC, Drummond HE, Anderson N, Smith LA, Arnott ID, Satsangi J. Does cigarette smoking influence the phenotype of Crohn’s disease? Analysis using the Montreal classification. Am J Gastroenterol. 2007;102(3):577–588. doi:10.1111/j.1572-0241.2007.01064.x
67. Chow DK, Sung JJ, Wu JC, Tsoi KK, Leong RW, Chan FK. Upper gastrointestinal tract phenotype of Crohn’s disease is associated with early surgery and further hospitalization. Inflamm Bowel Dis. 2009;15(4):551–557. doi:10.1002/ibd.20804
68. Magro F, Rodrigues-Pinto E, Coelho R, et al. Is it possible to change phenotype progression in Crohn’s disease in the era of immunomodulators? Predictive factors of phenotype progression. Am J Gastroenterol. 2014;109(7):1026–1036. doi:10.1038/ajg.2014.97
69. Crocco S, Martelossi S, Giurici N, Villanacci V, Ventura A. Upper gastrointestinal involvement in paediatric onset Crohn’s disease: prevalence and clinical implications. J Crohns Colitis. 2012;6(1):51–55. doi:10.1016/j.crohns.2011.06.013
70. Kim ES, Kwon Y, Choe YH, Kim MJ. Upper gastrointestinal tract involvement is more prevalent in Korean patients with pediatric Crohn’s disease than in European patients. Sci Rep. 2020;10(1):19032. doi:10.1038/s41598-020-75938-1
71. Greuter T, Piller A, Fournier N, et al. Upper gastrointestinal tract involvement in Crohn’s Disease: frequency, risk factors, and disease course. J Crohns Colitis. 2018;12(12):1399–1409. doi:10.1093/ecco-jcc/jjy121
72. Polito JM
73. Beaugerie L, Seksik P, Nion–Larmurier I, Gendre JP, Cosnes J. Predictors of Crohn’s disease. Gastroenterology. 2006;130(3):650–656. doi:10.1053/j.gastro.2005.12.019
74. Pigneur B, Seksik P, Viola S, et al. Natural history of Crohn’s disease: comparison between childhood- and adult-onset disease. Inflamm Bowel Dis. 2010;16(6):953–961. doi:10.1002/ibd.21152
75. Ministro P, Dias CC, Portela F, et al. Age at diagnosis is determinant for the outcome of inflammatory bowel disease: is it a myth?. Clin Transl Gastroenterol. 2021;12(2):e00309. doi:10.14309/ctg.0000000000000309
76. Vavricka SR, Brun L, Ballabeni P, et al. Frequency and risk factors for extraintestinal manifestations in the Swiss inflammatory bowel disease cohort. Am J Gastroenterol. 2011;106(1):110–119. doi:10.1038/ajg.2010.343
77. Bernstein CN, Blanchard JF, Rawsthorne P, Yu N. The prevalence of extraintestinal diseases in inflammatory bowel disease: a population-based study. Am J Gastroenterol. 2001;96(4):1116–1122. doi:10.1111/j.1572-0241.2001.03756.x
78. Yuksel I, Ataseven H, Basar O, et al. Peripheral arthritis in the course of inflammatory bowel diseases. Dig Dis Sci. 2011;56(1):183–187. doi:10.1007/s10620-010-1260-z
79. Simsek H, Schuman BM. Inflammatory bowel disease in 64 black patients: analysis of course, complications, and surgery. J Clin Gastroenterol. 1989;11(3):294–298. doi:10.1097/00004836-198906000-00010
80. Goldman CD, Kodner IJ, Fry RD, MacDermott RP. Clinical and operative experience with non-Caucasian patients with Crohn’s disease. Dis Colon Rectum. 1986;29(5):317–321. doi:10.1007/BF02554120
81. Cross RK, Jung C, Wasan S, Joshi G, Sawyer R, Roghmann MC. Racial differences in disease phenotypes in patients with Crohn’s disease. Inflamm Bowel Dis. 2006;12(3):192–198. doi:10.1097/01.MIB.0000217767.98389.20
82. Flasar MH, Quezada S, Bijpuria P, Cross RK. Racial differences in disease extent and severity in patients with ulcerative colitis: a retrospective cohort study. Dig Dis Sci. 2008;53(10):2754–2760. doi:10.1007/s10620-007-0190-x
83. Bernstein CN, Walld R, Marrie RA. Social determinants of outcomes in inflammatory bowel disease. Am J Gastroenterol. 2020;115(12):2036–2046. doi:10.14309/ajg.0000000000000794
84. Nahon S, Lahmek P, Macaigne G, et al. Socioeconomic deprivation does not influence the severity of Crohn’s disease: results of a prospective multicenter study. Inflamm Bowel Dis. 2009;15(4):594–598. doi:10.1002/ibd.20794
85. Cosnes J, Bourrier A, Nion-Larmurier I, Sokol H, Beaugerie L, Seksik P. Factors affecting outcomes in Crohn’s disease over 15 years. Gut. 2012;61(8):1140–1145. doi:10.1136/gutjnl-2011-301971
86. Zallot C, Peyrin-Biroulet L. Clinical risk factors for complicated disease: how reliable are they?. Dig Dis. 2012;30 Suppl 3:67–72. doi:10.1159/000342608
87. Lakatos PL, Szamosi T, Lakatos L. Smoking in inflammatory bowel diseases: good, bad or ugly?. World J Gastroenterol. 2007;13(46):6134–6139. doi:10.3748/wjg.v13.i46.6134
