Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 14
Personalized medicine for patients with COPD: where are we?
Authors Franssen FME , Alter P , Bar N, Benedikter BJ, Iurato S, Maier D, Maxheim M, Roessler FK, Spruit MA , Vogelmeier CF, Wouters EFM , Schmeck B
Received 10 March 2019
Accepted for publication 5 June 2019
Published 9 July 2019 Volume 2019:14 Pages 1465—1484
DOI https://doi.org/10.2147/COPD.S175706
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Russell
Frits ME Franssen1,2, Peter Alter,3 Nadav Bar,4 Birke J Benedikter5,6, Stella Iurato,7 Dieter Maier,8 Michael Maxheim,3 Fabienne K Roessler,4 Martijn A Spruit,1–2,9 Claus F Vogelmeier,3 Emiel FM Wouters1,2, Bernd Schmeck3,5
On behalf of the SysMed-COPD consortium
1Department of Research and Education, CIRO, Horn, The Netherlands; 2Department of Respiratory Medicine, Maastricht University Medical Centre, NUTRIM School of Nutrition and Translational Research in Metabolism, Maastricht, The Netherlands; 3Department of Medicine, Pulmonary and Critical Care Medicine, University Medical Center Giessen and Marburg, Philipps University of Marburg (UMR), Member of the German Center for Lung Research (DZL), Marburg, Germany; 4Department of Chemical Engineering, Norwegian University of Science and Technology (NTNU), Trondheim, Norway; 5Institute for Lung Research, Universities of Giessen and Marburg Lung Centre, Philipps-University Marburg, Member of the German Center for Lung Research (DZL), Marburg, Germany; 6Department of Medical Microbiology, Maastricht University Medical Center (MUMC+), Maastricht, The Netherlands; 7Viscovery Software GmbH, Vienna, Austria; 8Biomax Informatics AG, Planegg, Germany; 9REVAL - Rehabilitation Research Center, BIOMED - Biomedical Research Institute, Faculty of Rehabilitation Sciences, Hasselt University, Diepenbeek, Belgium
Abstract: Chronic airflow limitation is the common denominator of patients with chronic obstructive pulmonary disease (COPD). However, it is not possible to predict morbidity and mortality of individual patients based on the degree of lung function impairment, nor does the degree of airflow limitation allow guidance regarding therapies. Over the last decades, understanding of the factors contributing to the heterogeneity of disease trajectories, clinical presentation, and response to existing therapies has greatly advanced. Indeed, diagnostic assessment and treatment algorithms for COPD have become more personalized. In addition to the pulmonary abnormalities and inhaler therapies, extra-pulmonary features and comorbidities have been studied and are considered essential components of comprehensive disease management, including lifestyle interventions. Despite these advances, predicting and/or modifying the course of the disease remains currently impossible, and selection of patients with a beneficial response to specific interventions is unsatisfactory. Consequently, non-response to pharmacologic and non-pharmacologic treatments is common, and many patients have refractory symptoms. Thus, there is an ongoing urgency for a more targeted and holistic management of the disease, incorporating the basic principles of P4 medicine (predictive, preventive, personalized, and participatory). This review describes the current status and unmet needs regarding personalized medicine for patients with COPD. Also, it proposes a systems medicine approach, integrating genetic, environmental, (micro)biological, and clinical factors in experimental and computational models in order to decipher the multilevel complexity of COPD. Ultimately, the acquired insights will enable the development of clinical decision support systems and advance personalized medicine for patients with COPD.
Keywords: chronic obstructive pulmonary disease, personalized medicine, systems medicine, review
Introduction
Chronic obstructive pulmonary disease (COPD) is one of the leading global causes of morbidity and mortality among the non-communicable chronic diseases (NCD).1 Despite preventive measures to reduce exposure to risk factors and therapeutic advances, the worldwide burden of COPD is estimated to increase in the next decades,2 and the disease cannot be cured.3 Chronic airflow limitation assessed by spirometry remains the defining feature of COPD in subjects with respiratory symptoms and a history of exposure to risk factors.3 However, it is well-recognized that different phenotypes can be distinguished4 and that disease trajectories differ between patients.5 Patients with a comparable degree of airflow limitation may differ considerably in symptoms, functional capacity, and other patient-related outcomes (PROs),6 implicating that additional factors, such as age and extra-pulmonary conditions, contribute to the burden of the disease in individual patients. Comprehensive lung function assessment, including the degrees of hyperinflation and diffusion impairment, blood gases and mouth pressures, does not adequately reflect these PROs in COPD.7 Also, differentiation of COPD from asthma is challenging, and these conditions may coexist.3 In the last decades, the diagnostic workup of COPD has been revised in order to account for the heterogeneity of the disease and thereby enable a more patient-tailored treatment.3 Current strategies, however, neglect the potential of personalized measures for disease prevention and mainly focus on individualized pharmacotherapies. In order to reduce the burden of COPD and change the natural course of the disease, it is essential to advance our understanding of the complex pathophysiology, develop diagnostic tools that reflect the heterogeneity of the disease and its associated conditions, and be able to predict the response to comprehensive interventions in individual patients. Also, from a health economic perspective, better selection of probable responders for specific or costly interventions is warranted. However, this might not automatically result in a reduction in costs, if diagnostic tools to identify these responders are advanced and expensive.
Systems medicine is the integrated study of disease networks at multiple levels, ranging from the molecular level, through cells, organs, to the population level,8 in order to yield a comprehensive understanding of disease. This approach is required to provide care that is predictive, preventive, personalized, and incorporates patient participation (P4 medicine).8 Indeed, a systems medicine approach, integrating genetic, (micro)biological, radiological, clinical, and environmental factors in experimental and computational models may advance personalized treatment of COPD.9 Recently, the “Systems Medicine-based clinical decision support for COPD patients” (SysMed-COPD) project was initiated by an international and interdisciplinary consortium combining clinicians, clinical and basic scientists, computational and systems biology researchers, and bioinformatics engineers.
The first part of this review focuses on the current status regarding personalized management of COPD, and identifies the unmet needs in the domains of prevention, diagnosis, and assessment and treatment. In the second part, we propose a systems medicine approach towards COPD and highlight its potential to unravel the complex pathophysiology of the disease and provide the basis for P4 medicine in COPD.
Development of COPD
Lung development starts prenatally and continues after birth until maximal lung function is attained in early adulthood. After a plateau phase of approximately a decade, this is ensued by an individual lung function decline.10 In some individuals, a unique combination of genetic, lifestyle, and environmental factors may prevent reaching the normal plateau or result in accelerated decline, which may eventually lead to the onset of COPD at around 40–50 years of age.11
Pathobiology of COPD
Fully-developed COPD is characterized by a combination of individual degrees of (small) airway disease (bronchitis) and destruction of alveolar tissue (emphysema). In most cases, its development is initiated by long-term inhalation of oxidative and cytotoxic substances (eg, cigarette smoke) which induce epithelial and endothelial cell apoptosis, pro-inflammatory signaling, and recruitment of circulating monocytes and neutrophils to the lungs.12–15 The activated immune cells secrete proteolytic enzymes including neutrophil elastase, which cause extracellular matrix degradation.16 Some individuals appear able to maintain normal lung structure and function by mounting an appropriate repair response. In contrast, an insufficient repair response results in disintegration of the lung parenchyma and a dysregulated response in remodeling of the small airways, including smooth muscle cell, goblet cell, and mucus gland metaplasia, as well as subepithelial extracellular matrix deposition.15 Eosinophils seem to contribute to innate airway inflammation in a sub-group of COPD patients. During later stages of COPD, autoimmune responses may become activated, as indicated by elevated TH17-lymphocytes, B lymphocytes, and antibodies against self-antigens that become exposed during tissue injury.15,17,18 Once established, the loop of tissue damage, inflammation, and remodeling may self-perpetuate, leading to progressive airflow limitation, even without continued exposure to environmental triggers.19
Predisposing factors
Genetic factors have been estimated to account for 40–55% of the variance in adult lung function.20 Polymorphisms in genes that influence smoking behavior, the protease-antiprotease balance, the oxidant-antioxidant balance, inflammatory processes, or bronchodilator-response contribute to COPD risk.21 The most well-known gene associated with COPD is SERPINA1, which codes for the serine protease inhibitor alpha1-antitrypsin.21 Alpha1-antitrypsin inhibits neutrophil elastase and thereby prevents tissue damage. Mutations and polymorphisms of SERPINA1 that lead to decreased expression or activity of alpha1-antitrypsin are associated with early onset COPD with a strongly emphysematous phenotype, especially in smokers.22
Next to genetics, factors encountered during embryonic development and childhood also influence adult lung function and, therefore, COPD risk.23,24 For instance, maternal smoking during pregnancy, pre-term birth or low birthweight, and childhood asthma are associated with low lung function in early adulthood.25 In a longitudinal prospective cohort study, 44% of children with severe asthma at age 10 were diagnosed with COPD by the age of 50, irrespective of their smoking history, corresponding to a 32-fold relative risk.26 Lower respiratory tract infections during early childhood are also associated with COPD.27 However, it remains to be clarified whether this is a causative relationship or whether the risks for respiratory infections and COPD are both elevated due to an underlying immune deficiency.
Initiating factors
In the majority of cases, COPD development is initiated by exposure to noxious particles or gases, with the most important risk factor being cigarette smoking.28 Related respiratory irritants arising eg, from passive exposure to tobacco smoke, occupational sources, indoor open fires, and outdoor pollution, are thought to account for a large fraction of the smoking-independent incidence of COPD.29 Most respiratory toxicants are complex mixtures containing up to several thousand chemically distinct compounds, and their exact composition varies considerably depending on their mode of generation.30,31 Therefore, pathological mechanisms are likely to differ according to the differences in toxicant composition. Indeed, the type of exposure may influence the clinical phenotype of COPD.32
Modulating factors
A number of factors do not directly contribute to the predisposition or initiation of COPD, yet influence the course of the disease. Gender is likely to be an important modulating factor, as women develop more severe airflow limitation and emphysema than men with a comparable smoking history.33 Yet, when comparing women and men with a similar degree of airflow limitation, women have less emphysema and better oxygenation, but more small airway involvement, more frequent exacerbations, and a poorer quality-of-life.33,34 Also, they have fewer and different comorbidities than men.34
Lifestyle factors, such as diet and physical activity, also modulate COPD development. A number of epidemiological studies have found that intake of diets rich in vitamin C, vitamin E, and β-carotenes (eg, fruit, vegetables, oily fish, whole grains) is positively associated with lung function and, therefore, protects against COPD.35 This may be attributed to the ability of these micronutrients to diminish oxidative stress and oxidative stress-induced inflammation. Higher cardiorespiratory fitness is associated with lower incidence of COPD in the general population,36 and it was shown that improving fitness during childhood and adolescence is associated with greater adult lung volumes.37 Concerning physical activity, active smokers with moderate or high physical activity show attenuated lung function decline and reduced risk of COPD during long-term follow-up compared to those with low levels of physical activity.38 Moreover, COPD patients with regular physical activity have lower rates of hospital admissions and mortality.39
Finally, it has recently been established that the lungs have a microbiome, which may influence the pathogenesis of COPD.40 While it is still unclear whether and how smoking affects lung microbiome composition, the microbiome of COPD patients has a decreased stability and diversity, promoting a disproportionate proliferation of potentially pathogenic species, such as Haemophilus influenzae, Moraxella catarrhalis, and Streptococcus pneumoniae in the lower respiratory tract.40 This outgrowth in turn promotes airway inflammation and, along with viral infections, constitutes an important trigger of acute exacerbations. Importantly, it is known that microbiome composition influences the inflammatory profile: when the microbiome is dominated by Proteobacteria (eg, Haemophilus spp.) or Firmicutes (eg, Streptococcus spp.), this is associated with mediators of neutrophilic or eosinophilic inflammation, respectively.41
Personalized prevention of COPD
In individuals with normal early-adult lung function, the most important strategy for preventing COPD is avoidance of exposure to respiratory irritants. Depending on the source of exposure, this may be achieved by occupational safety procedures such as breathing masks, policies for reduction of air pollution, providing alternatives to indoor open fire for cooking and heating and – most importantly – smoking prevention and cessation. Lifestyle interventions, including prevention of toxic exposures, dietary changes, and increased physical activity, would not only decrease the incidence of COPD but also that of other, often comorbid, chronic diseases.42 The challenge here is to motivate those at risk to actively participate in preventive measures and to overcome the high dropout rate of lifestyle interventions. A personalized motivational approach, taking into account the individual psychosocial background, would likely improve the intervention success.43 In addition, specific preventive measures against comorbid diseases (for example treatment of hypertension or dyslipidaemia) have to be taken into account, and alpha1 antitrypsin augmentation therapy may be considered in deficient individuals.
