Back to Journals » Pharmacogenomics and Personalized Medicine » Volume 16
Personalized Dietary Regimens for Inflammatory Bowel Disease: Current Knowledge and Future Perspectives
Authors Wellens J , Vissers E, Matthys C, Vermeire S, Sabino J
Received 23 August 2022
Accepted for publication 6 January 2023
Published 12 January 2023 Volume 2023:16 Pages 15—27
DOI https://doi.org/10.2147/PGPM.S359365
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Martin H Bluth
Judith Wellens,1,2 Eva Vissers,1 Christophe Matthys,3,4 Séverine Vermeire,1,2 João Sabino1,2
1KU Leuven Department of Chronic Diseases and Metabolism, Translational Research Center for Gastrointestinal Disorders (TARGID), Leuven, Belgium; 2Department of Gastroenterology and Hepatology, University Hospitals Leuven, Leuven, Belgium; 3Clinical Nutrition Unit, Department of Endocrinology, University Hospitals Leuven, Leuven, Belgium; 4KU Leuven Department of Chronic Diseases and Metabolism, Clinical and Experimental Endocrinology, Leuven, Belgium
Correspondence: João Sabino, Email [email protected]
Abstract: Inflammatory bowel diseases (IBD) are chronic and incurable conditions of the gastro-intestinal tract with an increasing incidence and prevalence worldwide. Common symptoms are abdominal pain, diarrhea, and weight loss. Despite recent advances in medical management, many patients fail to achieve clinical remission and healing of the mucosa of the bowel. The cause is thought to involve an inappropriate reaction of the immune system, the microbiome and the environment in genetically susceptible individuals, leading to chronic bowel inflammation. Evidence is emerging that diet is a key environmental factor that might influence disease onset and course, and therefore may become a therapeutic strategy to mitigate inflammation and symptoms. Since IBD is a heterogeneous disease on a clinical and a molecular level, personalizing dietary advice could be the crucial factor to achieve long-lasting changes in dietary behaviors that could not only improve nutritional status but also tackle gut inflammation and abdominal symptoms on an individual level. In this review, we first discuss different aspects of personalized nutrition, namely the level, focus, and scope of personalized dietary regimens. Then, we provide a framework for the different goals of nutritional therapy in IBD and current evidence for personalized dietary approaches. Lastly, we discuss the need for adequate trial designs, access to the right data types and the bioinformatic tools that are necessary to develop algorithms that will allow us to move from general “healthy eating” advice to truly personalized nutritional plans for the individual IBD patient.
Keywords: inflammatory bowel disease, precision nutrition, nutritional therapy, personalized nutrition
Introduction
Inflammatory bowel diseases (IBD) are chronic and incurable inflammatory conditions of the gastrointestinal tract encompassing two main clinical entities: Crohn’s disease (CD) and ulcerative colitis (UC).1 Both conditions are thought to originate from an inappropriate immune response to microbial and/or environmental factors in a genetically susceptible host.1 The stunning rise in incidence of IBD in Asian countries and the Middle East suggests that lifestyle and dietary changes towards a “Westernized diet” play an important role in disease pathogenesis. The Westernized diet is hallmarked by an increased consumption of animal fat, red and processed meat, and a reduced intake of fibre.2–4 Indeed, a high dietary intake of total fats, polyunsaturated fatty acids (PUFAs), omega-6 fatty acids, and meat has been associated with an increased risk of CD and UC in observational studies.5 It should, however, be noted that many associations have been inconsistent, which is possibly due to poor trial design, differences in study populations or retrospective designs that suffer from recall bias.6
Importantly, besides these broad categories of nutritional elements, there is also the indisputable rise in ultra-processed food (UPF) consumption, which has recently been associated with an increase in incident cases of persons with IBD, and particularly CD.7–9
Emulsifiers are a category of food additives commonly used in UPFs to improve food texture, palatability, and shelf life. Emulsifiers have been associated with IBD, both in animal and laboratory studies.10 Polysorbates for example affect permeability of epithelial cell cultures,11 and induce intestinal inflammation in susceptible mice.10,12 For other emulsifiers such as carrageenan, administration through drinking water in guinea pigs resulted in colonic ulcerations resembling the ulcerations seen in humans. In colonic cell lines, this emulsifier induced tumor necrosis factor (TNF) production.13,14 Moreover, a study assessing the effect of carboxymethyl cellulose (CMC) in healthy volunteers found a perturbation in the faecal microbiota with a reduced diversity and changes in the metabolome with, amongst other changes, a decrease in short chain fatty acids.15 Interestingly, although this was a small study, clear intersubject variability in response to CMC was observed, with encroachment of microbiota in the gut epithelium in some, but not all, participants.