88. Mahid SS, Minor KS, Soto RE, Hornung CA, Galandiuk S. Smoking and inflammatory bowel disease: a meta-analysis. Mayo Clin Proc. 2006;81(11):1462–1471. doi:10.4065/81.11.1462
89. Lakatos PL, Czegledi Z, Szamosi T, et al. Perianal disease, small bowel disease, smoking, prior steroid or early azathioprine/biological therapy are predictors of disease behavior change in patients with Crohn’s disease. World J Gastroenterol. 2009;15(28):3504–3510. doi:10.3748/wjg.15.3504
90. D’Haens GR, Sartor RB, Silverberg MS, Petersson J, Rutgeerts P. Future directions in inflammatory bowel disease management. J Crohns Colitis. 2014;8(8):726–734. doi:10.1016/j.crohns.2014.02.025
91. Palmela C, Torres J, Cravo M. New Trends in inflammatory bowel disease. GE Port J Gastroenterol. 2015;22(3):103–111. doi:10.1016/j.jpge.2015.03.009
92. Dignass A, Van Assche G, Lindsay JO, et al. The second European evidence-based consensus on the diagnosis and management of Crohn’s disease: current management. J Crohns Colitis. 2010;4(1):28–62. doi:10.1016/j.crohns.2009.12.002
93. Bouguen G, Levesque BG, Pola S, Evans E, Sandborn WJ. Endoscopic assessment and treating to target increase the likelihood of mucosal healing in patients with Crohn’s disease. Clin Gastroenterol Hepatol. 2014;12(6):978–985. doi:10.1016/j.cgh.2013.11.005
94. Colombel JF, Narula N, Peyrin-Biroulet L. Management strategies to improve outcomes of patients with inflammatory bowel diseases. Gastroenterology. 2017;152(2):351–361 e5. doi:10.1053/j.gastro.2016.09.046
95. Peyrin-Biroulet L, Sandborn W, Sands BE, et al. Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE): determining therapeutic goals for treat-to-target. Am J Gastroenterol. 2015;110(9):1324–1338. doi:10.1038/ajg.2015.233
96. Turner D, Ricciuto A, Lewis A, et al. STRIDE-II: an update on the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) Initiative of the International Organization for the Study of IBD (IOIBD): determining therapeutic goals for treat-to-target strategies in IBD. Gastroenterology. 2021;160(5):1570–1583. doi:10.1053/j.gastro.2020.12.031
97. Waljee AK, Wiitala WL, Govani S, et al. Corticosteroid use and complications in a US inflammatory bowel disease cohort. PLoS One. 2016;11(6):e0158017. doi:10.1371/journal.pone.0158017
98. Steinhart AH, Ewe K, Griffiths AM, Modigliani R, Thomsen OO. Corticosteroids for maintenance of remission in Crohn’s disease. Cochrane Database Syst Rev. 2003;4:CD000301. doi:10.1002/14651858.CD000301
99. Waljee AK, Rogers MA, Lin P, et al. Short term use of oral corticosteroids and related harms among adults in the United States: population based cohort study. BMJ. 2017;357:j1415. doi:10.1136/bmj.j1415
100. Kundhal P, Zachos M, Holmes JL, Griffiths AM. Controlled ileal release budesonide in pediatric Crohn disease: efficacy and effect on growth. J Pediatr Gastroenterol Nutr. 2001;33(1):75–80. doi:10.1097/00005176-200107000-00013
101. Ford AC, Bernstein CN, Khan KJ, et al. Glucocorticosteroid therapy in inflammatory bowel disease: systematic review and meta-analysis. Am J Gastroenterol. 2011;106(4):590–9; quiz 600. doi:10.1038/ajg.2011.70
102. Rutgeerts P, Lofberg R, Malchow H, et al. A comparison of budesonide with prednisolone for active Crohn’s disease. N Engl J Med. 1994;331(13):842–845. doi:10.1056/NEJM199409293311304
103. Papi C, Luchetti R, Gili L, Montanti S, Koch M, Capurso L. Budesonide in the treatment of Crohn’s disease: a meta-analysis. Aliment Pharmacol Ther. 2000;14(11):1419–1428. doi:10.1046/j.1365-2036.2000.00867.x
104. Dignass A, Lindsay JO, Sturm A, et al. Second European evidence-based consensus on the diagnosis and management of ulcerative colitis part 2: current management. J Crohns Colitis. 2012;6(10):991–1030. doi:10.1016/j.crohns.2012.09.002
105. Wang Y, Parker CE, Bhanji T, Feagan BG, MacDonald JK. Oral 5-aminosalicylic acid for induction of remission in ulcerative colitis. Cochrane Database Syst Rev. 2016;4(4):CD000543. doi:10.1002/14651858.CD000543.pub4
106. Steinhart AH, Hemphill D, Greenberg GR. Sulfasalazine and mesalazine for the maintenance therapy of Crohn’s disease: a meta-analysis. Am J Gastroenterol. 1994;89(12):2116–2124.
107. Messori A, Brignola C, Trallori G, et al. Effectiveness of 5-aminosalicylic acid for maintaining remission in patients with Crohn’s disease: a meta-analysis. Am J Gastroenterol. 1994;89(5):692–698.