Preventive measures are particularly important for vulnerable individuals, for instance with a family history of COPD, alpha1-antitrypsin-deficiency, or who have experienced considerable early life disadvantage (maternal smoking, low birth weight, asthma, frequent and severe respiratory infections, etc.). It can be hypothesized that influenza and pneumococcal vaccination, by avoiding lower respiratory tract infections and associated inflammation, may attenuate lung function decline and lower COPD incidence; however, evidence is currently lacking to support this hypothesis.
Unmet needs
Currently, we are lacking effective screening tools to identify people at risk of developing COPD at an early stage. Ideally, a vulnerable population should be identified using a risk score comprising information on the family history of COPD, relevant early life factors, lung function in early adulthood,42 and lifestyle. Second, we need a comprehensive understanding of the different molecular and clinical disease subtypes, as well as correlated specific (companion) diagnostic or therapeutic measures. Patients and people at risk need to be screened over time for activation of pathobiological modules, such as oxidative stress, extracellular matrix degradation, neutrophilic or eosinophilic inflammation, autoimmune effects, and microbiome dysbiosis, to name just a few. Before this is possible, we need to expand our knowledge on the pathobiological modules and to identify corresponding biomarkers. These markers should be measurable with a cost-efficient test that allows screening of large at-risk populations, and should be detectable in biological specimens that can be collected easily during routine visits to the general practitioner, such as exhaled breath, saliva, sputum, or blood. We also need to identify strategies for modification of the pathobiological modules, including those that contribute to comorbidities. Besides the above-mentioned lifestyle interventions, these may include antioxidant and anti-inflammatory drugs, but also anti- or probiotics to induce shifts in the microbiome composition.
Diagnosis and assessment of COPD
Obstructive post-bronchodilator spirometry is the defining criterion for COPD in subjects with chronic respiratory symptoms.3 The Global initiative for Obstructive Lung Disease (GOLD) recommends a fixed ratio of forced expiratory volume in one second (FEV1), and forced vital capacity (FVC) <0.7 to diagnose the disease and FEV1 as percent predicted is used for spirometric classification of severity of airflow limitation.3 Since 2011, the current severity of symptoms and the history of moderate or severe exacerbations within the previous 12 months are assessed to allocate patients to groups A/B/C/D and guide therapy.3 Although the classification according to these three domains of the disease enables more personalized treatment recommendations, substantial heterogeneity in clinical characteristics is observed in patients within the same GOLD quadrant.44,45 Also, the GOLD A/B/C/D system does not discriminate between patients in which other factors, for example age or comorbidities, contribute to the burden of disease, and does not adequately differentiate patients with respect to mortality risk.46 Alternative tools to predict outcomes, including the BODE (body mass index, airflow obstruction, dyspnea, and exercise capacity) and ADO (age, dyspnea, and airflow obstruction) indices have been developed and validated in COPD, but cannot be used to guide therapy. In order to overcome the dynamic complexity and heterogeneity of the disease and facilitate treatments targeting the individual needs of patients, the “treatable traits” concept was recently proposed.47 In this concept, all manageable pulmonary and extra-pulmonary features of COPD are identified in individual patients and then treated.
Unmet needs
While management of stable COPD became more personalized over the last decades, by incremented understanding of the determinants of the individual burden of disease, current disease management does not meet the demands of P4 medicine. Predicting a beneficial or adverse individual response to specific interventions remains impossible for most; despite our increased understanding of the pathophysiology of COPD, primary or secondary prevention remains problematic. Also, it is not feasible to assess all treatable traits in every individual with COPD, and this will result in substantial costs for additional diagnostics and treatments, so there is a need for diagnostic algorithms for subgroups of homogeneous patients.48
Another unmet need is the high rate of underdiagnosis of COPD in the general population. While approximately half of patients with severe airflow limitation have a physician-based diagnosis of COPD, only 5% of those with mild lung function abnormality reported a diagnosis of COPD.49 Timely identification and pharmacologic treatment may ameliorate the progressive decline in lung function in mild disease.50 In addition, there is a significant group of symptomatic smokers with preserved spirometry, formerly referred to as GOLD stage 0.51 While only a minority of this group will progress to develop chronic airflow limitation,52 these symptomatic smokers have significant morbidity and healthcare utilization.53 Also, radiologic abnormalities including emphysema and air trapping are common in these subjects.54 Since emphysematous abnormalities predict accelerated loss of lung function55 and increased mortality,56 there is a need for early identification of these subjects. Computed tomography-based measurement of total airway count (TAC) was recently identified as a potential biomarker for airway-related disease changes in early/mild COPD.57 However, other than smoking cessation, no effective treatments are currently available for GOLD stage 0.
Exacerbations
Diagnostics and current treatment
COPD exacerbations are defined as acute worsenings of respiratory symptoms resulting in additional therapy.3 There is large variability in the frequency of exacerbations between patients.58 While a frequent-exacerbator phenotype was previously identified, the proportion of patients with two or more events in consecutive years is very small.59 This observation limits the clinical value of the assessment of future exacerbation risk as currently proposed by GOLD.60 Currently, a history of exacerbations and poor health status remain the best clinical predictors of future events59 and, thus, may drive pharmacotherapeutic decisions.
Although exacerbations are considered pivotal events in the natural course of disease, until recently, there was very little focus on characterizing these events and their triggers. The heterogeneous nature of exacerbations is increasingly recognized; distinct biological clusters have been identified based on sputum biomarkers, namely those associated with bacteria, viruses, or eosinophilic airway inflammation.61 In addition, a further cluster associated with limited changes in the inflammatory profile was identified. Although the pathophysiology of this cluster is largely unknown, it is very well possible that these “pauci-inflammatory” exacerbations are in fact other events mimicking the symptomatology of exacerbations, such as acute cardiovascular diseases, pulmonary embolism, acute psychological distress, or mechanical ventilatory constraints. It is increasingly advocated, therefore, that exacerbations must be differentiated from other events in order to enable a precision medical management of a patient presenting with a flare up of respiratory symptoms.62
Personalized exacerbation management
Bronchodilators, systemic corticosteroids, and antibiotics are central interventions in the management of exacerbations,60 and are broadly prescribed. Strategies to personalize the treatment of exacerbations are not available; sputum cultures may be used to guide antibiotics, but are not feasible in most clinical settings. Use of biomarkers of airway infection, including C-reactive protein (CRP) and procalcitonin, in the decision for antibiotics is not recommended.60 Blood eosinophilia is a promising biomarker for biomarker-directed systemic corticosteroid therapy during exacerbations,63 but warrants further investigation. Obviously, microbiologic, inflammatory, and clinical characterization of exacerbations, more details on pathophysiological interactions and potential treatment targets are needed to tailor more specific, individualized treatment strategies. Moreover, increased understanding of individual genetic variants modulating the immune response involved in exacerbation susceptibility64 and response to treatment65 may enable personalized exacerbation management in future patients.
Comorbidities
Concomitant diseases are common in patients with COPD, and contribute to the burden of disease and mortality in individual patients. They comprise a wide range of diseases, including cardiovascular disease in terms of atherosclerosis, heart failure, arrhythmias, hypertension, peripheral vascular disease and stroke, metabolic disorders including hyperglycemia and diabetes, hyperlipidemia, hyperuricemia, as well as gastroesophageal reflux disease, osteoporosis, rheumatoid diseases, cachexia, and mental disorders such as anxiety and depression.66–71 In addition, there is a substantial overlap with asthma, bronchiectasis, pneumonia, sleep disordered breathing, as well as an increased lung cancer risk.3 Almost all patients with COPD exhibit at least one comorbidity, while the majority of patients have four or more other diseases.72
In particular, coexistence of COPD and cardiovascular disease is associated with a worse prognosis, ie, increased morbidity and mortality.73,74 Moreover, reduction of FEV1 was shown as an independent unfavorable prognostic predictor in decompensated heart failure.75 Vice versa, the presence of COPD also worsens the prognosis of cardiac disease, as shown in patients hospitalized for acute myocardial infarction76 or decompensated heart failure.77
Recently, it was shown that plasma troponin I concentrations are an indicator of future cardiovascular events and cardiovascular death in patients with COPD and an increased cardiovascular risk, even when in the normal clinical range.78 Noteworthy, inhaled therapies affect neither troponin concentrations nor mortality or cardiovascular outcomes.78
Current concepts and management of lung-heart interactions
Occurrence of an increased right heart load in COPD is well known since decades and referred to as Cor pulmonale. In contrast, also a reduced right heart size has been reported recently, named Cor pulmonale parvus.79 Right ventricular volumes were lower and associated with the degree of pulmonary emphysema. In stable COPD, the degree of airflow limitation and hyperinflation are linked to left ventricular volume, wall stress,80 and an impaired diastolic filling.81 Potential mechanisms are a reduced left ventricular mass,82 and increased thoracic pressure oscillations during breathing in COPD, aggravated at exercise.83
Current interventional COPD studies on inhaled corticosteroid (ICS)/long-acting beta2-agonist (LABA) therapy showed short-term increases of left and right ventricular volumes and an improved ventricular strain.84 The CLAIM study on dual bronchodilation, using combined LABA and long-acting muscarinic antagonist (LAMA) therapy, showed an improved cardiac function, as indicated by left-ventricular end-diastolic volume.85 Recently, it was shown in this cohort with marked hyperinflation that dual bronchodilation led to an improved pulmonary microvascular blood flow and regional ventilation, as assessed by magnetic resonance imaging.86 However, it remains to be shown whether these interventional short-term effects translate into persistent cardiac changes during long-term treatment of COPD.
Personalized management of comorbidities and unmet needs
The list of comorbidities observed in patients with COPD is extensive. Currently, most comorbidities remain undiagnosed and untreated however.87,88 Also, in contrast to international recommendations, comorbidities are differently treated in COPD. For example, COPD patients with decompensated heart failure are prone to receive an inappropriate heart failure therapy, which is associated with worse long-term prognosis.77 Particularly, prescription rates of betablockers are insufficient, although their beneficial effects in COPD patients with cardiovascular comorbidity have been shown.76
While it is not feasible to screen for all comorbidities in every patient, there is a need for guidance in the diagnostic approach towards these. Identification of subgroups of patients with an increased risk of specific comorbidities may be helpful. Five patient clusters with distinct comorbidity profiles were previously reported in COPD, including metabolic, cardiovascular, cachectic, psychological, and less comorbidity clusters.72 While the degree of airflow limitation was comparable, there were significant differences in other lung function parameters, body weight, fat-free mass, and health status between clusters which may guide risk stratification of comorbidities.72 For example, the proportion of patients with comorbid osteoporosis was highest in the cachectic cluster, while health status was worst in patients in the psychological cluster. Neither circulating inflammatory biomarkers nor pack years of smoking differed between clusters, indicating that the origin of these profiles is multifactorial and largely unknown. Integrated analyses of not only the clinical disease network of COPD, but also incorporating endogenous (genetics, inflammation, oxidative stress, microbiology, aging, repair mechanisms), environmental (smoking, air pollution, physical activity, diet), and socio-economic factors (preterm birth, family size, employment) is warranted. A systems medicine approach for COPD provides this opportunity.
Pharmacologic management
Historic and current treatment algorithms
In the context of this document, it seems preferable to focus not so much on specific drugs but on treatment concepts. These are nicely reflected in the GOLD documents.89 In 2001, the first GOLD statement was published.51 The committee suggested to base treatment recommendations primarily on the level of airflow limitation. Regarding treatment options, the following proceedings were proposed: first, bronchodilators come first. Combinations of bronchodilators with different mechanisms of action may be preferable. Second, inhaled corticosteroids (ICS) can be given if significant symptoms and a significant lung function response (in a 6 week to 3 months trial with ICS) and/or repeated exacerbations are present.