Strategies to mitigate the detrimental effect of emulsifiers might include the use of plantain fibre such as broccoli that showed to reduce translocation of E. coli across bowel-specific M-cells in human cell cultures, thereby effectively counteracting the effects of polysorbate-80.16 Moreover, subanalyses of large randomized controlled trials on the effects of fibre in IBD patients have shown improvements in inflammatory markers and a more beneficial composition of the microbiota.17 These examples illustrate that dietary habits, combined with a personal propensity to develop disease, might play a role in disease onset and propagation of the inflammation through the interaction with the gut barrier.
Therefore, dietary strategies to prevent and reverse these detrimental effects and contribute to therapeutic management of IBD are needed, preferentially in a personalized way.
In this review, we will discuss the current knowledge and future perspectives of personalized dietary therapy in IBD, as well as the research avenues that need to be explored in order to get there.
What are Personalized Dietary Regimens?
Personalized nutrition is an approach that uses information on individual characteristics such as age, insulin sensitivity or the gut microbiota to develop targeted nutritional advice to assist patients in achieving a long-lasting and beneficial change in dietary behavior.18 This is partly based on the concept that individualized nutritional advice will be more effective than the traditional generic approaches in achieving long-term lifestyle changes.18 The increased initial and sustained dietary changes that can be realized are probably due to the more personal approach which might improve motivation. Furthermore, this personal advice has proven to outperform the conventional “one size fits all” approach even when provided through an internet-delivered intervention.19 Precision nutrition goes one step further and postulates that we can offer individual dietary advice that is known to be individually beneficial, based on a quantitative understanding of the relationship between the individual, phenotype, and food consumption.18 In order to implement these concepts, emerging -omic technologies will continue to gain importance and may aid in decision-making. Two important players are nutrigenetics that try to understand the different phenotypic responses to a specific diet, depending on the individual genotype, and nutrigenomics that focus on how nutrients might affect gene expression.18,20
Furthermore, there are three aspects of personalized dietary regimens or personalized nutrition that need to be considered: 1) the level of personalization, 2) the focus of personalization, and 3) the scope of personalization (Figure 1).
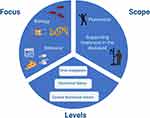 |
Figure 1 Aspects of personalized nutrition. Created with Biorender.com. |
The levels of personalization are again threefold.18 On the first or ground level, we find the conventional nutritional advice that is based on general guidelines for population groups by age and gender. This coincides with the traditional focus of nutritional sciences on the average response of a population to a certain diet or nutrient, and could be considered as primary prevention interventions.20 On the second level, we add a layer of individualization by adding phenotypic information about the individual’s nutritional status (such as biochemical and anthropometric data). At the third level, we aim to reach a level of personalized (or precision) nutrition that takes several aspects into account, such as the genotype, the gut microbiota or the metabolome. Similarly to drugs, nutrients have the ability to interact with and modulate molecular mechanisms underlying an organism’s physiological functions, and therefore have the potential to aid in individually tailored dietary advice.20
This brings us to the focus of personalized nutrition: biology or behavior. In precision medicine, we aim to understand the differential responses to diets and nutrients based on genetic, epigenetic, and gut microbial profiles, amongst others. This degree of biological understanding can also guide nutritional advice. For example, improved understanding of how and which specific nutrients and non-nutritional components might induce an intestinal inflammatory response when meeting specific strains of gut bacteria, might be key to personal advice in IBD. However, making changes to one’s dietary pattern should include a thorough assessment of current behavior, preferences, barriers, and objectives.18 In IBD, a patient can opt for a treatment with infliximab, which requires him or her to attend the infusion center every 8 weeks. It is needless to say that this eight-weekly “reminder” of treatment (and perhaps disease), is very different to adhering to a specific diet, that requires daily and continuous attention and effort from the patient. Besides, and contrary to medical treatment regimens, many processes other than intention drive hunger and energy intake and should thus be taken into account when designing a nutritional plan. In that regard, the PREDICT study showed that postprandial glucose dips predicted an increase in hunger, a shorter time until the next meal, and greater 24-hour energy intake in the general population.21 In addition, as eating behaviors are often embedded within a social environment, peer-supported interventions could be considered to encourage dietary behavioral changes.22
The last aspect of personalized nutrition is its scope. In addition to the different levels of personalization, the scope can be to address emerging problems of public health care, and prevention of disease, or can be aimed at supporting treatment in the diseased. In this review, we will primarily focus on personalized nutrition as an aid in the treatment of IBD, rather than its prevention.