108. Camma C, Giunta M, Rosselli M, Cottone M. Mesalamine in the maintenance treatment of Crohn’s disease: a meta-analysis adjusted for confounding variables. Gastroenterology. 1997;113(5):1465–1473. doi:10.1053/gast.1997.v113.pm9352848
109. Lim WC, Hanauer S. Aminosalicylates for induction of remission or response in Crohn’s disease. Cochrane Database Syst Rev. 2010;12:CD008870. doi:10.1002/14651858.CD008870
110. Akobeng AK, Zhang D, Gordon M, MacDonald JK. Oral 5-aminosalicylic acid for maintenance of medically-induced remission in Crohn’s disease. Cochrane Database Syst Rev. 2016;9(9):CD003715. doi:10.1002/14651858.CD003715.pub3
111. Lichtenstein GR, Loftus EV, Isaacs KL, Regueiro MD, Gerson LB, Sands BE. ACG clinical guideline: management of Crohn’s disease in adults. Am J Gastroenterol. 2018;113(4):481–517. doi:10.1038/ajg.2018.27
112. Chande N, Patton PH, Tsoulis DJ, Thomas BS, MacDonald JK. Azathioprine or 6-mercaptopurine for maintenance of remission in Crohn’s disease. Cochrane Database Syst Rev. 2015;2015(10):CD000067. doi:10.1002/14651858.CD000067.pub3
113. Feagan BG, Fedorak RN, Irvine EJ, et al. A comparison of methotrexate with placebo for the maintenance of remission in Crohn’s disease. North American Crohn’s Study Group Investigators. N Engl J Med. 2000;342(22):1627–1632. doi:10.1056/NEJM200006013422202
114. Pearson DC, May GR, Fick G, Sutherland LR. Azathioprine for maintaining remission of Crohn’s disease. Cochrane Database Syst Rev. 2000;2:CD000067. doi:10.1002/14651858.CD000067
115. Lemann M, Zenjari T, Bouhnik Y, et al. Methotrexate in Crohn’s disease: long-term efficacy and toxicity. Am J Gastroenterol. 2000;95(7):1730–1734. doi:10.1111/j.1572-0241.2000.02190.x
116. Colombel JF, Sandborn WJ, Reinisch W, et al. Infliximab, azathioprine, or combination therapy for Crohn’s disease. N Engl J Med. 2010;362(15):1383–1395. doi:10.1056/NEJMoa0904492
117. Colombel JF, Adedokun OJ, Gasink C, et al. Combination therapy with infliximab and azathioprine improves infliximab pharmacokinetic features and efficacy: a post hoc analysis. Clin Gastroenterol Hepatol. 2019;17(8):1525–1532 e1. doi:10.1016/j.cgh.2018.09.033
118. Kopylov U, Al-Taweel T, Yaghoobi M, et al. Adalimumab monotherapy versus combination therapy with immunomodulators in patients with Crohn’s disease: a systematic review and meta-analysis. J Crohns Colitis. 2014;8(12):1632–1641. doi:10.1016/j.crohns.2014.07.003
119. Chalhoub JM, Rimmani HH, Gumaste VV, Sharara AI. Systematic review and meta-analysis: adalimumab monotherapy versus combination therapy with immunomodulators for induction and maintenance of remission and response in patients with Crohn’s disease. Inflamm Bowel Dis. 2017;23(8):1316–1327. doi:10.1097/MIB.0000000000001203
120. Vermeire S, Noman M, Van Assche G, Baert F, D’Haens G, Rutgeerts P. Effectiveness of concomitant immunosuppressive therapy in suppressing the formation of antibodies to infliximab in Crohn’s disease. Gut. 2007;56(9):1226–1231. doi:10.1136/gut.2006.099978
121. Baert F, Noman M, Vermeire S, et al. Influence of immunogenicity on the long-term efficacy of infliximab in Crohn’s disease. N Engl J Med. 2003;348(7):601–608. doi:10.1056/NEJMoa020888
122. Van Assche G, Magdelaine-Beuzelin C, D’Haens G, et al. Withdrawal of immunosuppression in Crohn’s disease treated with scheduled infliximab maintenance: a randomized trial. Gastroenterology. 2008;134(7):1861–1868. doi:10.1053/j.gastro.2008.03.004
123. Targan SR, Hanauer SB, van Deventer SJ, et al. A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor alpha for Crohn’s disease. Crohn’s Disease cA2 Study Group. N Engl J Med. 1997;337(15):1029–1035. doi:10.1056/NEJM199710093371502
124. Hanauer SB, Feagan BG, Lichtenstein GR, et al. Maintenance infliximab for Crohn’s disease: the ACCENT I randomised trial. Lancet. 2002;359(9317):1541–1549. doi:10.1016/S0140-6736(02)08512-4
125. Colombel JF, Sandborn WJ, Rutgeerts P, et al. Adalimumab for maintenance of clinical response and remission in patients with Crohn’s disease: the CHARM trial. Gastroenterology. 2007;132(1):52–65. doi:10.1053/j.gastro.2006.11.041
126. Sandborn WJ, Feagan BG, Stoinov S, et al. Certolizumab pegol for the treatment of Crohn’s disease. N Engl J Med. 2007;357(3):228–238. doi:10.1056/NEJMoa067594
127. Present DH, Rutgeerts P, Targan S, et al. Infliximab for the treatment of fistulas in patients with Crohn’s disease. N Engl J Med. 1999;340(18):1398–1405. doi:10.1056/NEJM199905063401804
128. Sands BE, Anderson FH, Bernstein CN, et al. Infliximab maintenance therapy for fistulizing Crohn’s disease. N Engl J Med. 2004;350(9):876–885. doi:10.1056/NEJMoa030815
129. Sands BE, Blank MA, Patel K, van Deventer SJ, Study AI. Long-term treatment of rectovaginal fistulas in Crohn’s disease: response to infliximab in the ACCENT II Study. Clin Gastroenterol Hepatol. 2004;2(10):912–920. doi:10.1016/s1542-3565(04)00414-8
130. Colombel JF, Schwartz DA, Sandborn WJ, et al. Adalimumab for the treatment of fistulas in patients with Crohn’s disease. Gut. 2009;58(7):940–948. doi:10.1136/gut.2008.159251
131. Hanauer SB, Sandborn WJ, Rutgeerts P, et al. Human anti-tumor necrosis factor monoclonal antibody (adalimumab) in Crohn’s disease: the CLASSIC-I trial. Gastroenterology. 2006;130(2):323–33; quiz 591. doi:10.1053/j.gastro.2005.11.030
132. Sandborn WJ, Rutgeerts P, Enns R, et al. Adalimumab induction therapy for Crohn disease previously treated with infliximab: a randomized trial. Ann Intern Med. 2007;146(12):829–838. doi:10.7326/0003-4819-146-12-200706190-00159
133. Schreiber S, Khaliq-Kareemi M, Lawrance IC, et al. Maintenance therapy with certolizumab pegol for Crohn’s disease. N Engl J Med. 2007;357(3):239–250. doi:10.1056/NEJMoa062897
134. Kestens C, van Oijen MG, Mulder CL, et al. Adalimumab and infliximab are equally effective for Crohn’s disease in patients not previously treated with anti-tumor necrosis factor-alpha agents. Clin Gastroenterol Hepatol. 2013;11(7):826–831. doi:10.1016/j.cgh.2013.01.012
135. Osterman MT, Haynes K, Delzell E, et al. Comparative effectiveness of infliximab and adalimumab for Crohn’s disease. Clin Gastroenterol Hepatol. 2014;12(5):811–817 e3. doi:10.1016/j.cgh.2013.06.010
136. Stidham RW, Lee TC, Higgins PD, et al. Systematic review with network meta-analysis: the efficacy of anti-TNF agents for the treatment of Crohn’s disease. Aliment Pharmacol Ther. 2014;39(12):1349–1362. doi:10.1111/apt.12749
137. Colombel SBH JF, Sandborn W, Sands BE, et al. DOP86 Subcutaneous infliximab (CT-P13 SC) as maintenance therapy for Crohn’s disease: a phase 3, randomised, placebo-controlled study (LIBERTY-CD). J Crohn’s Colitis. 2023;17(1):i161–i162. doi:10.1093/ecco-jcc/jjac190.0126
138. Celltrion Wins FDA Approval for First Subcutaneous Version of IBD Drug Infliximab. BioSpace; 2023. Available from: https://www.biospace.com/article/celltrion-wins-fda-approval-for-first-subcutaneous-formulation-of-ibd-drug-infliximab/#:~:text=The%20FDA%20on%20Monday%20approved,ulcerative%20colitis%20and%20Crohn’s%20disease.