In the 2007 Executive Summary,90 a stage dependent step up of treatments was suggested. Now, the use of ICS should only be considered in patients with an FEV1 below 50% of predicted and a history of repeated exacerbations. This recommendation was mostly based on studies with fixed-dose LABA-ICS combinations.91,92 The abovementioned ICS test was no longer recommended.
In the 2011 document, the new assessment system based on spirometry, symptom load and exacerbation history was introduced,93 and patients were categorized into four groups (A, B, C, D) with separate treatment recommendations for each group. In this document the goals of treatment were defined as reduction of symptoms and reduction of future risk, in particular exacerbations.
In the 2017 version, the assessment system was refined.3 The A, B, C, D groups that define the pharmacological treatment were based on symptoms and exacerbation history only. The most relevant reason for this change was that the exacerbation history proved to be a better predictor of future risk of exacerbations than spirometric impairment.94 Now for each group treatment algorithms were introduced, including escalation and de-escalation (for ICS) concepts. Besides, the role of non-pharmacologic treatments including pulmonary rehabilitation (PR) and other measures that may increase physical activity is emphasized. It is stated that these treatments are equally important as drugs and that combining adequate pharmacological and non-pharmacological therapies is mandatory.
In the 2019 document, a further refinement was described.60 Groups A, B, C, D are now used for informing the initial treatment only. Regarding follow-up, two strata are suggested: patients that suffer primarily from symptoms (ie, dyspnoea) vs exacerbations. For both strata, separate treatment algorithms are proposed. Further, blood eosinophil counts are introduced as a biomarker for the likelihood that treating with an ICS may reduce the exacerbation rate. A management cycle (review – assess – adjust) is defined with the goal to identify reasons why patients are not doing well and adapt the treatments accordingly. Influenza vaccination is recommended as it reduces the incidence of lower respiratory tract infections95 and mortality96 in COPD, while pneumococcal vaccination decreases risk of pneumonia, especially in younger patients with severe COPD and those with comorbidities.97
This brief history nicely shows how the pharmacological COPD treatment has evolved. The mainstay of pharmacological treatment was and is that bronchodilators come first for most patients – either as mono-treatment or as a combination of two bronchodilators with different mechanisms. Because of a superior efficacy for long-term treatment, long-acting bronchodilators are preferred. Next, ICS are only considered in individuals with exacerbations or a history of asthma. ICS containing regimens are combinations of long acting ß2 agonists (LABA) and ICS or of long acting muscarinic antagonists (LAMA), LABA, and ICS. Third, besides ICS, other anti-inflammatory treatments (roflumilast, macrolides) may be used in selected patients.
Coming from an all-comers concept where treatment decisions were mostly based on spirometry data, we now choose drugs using a more tailored patient stratification system a) that is based on symptoms and exacerbation risk, where b) treatment is adapted to the predominant clinical problem (dyspnea and/or exacerbations), c) blood eosinophil counts are used as a biomarker when the use of an ICS is considered, and d) the combination of pharmacological and non-pharmacological treatments is advocated.
Unmet needs
The treatment concepts of the future have to be personal or at least better tailored to the patient's needs. In this context, open research questions have been summarized in an ATS/ERS statement.98 Based on this, there are a number of unmet needs that need further research. The first need relates to risk factors; are there differences regarding treatment effects between COPD caused by biomass exposure and smoking induced COPD? Do patients with reduced lung growth have a different treatment response than individuals with normally developed lungs? What is the role of gender and age? The second need concerns the treatable traits; to what extent is it useful and feasible to implement elements of treatable traits47 in treatment decisions? So far only airflow obstruction, exacerbation history, and blood eosinophil counts are taken into account. What about chronic bacterial colonization, bronchiectasis, emphysema, or asthmatic features? In order to make progress in this field there is an urgent need for (more) biomarkers. Finally, several questions in the field of drugs and non-pharmacological treatments remain unanswered; what are the benefits of pharmacological treatment of comorbidities (including cardiovascular disease and systemic inflammation) on respiratory outcomes and vice versa? What drugs are best to be combined with measures to improve physical activity for longer terms? What concepts regarding physical activation show the best results (hospital-based, ambulatory, internet-based)? How to incorporate patient preferences and priorities in the treatment algorithms for COPD?
Non-pharmacologic management
In addition to pharmacologic treatment, non-pharmacologic treatments play an important role in COPD management. Self-management education aims to adapt the health behaviour of patients and provide skills to personally manage the disease on an everyday basis. Reduction of behavioural risk factors, treatment adherence, and coping with symptoms are important aspects of self-management. Depending on the determinants of disease burden and complexity of interaction between these, additional non-pharmacological interventions may range from monodisciplinary interventions such as exercise training or nutritional supplementation to comprehensive interventions such as pulmonary rehabilitation (PR).99 Per definition, PR is a personalized intervention. Following a comprehensive assessment of the integrated health status of a patient with COPD, PR is a patient-tailored non-pharmacological intervention including exercise training, self-management education, nutritional counselling, psychological support, occupational therapy, and other treatments.100 PR is an evidence-based intervention associated with improvements in symptoms, health status, exercise tolerance, and healthcare utilization.100 Personalization of PR not only comprises variation in the components of the program between patients, but also flexibility of interventions within these components. While exercise training is considered the cornerstone of PR, this intervention may consist of endurance training, interval training, resistance training, nonlinear training, water-based training, neuromuscular electrical stimulation, or whole-body vibration training, based on the symptoms, degree of disability,101 and determinants of exercise intolerance in the individual patient.102
Unmet needs
As with pharmacological interventions in COPD, non-response to PR is common.103 Changes in exercise performance and health status are often used to qualify individuals with COPD as responders or non-responders to PR. However, it was shown that the response to PR may be differential; patients may improve health status without an improvement in exercise capacity or vice versa.104 Within the same domain, response may depend on the method of assessment.104 Using a non-parametric regression technique, four different clusters with a distinct multidimensional response to PR were identified,104 including groups of patients that show only a moderate (35.4%) or even a poor (10.5%) response. We need to better understand these groups as these may require an intensification or redesign of existing programs.
Interventional therapies
In COPD patients with pulmonary emphysema, air trapping and hyperinflation of the lungs contribute to dyspnoea, poor health status, and exercise intolerance.105 Despite optimal pharmacotherapy and PR, severe emphysema is associated with enormous refractory disability. Lung volume reduction is a treatment option for those with the most severe emphysema and results in improved health status, exercise tolerance, and even survival in carefully selected patients.106 As such, it is a nice example of personalized medicine in COPD. In contrast to other domains of disease management, predictors for beneficial response after lung volume reduction have been identified; patients with predominantly upper-lobe emphysema and low exercise capacity were shown to have a survival advantage following surgical lung volume reduction.107 In addition, absence of interlobar collateral ventilation is a predictor of response to endobronchial valve treatment.108
Unmet needs
Diagnostic workup of patients is complicated, techniques are expensive and require specific expertise, and most patients that are screened for lung volume reduction treatments do not meet the inclusion criteria for these interventions.108 However, the field of interventional pulmonology for COPD is expanding rapidly, and new techniques are being investigated.
Systems medicine model for COPD
As described before, current therapies for stable COPD and exacerbations are frequently inadequate to halt disease progression and unable to cure the disease. In addition, we lack sufficient tools to predict an individual’s disease progression or response to therapies. Systems medicine promises to improve our understanding of lung health and COPD. Thus, it has the potential to facilitate development of effective, personalized and ideally preventive interventions. The definition of systems medicine has in recent years been extended beyond an approach to medical understanding and treatment that is based on multi-disciplinary healthcare teams and that integrates biomolecular, psychological, and social dimensions. Now, systems medicine includes novel techniques for systematic assessment from molecular omics to physiome and diseasome, and the formalization of mechanistic hypotheses into computational predictive models.109 In this focus on mechanistic understanding, systems medicine differs from current data-heavy machine learning approaches.110 In the following we will focus on the research oriented part of systems medicine combing clinical, epidemiological, and experimental research with mathematical modeling, computer science, and machine learning110 to model specific pathophysiologic modules via computer simulation to test hypotheses, identify preventive measures, derive new biomarkers, and predict treatment outcomes.111
Recent clinical trials have shown that targeted therapies with small chemical compounds such as kinase inhibitors112 and therapeutic antibodies113 can serve as highly specific tools to manipulate inter- and intracellular regulatory pathways. Combined, both developments – in principle – allow correcting dysregulated physiological modules (protease-antiprotease balance, oxidant-antioxidant balance, inflammation, etc.) in a personalized way for every patient or at least for patient sub-group.114 As discussed above, PR and integrated care as well as preventive measures are further aspects relevant to implement a systems medicine-based approach to COPD. The big challenge to translation from research is to develop, test, and implement strategies and tools to gain clinically meaningful insights for everyday patient care. The systems medicine research and translation strategy critically depend on the involvement of patients and patient representatives, as well as all relevant stakeholders in the health systems.
Experimental models
Observational and interventional clinical studies are prerequisites for systems medicine approaches.110 However, certain analyses are necessary for the modeling process, but cannot be performed in humans, eg, because they are difficult to observe (eg, very early events in COPD development), technically not possible (eg, in vitro cellular imaging of the lung, very detailed time course measurements in the lung), or would be unethical (eg, early drug testing, genetic manipulation).115 Therefore, experimental models are indispensable in systems medicine. New and improved molecular technologies, like single cell sequencing116 and genome editing by CRISPR technology,117 enable us to deepen our pathophysiological insight to a detailed level.
Frequent models in biomedical research are inbred mice. For COPD research, mouse models of acute or chronic exposure to cigarette smoke are established and under investigation.118,119 Advantages are the analysis of whole organisms and the existence of knock-out mice and numerous molecular tools. Disadvantages are relevant differences in pulmonary anatomy, immunology, and the smoke-induced clinical phenotype in comparison to COPD patients. Complementary models are ex vivo cultures of surgically removed lung tissue,120 or air–liquid interface cultures121 of epithelial airway cells as obtained by bronchoscopy, which undergo differentiation in culture to all relevant cells types, produce mucus, and display ciliary beating. These models allow access to “real” human COPD, are relatively easy to work with, but lack crosstalk to other organ systems. Additionally, new organ-on-a-chip and bioreactor models allow the cellularization of microfluidic devices or tissue scaffolds with epithelial and endothelial cells, as well as flow of air at the apical compartment and buffer, including immune cells, in the endothelial compartment.122 Finally, primary human cells or cell lines can be exposed in vitro to cigarette smoke or cigarette smoke extracts.123
These models can be analyzed by manifold imaging (eg, high resolution lifetime microscopy), immunology (eg, flow cytometry), and molecular (eg, single-cell sequencing, proteomics, metabolomics) technologies. They complement clinical studies and provide comprehensive data sets for calibration, optimization, and validation of models derived from computer simulation.
Computational models
A computational model is a mathematical model aimed to investigate the behavior of a complex system. Due to the focus of systems medicine on the elucidation of medical questions, computational models usually have a certain predictive ability. Different sub-classes of computational models can be distinguished. Mechanistic models are traditionally based on the causal understanding of biological entities (eg, cells, proteins, organs) and their dynamic interactions. Basically, these models represent existing knowledge about biological systems in a form that can generate predictions on the behavior of the system. Statistical and machine-learning based models are based on associations rather than causal mechanisms, and can provide direct and strong predictions of (clinically) relevant outcomes.
Mechanistic models
Due to the complexity of COPD, which affects multiple molecules, cell-types, organs, and psycho-social factors, only few attempts to apply mechanistic models to predictive, personalization of treatment have so far been reported. Many of these models have been based on ordinary differential equations (ODE) to calculate the change over time in certain factors. Mostly, these aim to improve prediction of progression or exacerbation risk, for example by coupling exposure to inhaled substances, biological activity in terms of inflammation, and the degree of airflow limitation to progression.124 By doing so, they provide personalized estimates of ventilation/perfusion heterogeneity in COPD patients,125 mechanistically explain etiology from an imbalance of protease-antiprotease,126 leading to a cascade of positive feedback loops in tissue-degeneration, macrophage and neutrophil inflammation, and alveolar epithelial cell apoptosis,127 or generate a physiological model of breathlessness.128 None of these models have so far resulted in clinical application. However, models based on computational fluid dynamics have recently contributed to treatment optimization by predicting particle deposition in the airway tree,129 and are supported by the FDA as prognostic biomarkers in clinical trials.130,131 All models described so far were based on a single modeling technology such as ODE to describe effects on a single level, eg, molecular or cellular. In contrast to these, several approaches recently embarked on the generation of multi-scale, multi-physics models which combine different technologies such as ODE, agent based model, or partial differential equations (PDE). One such approach described the lung in a patient-specific way,132,133 from its molecular constituents to its cell types134 and overall biophysical structure and properties.135,136 This multi-scale model already created clinical impact by debunking thermoplasty as biologically meaningful intervention to asthma.137 Coupling such a patient specific lung model to a multi-scale full-body model to explain muscle wasting in COPD and suggest specific PR approaches was recently reported138 by integrating the ventilation/perfusion lung model with a physiological gas-exchange and distribution model,139 and a model of muscle cell energy generation140,141 and regulation of re-modeling.142 In summary, mechanistic models make inroads to generate patient specific predictions to aid clinical decisions, but have not yet reached impact beyond clinical trial stage.
Machine learning models
The increase in size and availability of patient datasets related to COPD enables us to employ statistical tools such as machine learning (ML) algorithms in order to solve scientific and clinical problems. ML algorithms use data to predict certain outcomes, and algorithms can learn and improve their predictions. ML methods are divided into two types. Supervised ML algorithms learn rules from annotated data (eg, labeled emphysema in CT-images) and apply them to classify new data samples (eg, unlabeled CT-image of emphysematous lung). Unsupervised ML algorithms use unlabeled data to detect patterns, eg, principle component analysis (PCA) or clustering. Unsupervised ML algorithms are well known in medical research, while supervised ML algorithms became popular in the last two decades and achieved promising results, for example in skin cancer classification143 and diabetic retinopathy detection.144
One main advantage of using ML algorithms (instead of traditional mechanistic models) is that they are easily adaptable and do not require a deep understanding of the underlying mechanisms of the system. Supervised ML algorithms can learn with the arrival of new data and improve the outcomes with minimal effort. In contrast, dynamic models need to be re-examined, the mechanisms and pathways need changes, and these new models must be re-analyzed to understand the outcomes, including re-estimation of the parameters using the new data. This re-construction of the mechanistic models is often a difficult and expensive process.
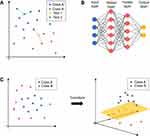
We review here a number of publications focusing on supervised ML algorithms that support clinical decisions for COPD (Table 1). The most prominently used ML algorithms are k-nearest neighbor (KNN), support vector machine (SVMs), and artificial neural network (ANN). KNNs predict the label of a new data sample by finding their closest data points (nearest neighbors) in the training dataset. The nearest neighbors then classify the new data point by vote (Figure 1A). SVMs try to find a line or plane that separates data samples with different labels. If that is not possible, the data points are transformed into a new dimension (Figure 1C). This makes it possible to separate the data samples with, for example, a two-dimensional plane. The ANNs are assembled by neurons organized in layers (Figure 1B). Each neuron sums up its weighted inputs and applies a non-linear activation function on the sum. The calculated value either functions as an input for the neurons in the next layer or as an output of the ANN. Using non-linear activation functions, ANNs are able to find complex boundaries to separate differentially labeled data points.
 |
Table 1 Overview of the reviewed publications focusing on machine learning in clinical decision support for COPD |
Most of the reviewed publications report high performance results of their tested ML algorithms, for example a classification accuracy >90% or even >98%. However, we must consider several important issues in these COPD ML studies. First, many studies use additional “feature selection” to decrease the complexity of their data, and thus focus only on the relevant data variables. Feature selection methods are often used to remove irrelevant and redundant attributes that do not contribute to the accuracy of the outcome or may in fact decrease the accuracy of the predictive model. Feature selection, therefore, improves the performance of their ML algorithm, but it should be used on an independent dataset rather than the entire training data. This is not possible in most COPD ML studies that have access only to small datasets (<300 patients). Using feature selection on the entire training set may yield a model that is enhanced by the selected features over other models being tested to get seemingly better results, when in fact it is a biased result. Second, when we examined the system performance, we found that more than half of the studies did not report the use of an independent test dataset (data that was not used in the training procedure). A missing independent test dataset can lead to an overestimation in the performance of the ML algorithms and should always be implemented in the development and evaluation of a ML-based system. Also, to address the multi-component background of COPD, scientists should incorporate a variety of independent measurement techniques. Despite that, we found that most of the reviewed publications focused on one or two different data types (source of the data such as measurement type). Only some145–152 reported the use of a broader patient health assessment (eg, spirometry, blood analysis, CT images, etc.). Finally, it is generally known that a limited training dataset results in poor approximation, especially for complex ML algorithms and systems.153 Most of the COPD ML studies we reviewed have limited access to data, and their training datasets range from 16–300 patients. This may be one reason why none of the studies154,155 reported testing their algorithm in a real-world clinical setting, suggesting a gap between research in COPD ML algorithms and applications in daily clinical settings. To close this gap, a representative training dataset is needed. The data should also be based on a detailed patient assessment to reflect the multi-component background of COPD.
Statistical models with clinical application
Many approaches to the personalization of COPD risk assessment or treatment suggestion are based on multilevel models, combining classical statistical analysis and machine learning approaches to clinical data with mechanistic modeling of biomedical research data. For example, they use a Bayesian network algorithm to derive clinical variables predictive of exacerbation risk and suggest context-aware preventive action183 or identify survival risk factor attributes by univariate analysis to generate probability distribution models to predict ICU COPD mortality risk.184 Many of these combined models straddle the border between personalized health-behavior advice and public health preventive policy advice such as the personal air-exposure monitoring and exposure-health-association analysis and exacerbation risk prediction model for London which couples exposure-health-association to a time-activity exposure multi-scale ODE model.185 A linked-equation model enabled direct estimation of health service costs and quality-adjusted life years (QALYs) for COPD patients over their lifetimes and was validated for predicted annual exacerbation rate and annual decline in FEV1 with a 3-year longitudinal clinical study186,187 to focus treatment attention on relevant factors. Recently, a patient-level, health economic simulation model including a large number of patient characteristics and relevant outcomes was developed, which can be used to personalize treatment decisions in COPD.188 While influence of physical activity189 has resulted only in a few models, the personalized prediction of exacerbation risk is a major focus of statistical models.152,190–193
Unmet needs
FAIR (findable, accessible, interoperable, reusable) models are required that bridge association-based (machine learning, statistical) and mechanism-based modeling.194 In addition, we need multi-scale, integrated computational models with medically relevant outputs on the level of the individual and focused on specific medical questions.
Access to relevant, high quality data and gold standards for testing and validation is essential to build association-based models. Large scale real-world data access efforts are ongoing in public health systems and will greatly improve our ability to learn.195 As data infrastructures become more important for the ability to improve healthcare, we believe clinics and health systems embracing them will flourish.
Beyond infrastructural and methodological tasks, we need clinical modelers. Too often, even in systems medicine, the disciplines are still separated due to experts staying in their own area; clinicians posing diagnostic and treatment questions and computational and mathematical experts focusing on methodological breakthroughs and fundamental insights. However, as long as “translators” intervene between the clinician and the model, we will lack the direct interaction giving rise to intuitive understanding and explorative generation of new questions, ideas, and hypotheses for clinical research. Therefore, we need models that are user friendly and accessible to clinical researchers to enable them to explore and pose their questions directly in-silico.
Towards a clinical decision support system for COPD
Given the availability of very large datasets and the increasing capability of machine learning approaches, clinical benefit could be optimized and patient risk minimized, developing a dynamic clinical decision support system (CDSS).196 In the clinical context, the term CDSS is often used for any computational system that provides direct aid to clinical decision-making, such as dashboards that present information in a comprehensive, actionable form and, therefore, help clinicians to integrate and prioritize multiple, diverse evidence.197,198
However, in the context of systems medicine and computational modelling, we regard CDSS as software that matches patient characteristics to a computerized clinical knowledge base (KB) and then presents patient-specific assessments or recommendations to the clinician and/or the patient for direct clinical decision-making.199 These systems interpret or advise for action and, therefore, are, at least in Europe, regulated as “medical devices” (EC 2017/745). Existing systems range from CDSS for spirometry quality control,200 to integrated-care based applications creating individual treatment pathways from multiple intervention modules based on a broad biopsychosocial patient assessment.48,201 The results showed by the available CDSS for the detection and diagnosis of COPD are promising and can be used in combination with the existing protocols to facilitate disease management.202 However, none of these COPD-specific systems has yet reached regulatory approval for clinical practice. Outside the field of COPD, examples of systems start to appear which support complex intervention plans, such as multi-perturbation treatment203 or dynamic adaptation of biopsychosocial factors (lifestyle, environment, medication).204
Too many CDSS have been proposed but never underwent dedicated validation trials at the point-of-care, and even less are enabled for continuous improvement on real world data from clinical practice. One of the difficulties to this is the lack of protocols and processes for regulatory approval of continuously adapting CDSS as a medical device.205 In addition, integration of CDSS in healthcare systems where patient participation and shared decision-making are increasingly important is a major challenge.206
Conclusion
As a consequence of advances in our understanding of the pathophysiology of COPD at multiple levels and in comprehensive diagnostic and therapeutic strategies over the last decades, disease management transformed from a “one size fits all” towards a more personalized approach. However, additional, far-reaching efforts are required to transform care into actual P4 medicine for COPD, where the emphasis is not only on personalized management, but also on predictive and preventive healthcare in which individual patients are actively involved. A multidisciplinary systems medicine approach may reveal the multilevel complexity of COPD and fill current gaps in optimization of treatment for individuals at risk and those with established airflow limitation.
Acknowledgments
We would like to apologize to all colleagues whose excellent contributions to the field of personalized COPD medicine could not be included in this text due to space constraints. Part of this work has been funded by German Ministry for Education and Research (BMBF) (ERACoSysMed2 SysMed-COPD-FKZ 031L0140, JPIAMR Pneumo-AMR-Protect-FKZ 01KI1702, e:Med CAPSYS-FKZ 01X1304E/01ZX1304F) to BS and by a Kootstra Talent Fellowship from the Center for Research Innovation, Support and Policy (CRISP) of Maastricht University Medical Center + to BJB. ZonMW (ERACoSysMed 90030355) funded the Dutch consortium partners to FMEF and EFMW. Austrian Science Fund FWF (ERACoSysMed I 3736-B30) funded the Austrian consortium partner SI. NB and FKR were funded by the Norwegian Research Council grant number 284045.
Disclosure
FMEF received personal fees for lectures and consultancies from AstraZeneca, Boehringer Ingelheim, Chiesi, TEVA, GlaxoSmithKline, and Novartis, outside of this work. He also received research grants from Novartis and MedImmune. BS received research funding from GlaxoSmithKline. SI reports grants from Austrian Science Fund FWF, during the conduct of the study. DM reports grants from Austrian Science Fund FWF, during the conduct of the study. MM reports grants from German Ministry for Education and Research (BMBF), during the conduct of the study. CFV reports grants and personal fees from AstraZeneca Boehringer Ingelheim, CSL Behring, Chiesi, GlaxoSmithKline, Grifols, Menarini, Mundipharma, Novartis, TEVA, Cipla, Bayer Schering, MSD, and Pfizer, outside the submitted work. BS reports grants from BMBF and GlaxoSmithKline, during the conduct of the study. The authors report no other conflicts of interest in this work.