139. Van Assche G, Vermeire S, Ballet V, et al. Switch to adalimumab in patients with Crohn’s disease controlled by maintenance infliximab: prospective randomised SWITCH trial. Gut. 2012;61(2):229–234. doi:10.1136/gutjnl-2011-300755
140. Burmester GR, Gordon KB, Rosenbaum JT, et al. Long-Term safety of adalimumab in 29,967 adult patients from global clinical trials across multiple indications: an updated analysis. Adv Ther. 2020;37(1):364–380. doi:10.1007/s12325-019-01145-8
141. Lichtenstein GR, Rutgeerts P, Sandborn WJ, et al. A pooled analysis of infections, malignancy, and mortality in infliximab- and immunomodulator-treated adult patients with inflammatory bowel disease. Am J Gastroenterol. 2012;107(7):1051–1063. doi:10.1038/ajg.2012.89
142. Grijalva CG, Chen L, Delzell E, et al. Initiation of tumor necrosis factor-alpha antagonists and the risk of hospitalization for infection in patients with autoimmune diseases. JAMA. 2011;306(21):2331–2339. doi:10.1001/jama.2011.1692
143. Mariette X, Matucci-Cerinic M, Pavelka K, et al. Malignancies associated with tumour necrosis factor inhibitors in registries and prospective observational studies: a systematic review and meta-analysis. Ann Rheum Dis. 2011;70(11):1895–1904. doi:10.1136/ard.2010.149419
144. Askling J, Fahrbach K, Nordstrom B, Ross S, Schmid CH, Symmons D. Cancer risk with tumor necrosis factor alpha (TNF) inhibitors: meta-analysis of randomized controlled trials of adalimumab, etanercept, and infliximab using patient level data. Pharmacoepidemiol Drug Saf. 2011;20(2):119–130. doi:10.1002/pds.2046
145. De Rycke L, Kruithof E, Van Damme N, et al. Antinuclear antibodies following infliximab treatment in patients with rheumatoid arthritis or spondylarthropathy. Arthritis Rheum. 2003;48(4):1015–1023. doi:10.1002/art.10876
146. Shin IS, Baer AN, Kwon HJ, Papadopoulos EJ, Siegel JN. Guillain-Barre and Miller Fisher syndromes occurring with tumor necrosis factor alpha antagonist therapy. Arthritis Rheum. 2006;54(5):1429–1434. doi:10.1002/art.21814
147. Chupin A, Perduca V, Meyer A, Bellanger C, Carbonnel F, Dong C. Systematic review with meta-analysis: comparative risk of lymphoma with anti-tumour necrosis factor agents and/or thiopurines in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2020;52(8):1289–1297. doi:10.1111/apt.16050
148. Luzentales-Simpson M, Pang YCF, Zhang A, Sousa JA, Sly LM. Vedolizumab: potential mechanisms of action for reducing pathological inflammation in inflammatory bowel diseases. Front Cell Dev Biol. 2021;9:612830. doi:10.3389/fcell.2021.612830
149. Sandborn WJ, Feagan BG, Rutgeerts P, et al. Vedolizumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med. 2013;369(8):711–721. doi:10.1056/NEJMoa1215739
150. Sands BE, Feagan BG, Rutgeerts P, et al. Effects of vedolizumab induction therapy for patients with Crohn’s disease in whom tumor necrosis factor antagonist treatment failed. Gastroenterology. 2014;147(3):618–627 e3. doi:10.1053/j.gastro.2014.05.008
151. Chandar AK, Singh S, Murad MH, Peyrin-Biroulet L, Loftus EV
152. Lin L, Liu X, Wang D, Zheng C. Efficacy and safety of antiintegrin antibody for inflammatory bowel disease: a systematic review and meta-analysis. Medicine. 2015;94(10):e556. doi:10.1097/MD.0000000000000556
153. Vermeire S, D’Haens G, Baert F, et al. Efficacy and safety of subcutaneous vedolizumab in patients with moderately to severely active crohn’s disease: results from the VISIBLE 2 randomised trial. J Crohns Colitis. 2022;16(1):27–38. doi:10.1093/ecco-jcc/jjab133
154. Takeda Announces FDA Acceptance of BLA for Subcutaneous Administration of ENTYVIO® (vedolizumab) for Maintenance Therapy in Moderately to Severely Active Crohn’s Disease; 2023. Available from: https://www.takeda.com/newsroom/newsreleases/2023/Takeda-Announces-FDA-Acceptance-of-BLA-for-Subcutaneous-Administration-of-ENTYVIO-vedolizumab-for-Maintenance-Therapy-in-Moderately-to-Severely-Active-Crohn-Disease/.