References
1. Collaborators GBDCoD. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1736–1788. doi:10.1016/S0140-6736(18)32203-7
2. Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3(11):e442. doi:10.1371/journal.pmed.0030442
3. Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. GOLD executive summary. Am J Respir Crit Care Med. 2017;195(5):557–582. doi:10.1164/rccm.201701-0218PP
4. Rennard SI, Locantore N, Delafont B, et al. Identification of five chronic obstructive pulmonary disease subgroups with different prognoses in the ECLIPSE cohort using cluster analysis. Ann Am Thorac Soc. 2015;12(3):303–312. doi:10.1513/AnnalsATS.201403-125OC
5. Duffy S, Weir M, Criner GJ. The complex challenge of chronic obstructive pulmonary disease. Lancet Respir Med. 2015;3(12):917–919. doi:10.1016/S2213-2600(15)00480-4
6. Agusti A, Calverley PM, Celli B, et al. Characterisation of COPD heterogeneity in the ECLIPSE cohort. Respir Res. 2010;11:122. doi:10.1186/1465-9921-11-62
7. Augustin IML, Spruit MA, Houben-Wilke S, et al. The respiratory physiome: clustering based on a comprehensive lung function assessment in patients with COPD. PLoS One. 2018;13(9):e0201593. doi:10.1371/journal.pone.0201593
8. Flores M, Glusman G, Brogaard K, Price ND, Hood L. P4 medicine: how systems medicine will transform the healthcare sector and society. Per Med. 2013;10(6):565–576. doi:10.2217/pme.13.57
9. Agusti A, Sobradillo P, Celli B. Addressing the complexity of chronic obstructive pulmonary disease: from phenotypes and biomarkers to scale-free networks, systems biology, and P4 medicine. Am J Respir Crit Care Med. 2011;183(9):1129–1137. doi:10.1164/rccm.201009-1414PP
10. Fletcher C, Peto R. The natural history of chronic airflow obstruction. Br Med J. 1977;1(6077):1645–1648. doi:10.1136/bmj.1.6077.1645
11. Lange P, Celli B, Agustí A, et al. Lung-Function Trajectories Leading to Chronic Obstructive Pulmonary Disease. N Engl J Med. 2015;373(2):111–122. doi:10.1056/NEJMoa1411532
12. Serikov VB, Leutenegger C, Krutilina R, et al. Cigarette smoke extract inhibits expression of peroxiredoxin V and increases airway epithelial permeability. Inhal Toxicol. 2006;18(1):79–92. doi:10.1080/08958370500282506
13. Schweitzer KS, Hatoum H, Brown MB, et al. Mechanisms of lung endothelial barrier disruption induced by cigarette smoke: role of oxidative stress and ceramides. Am J Physiol Lung Cell Mol Physiol. 2011;301(6):L836–846. doi:10.1152/ajplung.00385.2010
14. Vernooy JH, Bracke KR, Drummen NE, et al. Leptin modulates innate and adaptive immune cell recruitment after cigarette smoke exposure in mice. J Immunol. 2010;184(12):7169–7177. doi:10.4049/jimmunol.0900963
15. Hogg JC, Chu F, Utokaparch S, et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med. 2004;350(26):2645–2653. doi:10.1056/NEJMoa032158
16. Chung KF, Adcock IM. Multifaceted mechanisms in COPD: inflammation, immunity, and tissue repair and destruction. Eur Respir J. 2008;31(6):1334–1356. doi:10.1183/09031936.00018908
17. Nunez B, Sauleda J, Anto JM, et al. Anti-tissue antibodies are related to lung function in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2011;183(8):1025–1031. doi:10.1164/rccm.201001-0029OC
18. Brusselle GG, Joos GF, Bracke KR. New insights into the immunology of chronic obstructive pulmonary disease. Lancet. 2011;378(9795):1015–1026. doi:10.1016/S0140-6736(11)60988-4
19. Tuder RM, Petrache I. Pathogenesis of chronic obstructive pulmonary disease. J Clin Invest. 2012;122(8):2749–2755. doi:10.1172/JCI60324
20. Klimentidis YC, Vazquez AI, de Los Campos G, Allison DB, Dransfield MT, Thannickal VJ. Heritability of pulmonary function estimated from pedigree and whole-genome markers. Front Genet. 2013;4:174. doi:10.3389/fgene.2013.00174
21. Yuan C, Chang LG, Deng X. Genetic polymorphism and chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2017;12:1385–1393. doi:10.2147/COPD.S134161
22. Janus ED, Phillips NT, Carrell RW. Smoking, lung function, and alpha 1-antitrypsin deficiency. Lancet. 1985;1(8421):152–154.
23. Martinez FD. Early-life origins of chronic obstructive pulmonary disease. N Engl J Med. 2016;375(9):871–878. doi:10.1056/NEJMra1603287
24. Stern DA, Morgan WJ, Wright AL, Guerra S, Martinez FD. Poor airway function in early infancy and lung function by age 22 years: a non-selective longitudinal cohort study. Lancet. 2007;370(9589):758–764. doi:10.1016/S0140-6736(07)61379-8
25. Beyer D, Mitfessel H, Gillissen A. Maternal smoking promotes chronic obstructive lung disease in the offspring as adults. Eur J Med Res. 2009;14(Suppl 4):27–31.
26. Tai A, Tran H, Roberts M, Clarke N, Wilson J, Robertson CF. The association between childhood asthma and adult chronic obstructive pulmonary disease. Thorax. 2014;69(9):805–810. doi:10.1136/thoraxjnl-2013-204815
27. Edmond K, Scott S, Korczak V, et al. Long term sequelae from childhood pneumonia; systematic review and meta-analysis. PLoS One. 2012;7(2):e31239. doi:10.1371/journal.pone.0031239
28. Adeloye D, Chua S, Lee C, et al. Global and regional estimates of COPD prevalence: systematic review and meta-analysis. J Glob Health. 2015;5(2):020415. doi:10.7189/jogh.05.020415
29. Salvi SS, Barnes PJ. Chronic obstructive pulmonary disease in non-smokers. Lancet. 2009;374(9691):733–743. doi:10.1016/S0140-6736(09)61303-9
30. Pauwels C, Klerx WNM, Pennings JLA, et al. Cigarette filter ventilation and smoking protocol influence aldehyde smoke yields. Chem Res Toxicol. 2018;31(6):462–471. doi:10.1021/acs.chemrestox.7b00342
31. Centers for Disease Control and Prevention.How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease: A Report of the Surgeon General. 3, Chemistry and Toxicology of Cigarette Smoke and Biomarkers of Exposure and Harm. Atlanta, GA: Centers for Disease Control and Prevention (US); National Center for Chronic Disease Prevention and Health Promotion (US); Office on Smoking and Health (US); 2010.
32. Camp PG, Ramirez-Venegas A, Sansores RH, et al. COPD phenotypes in biomass smoke- versus tobacco smoke-exposed Mexican women. Eur Respir J. 2014;43(3):725–734. doi:10.1183/09031936.00206112
33. Aryal S, Diaz-Guzman E, Mannino DM. Influence of sex on chronic obstructive pulmonary disease risk and treatment outcomes. Int J Chron Obstruct Pulmon Dis. 2014;9:1145–1154. doi:10.2147/COPD.S54476
34. de Torres JP, Casanova C, Hernandez C, Abreu J, Aguirre-Jaime A, Celli BR. Gender and COPD in patients attending a pulmonary clinic. Chest. 2005;128(4):2012–2016. doi:10.1378/chest.128.4.2012
35. Hanson C, Rutten EP, Wouters EF, Rennard S. Influence of diet and obesity on COPD development and outcomes. Int J Chron Obstruct Pulmon Dis. 2014;9:723–733. doi:10.2147/COPD.S50111
36. Steell L, Ho FK, Sillars A, et al. Dose-response associations of cardiorespiratory fitness with all-cause mortality and incidence and mortality of cancer and cardiovascular and respiratory diseases: the UK Biobank cohort study. Br J Sports Med. 2019. doi:10.1136/bjsports-2018-099093
37. Hancox RJ, Rasmussen F. Does physical fitness enhance lung function in children and young adults? Eur Respir J. 2018;51(2):1701374. doi:10.1183/13993003.01374-2017
38. Garcia-Aymerich J, Lange P, Benet M, Schnohr P, Anto JM. Regular physical activity modifies smoking-related lung function decline and reduces risk of chronic obstructive pulmonary disease: a population-based cohort study. Am J Respir Crit Care Med. 2007;175(5):458–463. doi:10.1164/rccm.200607-896OC
39. Garcia-Aymerich J, Lange P, Benet M, Schnohr P, Anto JM. Regular physical activity reduces hospital admission and mortality in chronic obstructive pulmonary disease: a population based cohort study. Thorax. 2006;61(9):772–778. doi:10.1136/thx.2006.060145
40. Shukla SD, Budden KF, Neal R, Hansbro PM. Microbiome effects on immunity, health and disease in the lung. Clin Transl Immunology. 2017;6(3):e133. doi:10.1038/cti.2017.6
41. Wang Z, Bafadhel M, Haldar K, et al. Lung microbiome dynamics in COPD exacerbations. Eur Respir J. 2016;47(4):1082–1092. doi:10.1183/13993003.01406-2015
42. Agusti A, Noell G, Brugada J, Faner R. Lung function in early adulthood and health in later life: a transgenerational cohort analysis. Lancet Respir Med. 2017;5(12):935–945.
43. Joly B, Perriot J, d’Athis P, Chazard E, Brousse G, Quantin C. Success rates in smoking cessation: psychological preparation plays a critical role and interacts with other factors such as psychoactive substances. PLoS One. 2017;12(10):e0184800. doi:10.1371/journal.pone.0184800
44. Sillen MJ, Franssen FM, Delbressine JM, et al. Heterogeneity in clinical characteristics and co-morbidities in dyspneic individuals with COPD GOLD D: findings of the DICES trial. Respir Med. 2013;107(8):1186–1194. doi:10.1016/j.rmed.2013.04.020
45. Agusti A, Rennard S, Edwards LD, et al. Clinical and prognostic heterogeneity of C and D GOLD groups. Eur Respir J. 2015;46(1):250–254. doi:10.1183/09031936.00012215
46. Cabrera Lopez C, Casanova Macario C, Marin Trigo JM, et al. Comparison of the 2017 and 2015 global initiative for chronic obstructive lung disease reports. impact on grouping and outcomes. Am J Respir Crit Care Med. 2018;197(4):463–469. doi:10.1164/rccm.201707-1363OC
47. Agusti A, Bel E, Thomas M, et al. Treatable traits: toward precision medicine of chronic airway diseases. Eur Respir J. 2016;47(2):410–419. doi:10.1183/13993003.01359-2015
48. Vanfleteren LEGW, Spruit MA, Wouters EFM, Franssen FME. Management of chronic obstructive pulmonary disease beyond the lungs. Lancet Respir Med. 2016;4(11):911–924. doi:10.1016/S2213-2600(16)00097-7
49. Lindberg A, Bjerg A, Ronmark E, Larsson LG, Lundback B. Prevalence and underdiagnosis of COPD by disease severity and the attributable fraction of smoking Report from the Obstructive Lung Disease in Northern Sweden Studies. Respir Med. 2006;100(2):264–272. doi:10.1016/j.rmed.2005.04.029
50. Zhou Y, Zhong NS, Li X, et al. Tiotropium in Early-Stage Chronic Obstructive Pulmonary Disease. N Engl J Med. 2017;377(10):923–935. doi:10.1056/NEJMoa1700228
51. Pauwels RA, Buist AS, Calverley PM, Jenkins CR, Hurd SS. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop summary. Am J Respir Crit Care Med. 2001;163(5):1256–1276. doi:10.1164/ajrccm.163.5.2101039
52. Vestbo J, Lange P. Can GOLD Stage 0 provide information of prognostic value in chronic obstructive pulmonary disease? Am J Respir Crit Care Med. 2002;166(3):329–332. doi:10.1164/rccm.2112048
53. Woodruff PG, Barr RG, Bleecker E, et al. Clinical significance of symptoms in smokers with preserved pulmonary function. N Engl J Med. 2016;374(19):1811–1821. doi:10.1056/NEJMoa1505971
54. Regan EA, Lynch DA, Curran-Everett D, et al. Clinical and radiologic disease in smokers with normal spirometry. JAMA Intern Med. 2015;175(9):1539–1549. doi:10.1001/jamainternmed.2015.2735
55. Nishimura M, Makita H, Nagai K, et al. Annual change in pulmonary function and clinical phenotype in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012;185(1):44–52. doi:10.1164/rccm.201106-0992OC
56. Johannessen A, Skorge TD, Bottai M, et al. Mortality by level of emphysema and airway wall thickness. Am J Respir Crit Care Med. 2013;187(6):602–608. doi:10.1164/rccm.201209-1722OC
57. Kirby M, Tanabe N, Tan WC, et al. Total airway count on computed tomography and the risk of chronic obstructive pulmonary disease progression. Findings from a population-based study. Am J Respir Crit Care Med. 2018;197(1):56–65. doi:10.1164/rccm.201704-0692OC
58. Hurst JR, Vestbo J, Anzueto A, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010;363(12):1128–1138.
59. Han MK, Quibrera PM, Carretta EE, et al. Frequency of exacerbations in patients with chronic obstructive pulmonary disease: an analysis of the SPIROMICS cohort. Lancet Respir Med. 2017;5(8):619–626. doi:10.1016/S2213-2600(17)30207-2
60. Global Strategy for the diagnosis, managment, and prevention of chronic obstructive lung disease 2019 Report. Available from: www.goldcopd.com. Accessed June 01, 2019.
61. Bafadhel M, McKenna S, Terry S, et al. Acute exacerbations of chronic obstructive pulmonary disease: identification of biologic clusters and their biomarkers. Am J Respir Crit Care Med. 2011;184(6):662–671. doi:10.1164/rccm.201104-0597OC
62. Wedzicha JAE-C-C, Miravitlles M, Hurst JR, et al. Management of COPD exacerbations: a European Respiratory Society/American Thoracic Society guideline. Eur Respir J. 2017;49:3. doi:10.1183/13993003.00791-2016
63. Bafadhel M, McKenna S, Terry S, et al. Blood eosinophils to direct corticosteroid treatment of exacerbations of chronic obstructive pulmonary disease: a randomized placebo-controlled trial. Am J Respir Crit Care Med. 2012;186(1):48–55. doi:10.1164/rccm.201108-1553OC
64. Ishii T, Angata T, Wan ES, et al. Influence of SIGLEC9 polymorphisms on COPD phenotypes including exacerbation frequency. Respirology. 2017;22(4):684–690. doi:10.1111/resp.12952
65. Lee SW, Hwang HH, Hsu PW, Chuang TY, Liu CW, Wu LS. Whole-genome methylation profiling from PBMCs in acute-exacerbation COPD patients with good and poor responses to corticosteroid treatment. Genomics. 2018. doi:10.1016/j.ygeno.2018.09.010
66. Hillas G, Perlikos F, Tsiligianni I, Tzanakis N. Managing comorbidities in COPD. Int J Chron Obstruct Pulmon Dis. 2015;10:95–109. doi:10.2147/COPD.S54473
67. Gershon AS, Mecredy GC, Guan J, Victor JC, Goldstein R, To T. Quantifying comorbidity in individuals with COPD: a population study. Eur Respir J. 2015;45(1):51–59. doi:10.1183/09031936.00061414
68. Greulich T, Weist BJD, Koczulla AR, et al. Prevalence of comorbidities in COPD patients by disease severity in a German population. Respir Med. 2017;132:132–138. doi:10.1016/j.rmed.2017.10.007
69. Kahnert K, Lucke T, Huber RM, et al. Relationship of hyperlipidemia to comorbidities and lung function in COPD: results of the COSYCONET cohort. PLoS One. 2017;12(5):e0177501. doi:10.1371/journal.pone.0177501
70. Mantoani LC, Dell’Era S, MacNee W, Rabinovich RA. Physical activity in patients with COPD: the impact of comorbidities. Expert Rev Respir Med. 2017;11(9):685–698. doi:10.1080/17476348.2017.1354699
71. Kahnert K, Alter P, Welte T, et al. Uric acid, lung function, physical capacity and exacerbation frequency in patients with COPD: a multi-dimensional approach. Respir Res. 2018;19(1):110. doi:10.1186/s12931-018-0815-y
72. Vanfleteren LE, Spruit MA, Groenen M, et al. Clusters of comorbidities based on validated objective measurements and systemic inflammation in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2013;187(7):728–735. doi:10.1164/rccm.201209-1665OC
73. Mullerova H, Agusti A, Erqou S, Mapel DW. Cardiovascular comorbidity in COPD: systematic literature review. Chest. 2013;144(4):1163–1178. doi:10.1378/chest.12-2847
74. Miller J, Edwards LD, Agusti A, et al. Comorbidity, systemic inflammation and outcomes in the ECLIPSE cohort. Respir Med. 2013;107(9):1376–1384. doi:10.1016/j.rmed.2013.05.001
75. Iversen KK, Kjaergaard J, Akkan D, et al. The prognostic importance of lung function in patients admitted with heart failure. Eur J Heart Fail. 2010;12(7):685–691. doi:10.1093/eurjhf/hfq050
76. Hawkins NM, Huang Z, Pieper KS, et al. Chronic obstructive pulmonary disease is an independent predictor of death but not atherosclerotic events in patients with myocardial infarction: analysis of the Valsartan in Acute Myocardial Infarction Trial (VALIANT). Eur J Heart Fail. 2009;11(3):292–298. doi:10.1093/eurjhf/hfp001
77. Fisher KA, Stefan MS, Darling C, Lessard D, Goldberg RJ. Impact of COPD on the mortality and treatment of patients hospitalized with acute decompensated heart failure: the Worcester Heart Failure Study. Chest. 2015;147(3):637–645. doi:10.1378/chest.14-0607
78. Adamson PD, Anderson JA, Brook RD, et al. Cardiac troponin I and cardiovascular risk in patients with chronic obstructive pulmonary disease. J Am Coll Cardiol. 2018;72(10):1126–1137. doi:10.1016/j.jacc.2018.06.051
79. Kawut SM, Poor HD, Parikh MA, et al. Cor pulmonale parvus in chronic obstructive pulmonary disease and emphysema: the MESA COPD study. J Am Coll Cardiol. 2014;64(19):2000–2009. doi:10.1016/j.jacc.2014.07.991
80. Alter P, Jorres RA, Watz H, et al. Left ventricular volume and wall stress are linked to lung function impairment in COPD. Int J Cardiol. 2018;261:172–178. doi:10.1016/j.ijcard.2018.02.074
81. Alter P, Watz H, Kahnert K, et al. Airway obstruction and lung hyperinflation in COPD are linked to an impaired left ventricular diastolic filling. Respir Med. 2018;137:14–22. doi:10.1016/j.rmed.2018.02.011
82. Barr RG, Bluemke DA, Ahmed FS, et al. Percent emphysema, airflow obstruction, and impaired left ventricular filling. N Engl J Med. 2010;362(3):217–227. doi:10.1056/NEJMoa0808836
83. Dodd DS, Brancatisano T, Engel LA. Chest wall mechanics during exercise in patients with severe chronic air-flow obstruction. Am Rev Respir Dis. 1984;129(1):33–38. doi:10.1164/arrd.1984.129.1.33
84. Stone IS, Barnes NC, James WY, et al. Lung deflation and cardiovascular structure and function in chronic obstructive pulmonary disease. A randomized controlled trial. Am J Respir Crit Care Med. 2016;193(7):717–726. doi:10.1164/rccm.201508-1647OC
85. Hohlfeld JM, Vogel-Claussen J, Biller H, et al. Effect of lung deflation with indacaterol plus glycopyrronium on ventricular filling in patients with hyperinflation and COPD (CLAIM): a double-blind, randomised, crossover, placebo-controlled, single-centre trial. Lancet Respir Med. 2018;6(5):368–378. doi:10.1016/S2213-2600(18)30054-7
86. Vogel-Claussen J, Schonfeld CO, Kaireit TF, et al. Effect of indacaterol/glycopyrronium on pulmonary perfusion and ventilation in hyperinflated patients with Chronic Obstructive Pulmonary Disease (CLAIM). A double-blind, randomized, crossover trial. Am J Respir Crit Care Med. 2019;199(9):1086–1096. doi:10.1164/rccm.201805-0995OC
87. Rutten FH, Cramer MJ, Grobbee DE, et al. Unrecognized heart failure in elderly patients with stable chronic obstructive pulmonary disease. Eur Heart J. 2005;26(18):1887–1894. doi:10.1093/eurheartj/ehi291
88. Triest FJ, Franssen FM, Spruit MA, Groenen MT, Wouters EF, Vanfleteren LE. Poor agreement between chart-based and objectively identified comorbidities of COPD. Eur Respir J. 2015;46(5):1492–1495. doi:10.1183/13993003.00667-2015
89. Rodriguez-Roisin R, Rabe KF, Vestbo J, et al. Global Initiative for Chronic Obstructive Lung Disease (GOLD) 20th Anniversary: a brief history of time. Eur Respir J. 2017;50:1. doi:10.1183/13993003.00711-2017
90. Rabe KF, Hurd S, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007;176(6):532–555. doi:10.1164/rccm.200703-456SO
91. Calverley P, Pauwels R, Vestbo J, et al. Combined salmeterol and fluticasone in the treatment of chronic obstructive pulmonary disease: a randomised controlled trial. Lancet. 2003;361(9356):449–456. doi:10.1016/S0140-6736(03)12459-2
92. Szafranski W, Cukier A, Ramirez A, et al. Efficacy and safety of budesonide/formoterol in the management of chronic obstructive pulmonary disease. Eur Respir J. 2003;21(1):74–81.
93. Vestbo J, Hurd SS, Agusti AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187(4):347–365. doi:10.1164/rccm.201204-0596PP
94. Haughney J, Gruffydd-Jones K, Roberts J, Lee AJ, Hardwell A, McGarvey L. The distribution of COPD in UK general practice using the new GOLD classification. Eur Respir J. 2014;43(4):993–1002. doi:10.1183/09031936.00065013
95. Kopsaftis Z, Wood-Baker R, Poole P. Influenza vaccine for chronic obstructive pulmonary disease (COPD). Cochrane Database Syst Rev. 2018;6:CD002733.
96. Bekkat-Berkani R, Wilkinson T, Buchy P, et al. Seasonal influenza vaccination in patients with COPD: a systematic literature review. BMC Pulm Med. 2017;17(1):79. doi:10.1186/s12890-017-0500-9
97. Walters JA, Smith S, Poole P, Granger RH, Wood-Baker R. Injectable vaccines for preventing pneumococcal infection in patients with chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2010;(11):CD001390.