155. Loftus EV
156. Selewski DT, Shah GV, Segal BM, Rajdev PA, Mukherji SK. Natalizumab (Tysabri). AJNR Am J Neuroradiol. 2010;31(9):1588–1590. doi:10.3174/ajnr.A2226
157. MacDonald JK, McDonald JW. Natalizumab for induction of remission in Crohn’s disease. Cochrane Database Syst Rev. 2007;1:CD006097. doi:10.1002/14651858.CD006097.pub2
158. Bloomgren G, Richman S, Hotermans C, et al. Risk of natalizumab-associated progressive multifocal leukoencephalopathy. N Engl J Med. 2012;366(20):1870–1880. doi:10.1056/NEJMoa1107829
159. Benson JM, Peritt D, Scallon BJ, et al. Discovery and mechanism of ustekinumab: a human monoclonal antibody targeting interleukin-12 and interleukin-23 for treatment of immune-mediated disorders. MAbs. 2011;3(6):535–545. doi:10.4161/mabs.3.6.17815
160. Feagan BG, Sandborn WJ, Gasink C, et al. Ustekinumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med. 2016;375(20):1946–1960. doi:10.1056/NEJMoa1602773
161. Papp K, Gottlieb AB, Naldi L, et al. Safety surveillance for ustekinumab and other psoriasis treatments From the Psoriasis Longitudinal Assessment and Registry (PSOLAR). J Drugs Dermatol. 2015;14(7):706–714.
162. Khader SA, Thirunavukkarasu S. The tale of IL-12 and IL-23: a paradigm shift. J Immunol. 2019;202(3):629–630. doi:10.4049/jimmunol.1801603
163. D’Haens G, Panaccione R, Baert F, et al. Risankizumab as induction therapy for Crohn’s disease: results from the phase 3 ADVANCE and MOTIVATE induction trials. Lancet. 2022;399(10340):2015–2030. doi:10.1016/S0140-6736(22)00467-6
164. Ferrante M, Panaccione R, Baert F, et al. Risankizumab as maintenance therapy for moderately to severely active Crohn’s disease: results from the multicentre, randomised, double-blind, placebo-controlled, withdrawal phase 3 FORTIFY maintenance trial. Lancet. 2022;399(10340):2031–2046. doi:10.1016/S0140-6736(22)00466-4
165. Gordon KB, Lebwohl M, Papp KA, et al. Long-term safety of risankizumab from 17 clinical trials in patients with moderate-to-severe plaque psoriasis. Br J Dermatol. 2022;186(3):466–475. doi:10.1111/bjd.20818
166. Loftus EV
167. Sandborn WJ, Feagan BG, Loftus EV
168. Burmester GR, Cohen SB, Winthrop KL, et al. Safety profile of upadacitinib over 15,000 patient-years across rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis and atopic dermatitis. RMD Open. 2023;9(1):e002735. doi:10.1136/rmdopen-2022-002735
169. Ytterberg SR, Bhatt DL, Mikuls TR, et al. Cardiovascular and cancer risk with tofacitinib in rheumatoid arthritis. N Engl J Med. 2022;386(4):316–326. doi:10.1056/NEJMoa2109927
170. Sandborn WJ, D’Haens GR, Sands BE, et al. Tofacitinib for the treatment of ulcerative colitis: an integrated summary of up to 7.8 years of safety data from the global clinical programme. J Crohns Colitis. 2023;17(3):338–351. doi:10.1093/ecco-jcc/jjac141
171. Sands BE, Irving PM, Hoops T, et al. Ustekinumab versus adalimumab for induction and maintenance therapy in biologic-naive patients with moderately to severely active Crohn’s disease: a multicentre, randomised, double-blind, parallel-group, phase 3b trial. Lancet. 2022;399(10342):2200–2211. doi:10.1016/S0140-6736(22)00688-2
172. AbbVie’s SKYRIZI® (risankizumab) Met All Primary and Secondary Endpoints Versus Stelara® (ustekinumab) in Head-to-Head Study in Crohn’s Disease. AbbVie; 2023. Available from: https://news.abbvie.com/news/press-releases/abbvies-skyrizi-risankizumab-met-all-primary-and-secondary-endpoints-versus-stelara-ustekinumab-in-head-to-head-study-in-crohns-disease.htm.
173. Singh S, Murad MH, Fumery M, et al. Comparative efficacy and safety of biologic therapies for moderate-to-severe Crohn’s disease: a systematic review and network meta-analysis. Lancet Gastroenterol Hepatol. 2021;6(12):1002–1014. doi:10.1016/S2468-1253(21)00312-5
174. Waljee AK, Lipson R, Wiitala WL, et al. Predicting hospitalization and outpatient corticosteroid use in inflammatory bowel disease patients using machine learning. Inflamm Bowel Dis. 2017;24(1):45–53. doi:10.1093/ibd/izx007
175. Cuffari C. A Physician’s Guide to Azathioprine Metabolite Testing. Gastroenterol Hepatol. 2006;2(1):58–63.
176. Weinshilboum RM, Sladek SL. Mercaptopurine pharmacogenetics: monogenic inheritance of erythrocyte thiopurine methyltransferase activity. Am J Hum Genet. 1980;32(5):651–662.
177. Alves S, Prata MJ, Ferreira F, Amorim A. Screening of thiopurine S-methyltransferase mutations by horizontal conformation-sensitive gel electrophoresis. Hum Mutat. 2000;15(3):246–253. doi:10.1002/(SICI)1098-1004(200003)15:3<246::AID-HUMU5>3.0.CO;2-#
178. Evans WE, Horner M, Chu YQ, Kalwinsky D, Roberts WM. Altered mercaptopurine metabolism, toxic effects, and dosage requirement in a thiopurine methyltransferase-deficient child with acute lymphocytic leukemia. J Pediatr. 1991;119(6):985–989. doi:10.1016/s0022-3476(05)83063-x
179. Lennard L, Rees CA, Lilleyman JS, Maddocks JL. Childhood leukaemia: a relationship between intracellular 6-mercaptopurine metabolites and neutropenia. Br J Clin Pharmacol. 1983;16(4):359–363. doi:10.1111/j.1365-2125.1983.tb02178.x
180. Bostrom B, Erdmann G. Cellular pharmacology of 6-mercaptopurine in acute lymphoblastic leukemia. Am J Pediatr Hematol Oncol. 1993;15(1):80–86. doi:10.1097/00043426-199302000-00010
181. Cuffari C, Dassopoulos T, Turnbough L, Thompson RE, Bayless TM. Thiopurine methyltransferase activity influences clinical response to azathioprine in inflammatory bowel disease. Clin Gastroenterol Hepatol. 2004;2(5):410–417. doi:10.1016/s1542-3565(04)00127-2
182. Kaskas BA, Louis E, Hindorf U, et al. Safe treatment of thiopurine S-methyltransferase deficient Crohn’s disease patients with azathioprine. Gut. 2003;52(1):140–142. doi:10.1136/gut.52.1.140
183. Feuerstein JD, Nguyen GC, Kupfer SS, Falck-Ytter Y, Singh S. American gastroenterological association institute clinical guidelines C. American Gastroenterological Association Institute guideline on therapeutic drug monitoring in inflammatory bowel disease. Gastroenterology. 2017;153(3):827–834. doi:10.1053/j.gastro.2017.07.032
184. Dervieux T, Meyer G, Barham R, et al. Liquid chromatography-tandem mass spectrometry analysis of erythrocyte thiopurine nucleotides and effect of thiopurine methyltransferase gene variants on these metabolites in patients receiving azathioprine/6-mercaptopurine therapy. Clin Chem. 2005;51(11):2074–2084. doi:10.1373/clinchem.2005.050831
185. Dubinsky MC, Lamothe S, Yang HY, et al. Pharmacogenomics and metabolite measurement for 6-mercaptopurine therapy in inflammatory bowel disease. Gastroenterology. 2000;118(4):705–713. doi:10.1016/s0016-5085(00)70140-5
186. Osterman MT, Kundu R, Lichtenstein GR, Lewis JD. Association of 6-thioguanine nucleotide levels and inflammatory bowel disease activity: a meta-analysis. Gastroenterology. 2006;130(4):1047–1053. doi:10.1053/j.gastro.2006.01.046
187. Boccara G, Lopez S, Huguet M, Mann C, Colson P. Myopericardite aigue dans les suites d’une cure laparoscopique de reflux gastro-oesophagien [Acute myopericarditis following laparoscopic treatment of gastroesophageal reflux]. Ann Fr Anesth Reanim. 1998;17(9):1148–1151. doi:10.1016/s0750-7658(00)80010-6
188. Waljee AK, Sauder K, Patel A, et al. Machine learning algorithms for objective remission and clinical outcomes with thiopurines. J Crohns Colitis. 2017;11(7):801–810. doi:10.1093/ecco-jcc/jjx014
189. Albader F, Golovics PA, Gonczi L, Bessissow T, Afif W, Lakatos PL. Therapeutic drug monitoring in inflammatory bowel disease: the dawn of reactive monitoring. World J Gastroenterol. 2021;27(37):6231–6247. doi:10.3748/wjg.v27.i37.6231
190. Seow CH, Newman A, Irwin SP, Steinhart AH, Silverberg MS, Greenberg GR. Trough serum infliximab: a predictive factor of clinical outcome for infliximab treatment in acute ulcerative colitis. Gut. 2010;59(1):49–54. doi:10.1136/gut.2009.183095
191. Karmiris K, Paintaud G, Noman M, et al. Influence of trough serum levels and immunogenicity on long-term outcome of adalimumab therapy in Crohn’s disease. Gastroenterology. 2009;137(5):1628–1640. doi:10.1053/j.gastro.2009.07.062
192. Papamichael K, Juncadella A, Wong D, et al. Proactive therapeutic drug monitoring of adalimumab is associated with better long-term outcomes compared with standard of care in patients with inflammatory bowel disease. J Crohns Colitis. 2019;13(8):976–981. doi:10.1093/ecco-jcc/jjz018
193. Vaughn BP, Martinez-Vazquez M, Patwardhan VR, Moss AC, Sandborn WJ, Cheifetz AS. Proactive therapeutic concentration monitoring of infliximab may improve outcomes for patients with inflammatory bowel disease: results from a pilot observational study. Inflamm Bowel Dis. 2014;20(11):1996–2003. doi:10.1097/MIB.0000000000000156
194. Assa A, Matar M, Turner D, et al. Proactive monitoring of adalimumab trough concentration associated with increased clinical remission in children with Crohn’s disease compared with reactive monitoring. Gastroenterology. 2019;157(4):985–996 e2. doi:10.1053/j.gastro.2019.06.003
195. Fernandes SR, Bernardo S, Simoes C, et al. Proactive infliximab drug monitoring is superior to conventional management in inflammatory bowel disease. Inflamm Bowel Dis. 2020;26(2):263–270. doi:10.1093/ibd/izz131
196. Vande Casteele N, Ferrante M, Van Assche G, et al. Trough concentrations of infliximab guide dosing for patients with inflammatory bowel disease. Gastroenterology. 2015;148(7):1320–9 e3. doi:10.1053/j.gastro.2015.02.031
197. Syversen SW, Goll GL, Jorgensen KK, et al. Effect of therapeutic drug monitoring vs standard therapy during infliximab induction on disease remission in patients with chronic immune-mediated inflammatory diseases: a randomized clinical trial. JAMA. 2021;325(17):1744–1754. doi:10.1001/jama.2021.4172
198. Bossuyt P, Pouillon L, Claeys S, et al. Ultra-proactive therapeutic drug monitoring of infliximab based on point of care testing in inflammatory bowel disease: results of a pragmatic trial. J Crohns Colitis. 2022;16(2):199–206. doi:10.1093/ecco-jcc/jjab127
199. D’Haens GR, Sandborn WJ, Loftus EV
200. Colombel JF, Panaccione R, Bossuyt P, et al. Effect of tight control management on Crohn’s disease (CALM): a multicentre, randomised, controlled phase 3 trial. Lancet. 2017;390(10114):2779–2789. doi:10.1016/S0140-6736(17)32641-7