98. Celli BR, Decramer M, Wedzicha JA, et al. An official American Thoracic Society/European Respiratory Society statement: research questions in COPD. Eur Respir J. 2015;45(4):879–905. doi:10.1183/09031936.00009015
99. Spruit MA, Wouters EFM. Organizational aspects of pulmonary rehabilitation in chronic respiratory diseases. Respirology. 2019. doi:10.1111/resp.13512
100. Spruit MA, Singh SJ, Garvey C, et al. An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med. 2013;188(8):e13–64. doi:10.1164/rccm.201309-1634ST
101. Andrianopoulos V, Klijn P, Franssen FM, Spruit MA. Exercise training in pulmonary rehabilitation. Clin Chest Med. 2014;35(2):313–322. doi:10.1016/j.ccm.2014.02.013
102. Rocha A, Arbex FF, Sperandio PA, et al. Exercise intolerance in comorbid COPD-heart failure: the role of impaired aerobic function. Eur Respir J. 2019;53:1802386. doi:10.1183/13993003.02386-2018
103. Garrod R, Marshall J, Barley E, Jones PW. Predictors of success and failure in pulmonary rehabilitation. Eur Respir J. 2006;27(4):788–794. doi:10.1183/09031936.06.00130605
104. Spruit MA, Augustin IM, Vanfleteren LE, et al. Differential response to pulmonary rehabilitation in COPD: multidimensional profiling. Eur Respir J. 2015;46(6):1625–1635. doi:10.1183/13993003.00350-2015
105. Janssen R, Piscaer I, Franssen FM, Wouters EF. Emphysema: looking beyond alpha-1 antitrypsin deficiency. Expert Rev Respir Med. 2019;13:381–397. doi:10.1080/17476348.2019.1580575
106. van Geffen WH, Slebos DJ, Herth FJ, Kemp SV, Weder W, Shah PL. Surgical and endoscopic interventions that reduce lung volume for emphysema: a systemic review and meta-analysis. Lancet Respir Med. 2019;7(4):313–324. doi:10.1016/S2213-2600(18)30431-4
107. Fishman A, Martinez F, Naunheim K, et al. A randomized trial comparing lung-volume-reduction surgery with medical therapy for severe emphysema. N Engl J Med. 2003;348(21):2059–2073. doi:10.1056/NEJMoa030287
108. Klooster K, Ten Hacken NH, Hartman JE, Kerstjens HA, van Rikxoort EM, Slebos DJ. Endobronchial valves for emphysema without interlobar collateral ventilation. N Engl J Med. 2015;373(24):2325–2335. doi:10.1056/NEJMoa1507807
109. Maier D. Applying systems medicine in the clinic. Curr Opin Syst Biol. 2017;3:77–87. doi:10.1016/j.coisb.2017.04.014
110. Apweiler R, Beissbarth T, Berthold MR, et al. Whither systems medicine? Exp Mol Med. 2018;50(3):e453. doi:10.1038/emm.2017.290
111. Schmeck B, Bertrams W, Lai X, Vera J. Systems medicine for lung diseases: phenotypes and precision medicine in cancer, infection, and allergy. Methods Mol Biol. 2016;1386:119–133. doi:10.1007/978-1-4939-3283-2_8
112. Charron CE, Russell P, Ito K, et al. RV568, a narrow-spectrum kinase inhibitor with p38 MAPK-alpha and -gamma selectivity, suppresses COPD inflammation. Eur Respir J. 2017;50:4. doi:10.1183/13993003.00711-2017
113. Pavord ID, Chanez P, Criner GJ, et al. Mepolizumab for eosinophilic chronic obstructive pulmonary disease. N Engl J Med. 2017;377(17):1613–1629. doi:10.1056/NEJMoa1708208
114. Leung JM, Obeidat M, Sadatsafavi M, Sin DD. Introduction to precision medicine in COPD. Eur Respir J. 2019;53:4. doi:10.1183/13993003.01184-2018
115. Eberhardt M, Lai X, Tomar N, et al. Third-kind encounters in biomedicine: immunology meets mathematics and informatics to become quantitative and predictive. Methods Mol Biol. 2016;1386:135–179. doi:10.1007/978-1-4939-3283-2_9
116. Reyfman PA, Walter JM, Joshi N, et al. Single-cell transcriptomic analysis of human lung provides insights into the pathobiology of pulmonary fibrosis. Am J Respir Crit Care Med. 2018. doi:10.1164/rccm.201712-2410OC
117. Castaldi PJ, Guo F, Qiao D, et al. Identification of functional variants in the FAM13A chronic obstructive pulmonary disease genome-wide association study locus by massively parallel reporter assays. Am J Respir Crit Care Med. 2019;199(1):52–61. doi:10.1164/rccm.201802-0337OC
118. Seimetz M, Parajuli N, Pichl A, et al. Inducible NOS inhibition reverses tobacco-smoke-induced emphysema and pulmonary hypertension in mice. Cell. 2011;147(2):293–305. doi:10.1016/j.cell.2011.08.035
119. Jia J, Conlon TM, Sarker RS, et al. Cholesterol metabolism promotes B-cell positioning during immune pathogenesis of chronic obstructive pulmonary disease. EMBO Mol Med. 2018;10:5. doi:10.15252/emmm.201708349
120. Uhl FE, Vierkotten S, Wagner DE, et al. Preclinical validation and imaging of Wnt-induced repair in human 3D lung tissue cultures. Eur Respir J. 2015;46(4):1150–1166. doi:10.1183/09031936.00183214
121. Yang J, Zuo WL, Fukui T, et al. Smoking-dependent distal-to-proximal repatterning of the adult human small airway epithelium. Am J Respir Crit Care Med. 2017;196(3):340–352. doi:10.1164/rccm.201608-1672OC
122. Gkatzis K, Taghizadeh S, Huh D, Stainier DYR, Bellusci S. Use of three-dimensional organoids and lung-on-a-chip methods to study lung development, regeneration and disease. Eur Respir J. 2018;52:5. doi:10.1183/13993003.01675-2018
123. Benedikter BJ, Volgers C, van Eijck PH, et al. Cigarette smoke extract induced exosome release is mediated by depletion of exofacial thiols and can be inhibited by thiol-antioxidants. Free Radic Biol Med. 2017;108:334–344. doi:10.1016/j.freeradbiomed.2017.03.026
124. Agusti A, Compte A, Faner R, et al. The EASI model: A first integrative computational approximation to the natural history of COPD. PLoS One. 2017;12(10):e0185502. doi:10.1371/journal.pone.0185502
125. Loeppky JA, Icenogle MV, Caprihan A, Vidal Melo MF, Altobelli SA. CO2 rebreathing model in COPD: blood-to-gas equilibration. Eur J Appl Physiol. 2006;98(5):450–460. doi:10.1007/s00421-006-0288-4
126. Cox LAT. A mathematical model of protease-antiprotease homeostasis failure in chronic obstructive pulmonary disease (COPD). Risk Anal. 2009;29(4):576–586. doi:10.1111/j.1539-6924.2008.01152.x
127. Cox LAT. A causal model of chronic obstructive pulmonary disease (COPD) risk. Risk Anal. 2011;31(1):38–62. doi:10.1111/j.1539-6924.2010.01487.x
128. Jolley CJ, Moxham J. A physiological model of patient-reported breathlessness during daily activities in COPD. Eur Respir Rev. 2009;18(112):66–79. doi:10.1183/09059180.00000809
129. Zhang B, Qi S, Yue Y, et al. Particle disposition in the realistic airway tree models of subjects with tracheal bronchus and COPD. Biomed Res Int. 2018;2018:7428609.
130. De Backer LA, Vos W, De Backer J, Van Holsbeke C, Vinchurkar S, De Backer W. The acute effect of budesonide/formoterol in COPD: a multi-slice computed tomography and lung function study. Eur Respir J. 2012;40(2):298–305. doi:10.1183/09031936.00072511
131. De Backer JW, Vanderveken OM, Vos WG, et al. Functional imaging using computational fluid dynamics to predict treatment success of mandibular advancement devices in sleep-disordered breathing. J Biomech. 2007;40(16):3708–3714. doi:10.1016/j.jbiomech.2007.06.022
132. Burrowes KS, Doel T, Brightling C. Computational modeling of the obstructive lung diseases asthma and COPD. J Transl Med. 2014;12(Suppl 2):S5. doi:10.1186/1479-5876-12-S2-S5
133. Burrowes KS, De Backer J, Smallwood R, et al. Multi-scale computational models of the airways to unravel the pathophysiological mechanisms in asthma and chronic obstructive pulmonary disease (AirPROM). Interface Focus. 2013;3(2). doi:10.1098/rsfs.2012.0057.
134. Hiorns JE, Jensen OE, Brook BS. Static and dynamic stress heterogeneity in a multiscale model of the asthmatic airway wall. J Appl Physiol. 2016;121(1):233–247. doi:10.1152/japplphysiol.00715.2015
135. Bordas R, Lefevre C, Veeckmans B, et al. Development and analysis of patient-based complete conducting airways models. PLoS One. 2015;10(12):e0144105. doi:10.1371/journal.pone.0144105
136. Walters M, Wells AK, Jones IP, et al. Patient-specific simulation of tidal breathing.
137. Chernyavsky IL, Russell RJ, Saunders RM, et al. In vitro, in silico and in vivo study challenges the impact of bronchial thermoplasty on acute airway smooth muscle mass loss. Eur Respir J. 2018;51(5):1701680. doi:10.1183/13993003.01680-2017
138. Marín de Mas I, Fanchon E, Papp B, Kalko S, Roca J, Cascante M. Molecular mechanisms underlying COPD-muscle dysfunction unveiled through a systems medicine approach. Bioinformatics. 2017;33(1):95–103.
139. Cano I, Roca J, Wagner PD. Effects of lung ventilation-perfusion and muscle metabolism-perfusion heterogeneities on maximal O2 transport and utilization. J Physiol (Lond). 2015;593(8):1841–1856. doi:10.1113/jphysiol.2014.286492
140. Selivanov VA, Cascante M, Friedman M, Schumaker MF, Trucco M, Votyakova TV. Multistationary and oscillatory modes of free radicals generation by the mitochondrial respiratory chain revealed by a bifurcation analysis. PLoS Comput Biol. 2012;8(9):e1002700. doi:10.1371/journal.pcbi.1002700
141. Selivanov VA, Votyakova TV, Pivtoraiko VN, et al. Reactive oxygen species production by forward and reverse electron fluxes in the mitochondrial respiratory chain. PLoS Comput Biol. 2011;7(3):e1001115. doi:10.1371/journal.pcbi.1002244
142. Clarke K, Ricciardi S, Pearson T, et al. The role of Eif6 in skeletal muscle homeostasis revealed by endurance training co-expression networks. Cell Rep. 2017;21(6):1507–1520. doi:10.1016/j.celrep.2017.10.040
143. Esteva A, Kuprel B, Novoa RA, et al. Dermatologist-level classification of skin cancer with deep neural networks. Nature. 2017;542:115–118. doi:10.1038/nature21056
144. Abràmoff MD, Lavin PT, Birch M, Shah N, Folk JC. Pivotal trial of an autonomous AI-based diagnostic system for detection of diabetic retinopathy in primary care offices. NPJ Digit Med. 2018;1:39. doi:10.1038/s41746-018-0040-6
145. Bellos CC, Papadopoulos A, Rosso R, Fotiadis DI. Identification of COPD patients’ health status using an intelligent system in the CHRONIOUS wearable platform. IEEE J Biomed Health Inform. 2014;18:731–738. doi:10.1109/JBHI.2013.2293172
146. Er O, Sertkaya C, Temurtas F, Tanrikulu AC. A comparative study on chronic obstructive pulmonary and pneumonia diseases diagnosis using neural networks and artificial immune system. J Med Syst. 2009;33:485–492.
147. Er O, Yumusak N, Temurtas F. Chest diseases diagnosis using artificial neural networks. Expert Syst Appl. 2010;37:7648–7655. doi:10.1016/j.eswa.2010.04.078
148. Fernández-Granero MA, Sánchez-Morillo D, León-Jiménez A, Crespo LF. Automatic prediction of chronic obstructive pulmonary disease exacerbations through home telemonitoring of symptoms. Biomed Mater Eng. 2014;24:3825–3832. doi:10.3233/BME-141212
149. Deep Neural Networks for Prediction of Exacerbations of Patients with Chronic Obstructive Pulmonary Disease. Cham: Springer International Publishing; 19th International Conference, EANN 2018, September 3-5, 2018; Bristol, UK.
150. van der Heijden M, Lucas PJF, Lijnse B, Heijdra YF, Schermer TRJ. An autonomous mobile system for the management of COPD. J Biomed Inform. 2013;46:458–469. doi:10.1016/j.jbi.2013.03.003
151. Ying J, Dutta J, Guo N, et al. Classification of exacerbation frequency in the COPDGene cohort using deep learning with deep belief networks. IEEE J Biomed Health Inform. 2016;1. doi:10.1109/JBHI.2016.2642944
152. Amalakuhan B, Kiljanek L, Parvathaneni A, Hester M, Cheriyath P, Fischman D. A prediction model for COPD readmissions: catching up, catching our breath, and improving a national problem. J Community Hosp Intern Med Perspect. 2012;2(1).