201. Dulai PS, Boland BS, Singh S, et al. Development and validation of a scoring system to predict outcomes of vedolizumab treatment in patients with crohn’s disease. Gastroenterology. 2018;155(3):687–695 e10. doi:10.1053/j.gastro.2018.05.039
202. Waljee AK, Liu B, Sauder K, et al. Predicting corticosteroid-free endoscopic remission with vedolizumab in ulcerative colitis. Aliment Pharmacol Ther. 2018;47(6):763–772. doi:10.1111/apt.14510
203. Waljee AK, Wallace BI, Cohen-Mekelburg S, et al. Development and validation of machine learning models in prediction of remission in patients with moderate to severe Crohn disease. JAMA Netw Open. 2019;2(5):e193721. doi:10.1001/jamanetworkopen.2019.3721
204. Caviglia GP, Rosso C, Stalla F, et al. On-treatment decrease of serum interleukin-6 as a predictor of clinical response to biologic therapy in patients with inflammatory bowel diseases. J Clin Med. 2020;9(3):800. doi:10.3390/jcm9030800
205. Bek S, Nielsen JV, Bojesen AB, et al. Systematic review: genetic biomarkers associated with anti-TNF treatment response in inflammatory bowel diseases. Aliment Pharmacol Ther. 2016;44(6):554–567. doi:10.1111/apt.13736
206. Siegel CA, Horton H, Siegel LS, et al. A validated web-based tool to display individualised Crohn’s disease predicted outcomes based on clinical, serologic and genetic variables. Aliment Pharmacol Ther. 2016;43(2):262–271. doi:10.1111/apt.13460
207. Trikudanathan G, Venkatesh PG, Navaneethan U. Diagnosis and therapeutic management of extra-intestinal manifestations of inflammatory bowel disease. Drugs. 2012;72(18):2333–2349. doi:10.2165/11638120-000000000-00000
208. Peyrin-Biroulet L, Van Assche G, Gomez-Ulloa D, et al. Systematic review of tumor necrosis factor antagonists in extraintestinal manifestations in inflammatory bowel disease. Clin Gastroenterol Hepatol. 2017;15(1):25–36 e27. doi:10.1016/j.cgh.2016.06.025
209. Vavricka SR, Gubler M, Gantenbein C, et al. Anti-TNF treatment for extraintestinal manifestations of inflammatory bowel disease in the Swiss IBD Cohort Study. Inflamm Bowel Dis. 2017;23(7):1174–1181. doi:10.1097/MIB.0000000000001109
210. Tadbiri S, Peyrin-Biroulet L, Serrero M, et al. Impact of vedolizumab therapy on extra-intestinal manifestations in patients with inflammatory bowel disease: a multicentre cohort study nested in the OBSERV-IBD cohort. Aliment Pharmacol Ther. 2018;47(4):485–493. doi:10.1111/apt.14419
211. Chateau T, Bonovas S, Le Berre C, Mathieu N, Danese S, Peyrin-Biroulet L. Vedolizumab treatment in extra-intestinal manifestations in inflammatory bowel disease: a systematic review. J Crohns Colitis. 2019;13(12):1569–1577. doi:10.1093/ecco-jcc/jjz095
212. Liefferinckx C, Verstockt B, Gils A, et al. Long-term clinical effectiveness of ustekinumab in patients with crohn’s disease who failed biologic therapies: a National Cohort Study. J Crohns Colitis. 2019;13(11):1401–1409. doi:10.1093/ecco-jcc/jjz080
213. Pugliese D, Daperno M, Fiorino G, et al. Real-life effectiveness of ustekinumab in inflammatory bowel disease patients with concomitant psoriasis or psoriatic arthritis: an IG-IBD study. Dig Liver Dis. 2019;51(7):972–977. doi:10.1016/j.dld.2019.03.007
214. Phillips FM, Verstockt B, Sebastian S, et al. Inflammatory cutaneous lesions in inflammatory bowel disease treated with vedolizumab or ustekinumab: an ECCO CONFER multicentre case series. J Crohns Colitis. 2020;14(10):1488–1493. doi:10.1093/ecco-jcc/jjaa078
215. Spagnuolo R, Dastoli S, Silvestri M, et al. Anti-interleukin 12/23 in the treatment of erythema nodosum and Crohn disease: a case report. Dermatol Ther. 2019;32(2):e12811. doi:10.1111/dth.12811
216. Fahmy M, Ramamoorthy S, Hata T, Sandborn WJ. Ustekinumab for peristomal pyoderma gangrenosum. Am J Gastroenterol. 2012;107(5):794–795. doi:10.1038/ajg.2012.42
217. Nunes G, Patita M, Fernandes V. Refractory pyoderma gangrenosum in a patient with crohn’s disease: complete response to ustekinumab. J Crohns Colitis. 2019;13(6):812–813. doi:10.1093/ecco-jcc/jjy200
218. Gordon KB, Strober B, Lebwohl M, et al. Efficacy and safety of risankizumab in moderate-to-severe plaque psoriasis (UltIMMa-1 and UltIMMa-2): results from two double-blind, randomised, placebo-controlled and ustekinumab-controlled phase 3 trials. Lancet. 2018;392(10148):650–661. doi:10.1016/S0140-6736(18)31713-6
219. Colombel JF, Cao Q, Ghosh S, et al. OP33 Effect of upadacitinib (UPA) treatment on extraintestinal manifestations (EIMs) in patients with moderate-to-severe Ulcerative Colitis (UC): results from the UPA Phase 3 programme. J Crohn’s Colitis. 2022;16(Supplement_1):i036–i037. doi:10.1093/ecco-jcc/jjab232.032
220. Danese S, Vermeire S, Zhou W, et al. Upadacitinib as induction and maintenance therapy for moderately to severely active ulcerative colitis: results from three phase 3, multicentre, double-blind, randomised trials. Lancet. 2022;399(10341):2113–2128. doi:10.1016/S0140-6736(22)00581-5
221. Kooybaran NR, Korsten P, Schon MP, Mossner R. Response of rheumatoid arthritis-associated pyoderma gangrenous to the JAK1 inhibitor upadacitinib. J Dtsch Dermatol Ges. 2022;20(4):522–524. doi:10.1111/ddg.14716
222. Van Eycken L, Dens AC, de Vlam K, Neerinckx B, De Haes P. Resolution of therapy-resistant pyoderma gangrenosum with upadacitinib. JAAD Case Rep. 2023;37:89–91. doi:10.1016/j.jdcr.2023.05.016
223. Cross RK, Langenberg P, Regueiro M, et al. A Randomized controlled trial of TELEmedicine for Patients with Inflammatory Bowel Disease (TELE-IBD). Am J Gastroenterol. 2019;114(3):472–482. doi:10.1038/s41395-018-0272-8
224. Click B, Regueiro M. The inflammatory bowel disease medical home: from patients to populations. Inflamm Bowel Dis. 2019;25(12):1881–1885. doi:10.1093/ibd/izz062