153. Raudys SJ, Jain AK. Small sample size effects in statistical pattern recognition: recommendations for practitioners. IEEE Trans Pattern Anal Mach Intell. 1991;13:252–264. doi:10.1109/34.75512
154. Gurbeta L, Badnjevic A, Maksimovic M, Omanovic-Miklicanin E, Sejdic E. A telehealth system for automated diagnosis of asthma and chronical obstructive pulmonary disease. J Am Med Inform Assoc. 2018;25(9):1213–1217. doi:10.1093/jamia/ocy055
155. Badnjevic A, Cifrek M, Koruga D, Osmankovic D. Neuro-fuzzy classification of asthma and chronic obstructive pulmonary disease. BMC Med Inform Decis Mak. 2015;15(Suppl 3):S1. doi:10.1186/1472-6947-15-S3-S1
156. Amaral JLM, Lopes AJ, Jansen JM, Faria ACD, Melo PL. Machine learning algorithms and forced oscillation measurements applied to the automatic identification of chronic obstructive pulmonary disease. Comput Methods Programs Biomed. 2012;105:183–193. doi:10.1016/j.cmpb.2011.09.009
157. Amaral JLM, Lopes AJ, Faria ACD, Melo PL. Machine learning algorithms and forced oscillation measurements to categorise the airway obstruction severity in chronic obstructive pulmonary disease. Comput Methods Programs Biomed. 2015;118:186–197. doi:10.1016/j.cmpb.2014.11.002
158. Badnjevic A, Gurbeta L, Custovic E. An expert diagnostic system to automatically identify asthma and chronic obstructive pulmonary disease in clinical settings. Sci Rep. 2018;8:11645. doi:10.1038/s41598-018-30116-2
159. Spathis D, Vlamos P. Diagnosing asthma and chronic obstructive pulmonary disease with machine learning. Health Informatics J. 2017;146045821772316. doi:10.1177/1460458217723169
160. Topalovic M, Laval S, Aerts J-M, Troosters T, Decramer M, Janssens W. Automated interpretation of pulmonary function tests in adults with respiratory complaints. Respiration. 2017;93:170–178. doi:10.1159/000454956
161. Bodduluri S, Newell JD
162. Classification of COPD with Multiple Instance Learning. IEEE; 2014.22nd International Conference on Pattern Recognition, Stockholm, Sweden
163. Mets OM, Buckens CFM, Zanen P, et al. Identification of chronic obstructive pulmonary disease in lung cancer screening computed tomographic scans. JAMA. 2011;306:1775–1781. doi:10.1001/jama.2011.1531
164. Murphy K, Pluim JPW, van Rikxoort EM, et al. Toward automatic regional analysis of pulmonary function using inspiration and expiration thoracic CT. Med Phys. 2012;39:1650–1662. doi:10.1118/1.3687891
165. Sørensen L, Loog M, Lo P, et al. Image dissimilarity-based quantification of lung disease from CT.
166. Sørensen L, Nielsen M, Lo P, Ashraf H, Pedersen JH, de Bruijne M. Texture-based analysis of COPD: A data-driven approach. IEEE Trans Med Imaging. 2012;31:70–78. doi:10.1109/TMI.2011.2164931
167. Van Rikxoort EM, de Jong PA, Mets OM,van Ginneken B. Automatic classication of pulmonary function in COPD patients using trachea analysis in chest CT scans. SPIE Medical Imaging. 2012; 8315: MI. doi.org/10.1117/12.911603
168. Kuncheva LI, Rodriguez JJ, Syed YI, Phillips CO, Lewis KE. Classifier ensemble methods for diagnosing COPD from volatile organic compounds in exhaled air. Int J Knowledge Discovery Bioinf. 2012;3:1–15. doi:10.4018/jkdb.2012040101
169. Mieloszyk RJ, Verghese GC, Deitch K, et al. Automated quantitative analysis of capnogram shape for COPD–normal and COPD–CHF classification. IEEE Trans Biomed Eng. 2014;61:2882–2890. doi:10.1109/TBME.2014.2332954
170. Phillips CO, Syed Y, Parthaláin NM, Zwiggelaar R, Claypole TC, Lewis KE. Machine learning methods on exhaled volatile organic compounds for distinguishing COPD patients from healthy controls. J Breath Res. 2012;6:036003. doi:10.1088/1752-7155/6/3/036003
171. Van Berkel JJBN, Dallinga JW, Möller GM, et al. A profile of volatile organic compounds in breath discriminates COPD patients from controls. Respir Med. 2010;104:557–563. doi:10.1016/j.rmed.2009.10.018
172. Bermejo-Peláez D, Estepar RSJ, Ledesma-Carbayo MJ. Emphysema classification using a multi-view convolutional network. 2018 IEEE 15th International Symposium on Biomedical Imaging (ISBI 2018), Washington, DC;2018;519–522. doi:10.1109/ISBI.2018.8363629
173. Coppini G, Miniati M, Paterni M, Monti S, Ferdeghini EM. Computer-aided diagnosis of emphysema in COPD patients: neural-network-based analysis of lung shape in digital chest radiographs. Med Eng Phys. 2007;29:76–86. doi:10.1016/j.medengphy.2006.02.001
174. Friman O, Borga M, Lundberg M, Tylen U, Knutsson H. Recognizing emphysema - a neural network approach. Object recognition supported by user interaction for service robots. Quebec City, Quebec; 2002;1: 512-515. doi:10.1109/ICPR.2002.1044781.
175. Karabulut EM and Ibrikci T. Emphysema discrimination from raw HRCT images by convolutional neural networks. 9th International Conference on Electrical and Electronics Engineering (ELECO), Bursa, 2015, pp. 705-708. doi:10.1109/ELECO.2015.7394441
176. Mendoza CS, Washko GR, Ross JC, et al. Emphysema quantificaion in a multi-scanner HRCT cohort using local intensity distributions.
177. Prasad M, Sowmya A, Wilson P. Multi-level classification of emphysema in HRCT lung images. Pattern Anal Appl. 2009;12:9–20. doi:10.1007/s10044-007-0093-7
178. Sørensen L, Shaker SB, de Bruijne M. Quantitative analysis of pulmonary emphysema using local binary patterns. IEEE Trans Med Imaging. 2010;29:559–569.
179. Xu Y, Sonka M, McLennan G, Guo J, Hoffman EA. MDCT-based 3-D texture classification of emphysema and early smoking related lung pathologies. IEEE Trans Med Imaging. 2006;25:464–475. doi:10.1109/TMI.2006.870889
180. Fernandez-Granero MA, Sanchez-Morillo D, Leon-Jimenez A. Computerised analysis of telemonitored respiratory sounds for predicting acute exacerbations of COPD. Sensors. 2015;15:26978–26996. doi:10.3390/s151026978
181. Fernandez-Granero MA, Sanchez-Morillo D, Leon-Jimenez A. An artificial intelligence approach to early predict symptom-based exacerbations of COPD. Biotechnol Biotechnol Equip. 2018;32:778–784. doi:10.1080/13102818.2018.1437568
182. Mohktar MS, Redmond SJ, Antoniades NC, et al. Predicting the risk of exacerbation in patients with chronic obstructive pulmonary disease using home telehealth measurement data. Artif Intell Med. 2015;63:51–59. doi:10.1016/j.artmed.2014.12.003
183. McHeick H, Saleh L, Ajami H, Mili H. Context relevant prediction model for COPD domain using bayesian belief network. Sensors (Basel). 2017;17(7). doi:10.3390/s17050968
184. He H, Sun Y, Sun B, Zhan Q. Application of a parametric model in the mortality risk analysis of ICU patients with severe COPD. Clin Respir J. 2018;12(2):491–498. doi:10.1111/crj.12549
185. Moore E, Chatzidiakou L, Jones RL, et al. Linking e-health records, patient-reported symptoms and environmental exposure data to characterise and model COPD exacerbations: protocol for the COPE study. BMJ Open. 2016;6(7):e011330. doi:10.1136/bmjopen-2016-011330
186. Risebrough NA, Briggs A, Baker TM, et al. Validating A model to predict disease progression outcomes in patients with COPD. Value Health. 2014;17(7):A560–A561. doi:10.1016/j.jval.2014.08.1852
187. Briggs AH, Baker T, Risebrough NA, et al. Development of the Galaxy Chronic Obstructive Pulmonary Disease (COPD) model using data from ECLIPSE: internal validation of a linked-equations cohort model. Med Decis Making. 2017;37(4):469–480. doi:10.1177/0272989X16653118
188. Hoogendoorn M, Corro Ramos I, Baldwin M, Gonzalez-Rojas Guix N, Rutten-van Molken M. Broadening the perspective of cost-effectiveness modeling in chronic obstructive pulmonary disease: a new patient-level simulation model suitable to evaluate stratified medicine. Value Health. 2019;22(3):313–321. doi:10.1016/j.jval.2018.10.008
189. Altenburg WA, Bossenbroek L, de Greef MHG, Kerstjens HAM, Ten Hacken NHT, Wempe JB. Functional and psychological variables both affect daily physical activity in COPD: a structural equations model. Respir Med. 2013;107(11):1740–1747. doi:10.1016/j.rmed.2013.06.002
190. Nwaru BI, Simpson CR, Sheikh A, Kotz D. External validation of a COPD prediction model using population-based primary care data: a nested case-control study. Sci Rep. 2017;7:44702. doi:10.1038/srep44702
191. Kotz D, Simpson CR, Viechtbauer W, van Schayck OCP, Sheikh A. Development and validation of a model to predict the 10-year risk of general practitioner-recorded COPD. NPJ Prim Care Respir Med. 2014;24:14011. doi:10.1038/npjpcrm.2014.11
192. Lode H, Allewelt M, Balk S, et al. A prediction model for bacterial etiology in acute exacerbations of COPD. Infection. 2007;35(3):143–149. doi:10.1007/s15010-007-6078-z
193. Dal Negro RW, Micheletto C, Tognella S, Visconti M, Guerriero M, Sandri MF. A two-stage logistic model based on the measurement of pro-inflammatory cytokines in bronchial secretions for assessing bacterial, viral, and non-infectious origin of COPD exacerbations. COPD. 2005;2(1):7–16.
194. Erdemir A, Hunter PJ, Holzapfel GA, et al. Perspectives on sharing models and related resources in computational biomechanics research. J Biomech Eng. 2018;140(2):024701. doi:10.1115/1.4038768
195. Schneeweiss S, Eichler HG, Garcia-Altes A, et al. Real world data in adaptive biomedical innovation: a framework for generating evidence fit for decision-making. Clin Pharmacol Ther. 2016;100(6):633–646. doi:10.1002/cpt.512
196. Ryan D, Blakey J, Chisholm A, et al. Use of electronic medical records and biomarkers to manage risk and resource efficiencies. Eur Respir J. 2017;4(1):1293386. doi:10.1080/20018525.2017.1293386
197. Agusti A, MacNee W. The COPD control panel: towards personalised medicine in COPD. Thorax. 2013;68(7):687–690. doi:10.1136/thoraxjnl-2012-202772
198. Mattila J, Koikkalainen J, Virkki A, van Gils M, Lötjönen J. Design and application of a generic clinical decision support system for multiscale data. IEEE Trans Biomed Eng. 2012;59(1):234–240. doi:10.1109/TBME.2011.2170986
199. Sim I, Gorman P, Greenes RA, et al. Clinical decision support systems for the practice of evidence-based medicine. J Am Med Inform Assoc. 2001;8(6):527–534.
200. Burgos F, Melia U, Vallverdú M, et al. Clinical decision support system to enhance quality control of spirometry using information and communication technologies. JMIR Med Inform. 2014;2(2):e29. doi:10.2196/medinform.3179
201. Roca J, Cano I, Gomez-Cabrero D, Tegnér J. From systems understanding to personalized medicine: lessons and recommendations based on a multidisciplinary and translational analysis of COPD. Methods Mol Biol. 2016;1386:283–303. doi:10.1007/978-1-4939-3283-2_13
202. Velickovski F, Ceccaroni L, Roca J, et al. Clinical Decision Support Systems (CDSS) for preventive management of COPD patients. J Transl Med. 2014;12(Suppl 2):S9. doi:10.1186/1479-5876-12-S2-S9
203. Goldman AW, Burmeister Y, Cesnulevicius K, et al. Bioregulatory systems medicine: an innovative approach to integrating the science of molecular networks, inflammation and systems biology with the patient’s autoregulatory capacity? Front Physiol. 2015;6:225. doi:10.3389/fphys.2015.00098
204. Gustafson C, Kara N. Fitzgerald, ND: case reports-informing the practice of systems medicine. Integr Med (Encinitas). 2015;14(6):36–39.
205. Belard A, Buchman T, Forsberg J, et al. Precision diagnosis: a view of the clinical decision support systems (CDSS) landscape through the lens of critical care. J Clin Monit Comput. 2017;31(2):261–271. doi:10.1007/s10877-016-9849-1
206. McGinn T. Putting meaning into meaningful use: a roadmap to successful integration of evidence at the point of care. JMIR Med Inform. 2016;4(2):e16. doi:10.2196/medinform.5853
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.