225. O’Dell ML. What is a patient-centered medical home?. Mo Med. 2016;113(4):301–304.
226. Friedberg MW, Rosenthal MB, Werner RM, Volpp KG, Schneider EC. Effects of a medical home and shared savings intervention on quality and utilization of care. JAMA Intern Med. 2015;175(8):1362–1368. doi:10.1001/jamainternmed.2015.2047
227. Rosenthal MB, Sinaiko AD, Eastman D, Chapman B, Partridge G. Impact of the Rochester medical home initiative on primary care practices, quality, utilization, and costs. Med Care. 2015;53(11):967–973. doi:10.1097/MLR.0000000000000424
228. Regueiro M, Click B, Anderson A, et al. Reduced unplanned care and disease activity and increased quality of life after patient enrollment in an inflammatory bowel disease medical home. Clin Gastroenterol Hepatol. 2018;16(11):1777–1785. doi:10.1016/j.cgh.2018.04.007
229. Singh S, Brill JV, Proudfoot JA, et al. Project sonar: a community practice-based intensive medical home for patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol. 2018;16(12):1847–1850 e1. doi:10.1016/j.cgh.2018.08.052
230. Berinstein JA, Cohen-Mekelburg SA, Greenberg GM, et al. A care coordination intervention improves symptoms but not charges in high-risk patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2022;20(5):1029–1038 e9. doi:10.1016/j.cgh.2021.08.034
231. Oshi Health. Available from: https://oshihealth.com/.
232. Clinical Trial Results Demonstrate Oshi Health’s Multidisciplinary GI. Care significantly improves patient outcomes with savings greater than $10,000 per patient within six months. Oshi Health; 2023. Available from: https://oshihealth.com/clinical-trial-demonstrates-oshi-improves-patient-outcomes/.
233. Ungaro RC, Yzet C, Bossuyt P, et al. Deep remission at 1 year prevents progression of early Crohn’s disease. Gastroenterology. 2020;159(1):139–147. doi:10.1053/j.gastro.2020.03.039
234. Ribaldone DG, Caviglia GP, Pellicano R, et al. Effectiveness and safety of adalimumab biosimilar ABP 501 in Crohn’s disease: an observational study. Rev Esp Enferm Dig. 2020;112(3):195–200. doi:10.17235/reed.2020.6693/2019
235. FDA approves first oral treatment for moderately to severely active Crohn’s disease. U.S. Food and Drug Administration. Available from: https://www.fda.gov/drugs/news-events-human-drugs/fda-approves-first-oral-treatment-moderately-severely-active-crohns-disease.
236. Vogel CL, Cobleigh MA, Tripathy D, et al. Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J Clin Oncol. 2002;20(3):719–726. doi:10.1200/JCO.2002.20.3.719
237. Graham DB, Xavier RJ. Pathway paradigms revealed from the genetics of inflammatory bowel disease. Nature. 2020;578(7796):527–539. doi:10.1038/s41586-020-2025-2
238. Sazonovs A, Kennedy NA, Moutsianas L, et al. HLA-DQA1*05 carriage associated with development of anti-drug antibodies to infliximab and adalimumab in patients with Crohn’s disease. Gastroenterology. 2020;158(1):189–199. doi:10.1053/j.gastro.2019.09.041
239. West NR, Hegazy AN, Owens BMJ, et al. Oncostatin M drives intestinal inflammation and predicts response to tumor necrosis factor-neutralizing therapy in patients with inflammatory bowel disease. Nat Med. 2017;23(5):579–589. doi:10.1038/nm.4307
240. Verstockt B, Verstockt S, Dehairs J, et al. Low TREM1 expression in whole blood predicts anti-TNF response in inflammatory bowel disease. EBioMedicine. 2019;40:733–742. doi:10.1016/j.ebiom.2019.01.027
241. Verstockt B, Verstockt S, Veny M, et al. Expression levels of 4 genes in colon tissue might be used to predict which patients will enter endoscopic remission after vedolizumab therapy for inflammatory bowel diseases. Clin Gastroenterol Hepatol. 2020;18(5):1142–1151 e10. doi:10.1016/j.cgh.2019.08.030
242. Ananthakrishnan AN, Luo C, Yajnik V, et al. Gut microbiome function predicts response to anti-integrin biologic therapy in inflammatory bowel diseases. Cell Host Microbe. 2017;21(5):603–610 e3. doi:10.1016/j.chom.2017.04.010
243. Doherty MK, Ding T, Koumpouras C, et al.. Fecal microbiota signatures are associated with response to ustekinumab therapy among crohn’s disease patients. mBio. 2018;9(2). doi:10.1128/mBio.02120-17
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