Goals of Nutritional Therapy in IBD
Assess the Nutritional Status and Avoid Nutritional Deficiencies
Inflammation as seen in IBD can contribute to weight loss and malnutrition through inflammation-associated anorexia with decreased food intake, elevation of resting energy expenditure, and increased muscle catabolism. In addition, IBD-related factors, such as oral ulcers, diarrhea, bowel resections, and malabsorption, also contribute to the development of malnutrition.23,24
Therefore, IBD patients should be routinely screened for malnutrition using validated tools such as the Nutritional Risk Screening 200225 or the Malnutrition Universal Screening Tool,26 and when indicated, undergo a diagnostic assessment, as described by the global clinical nutrition community (GLIM) criteria.23 This assessment requires one positive etiological criterion (already met through the presence of the inflammatory condition), and one phenotypical criterion, which can be “non-volitional weight loss”, “low body mass index (BMI)”, or “reduced muscle mass”.23 It is important to note that low BMI can be a criterion, but that a malnourished phenotype can be present irrespective of body morphology: lean, normal, or obese.
In addition to malnourishment due to the inflammatory aspect of the disease, many IBD patients display a high prevalence of food avoidance (28–89%) and restrictive dietary behaviors (41–93%), and experience a profound impact on their food-related quality of life (QoL).27,28
This puts patients further at risk to develop nutritional deficiencies both in active and quiescent disease, as has previously been shown for vitamin C, copper, niacin, and zinc, among others.24,29
Taken together, when malnutrition is observed, and the diagnosis ascertained, the severity can be graded and complemented by a comprehensive assessment to provide an individual care plan to meet nutritional needs. For example, when a specific nutrient deficiency is noted such as iron, vitamin C or folic acid deficiency, this can be supplemented through medication or dietary alterations. The first goal of a personalized dietary regimen should therefore be the following: assess the nutritional status and avoid nutritional deficiencies (Figure 2). Of course, this first goal should already be part of the general IBD care plan, apart from any personalized dietary strategies. Other aspects of general nutritional care in IBD are outside the scope of this review and are excellently summarized by ESPEN.30
 |
Figure 2 Goals of nutritional therapy in IBD. Created with Biorender.com. |
Treat the Inflammation, Diet as a Leverage
Clearly, the inflammatory burden is an important factor in driving malnutrition, nutritional deficiencies, and (food-related) well-being.
First and foremost, the inflammatory burden of IBD should be treated lege artis following international guidelines, since malnutrition has been associated with many complications in IBD, including prolonged hospitalization, infection, greater need for surgery, development of venous thromboembolism, post-operative complications, and mortality.31–33 However, many patients only achieve partial control of their disease, and the question arises if dietary adjustments might leverage their response to treatment and aid in controlling the inflammatory burden.
As discussed earlier, IBD is a complex entity, encompassing derangements in inflammatory pathways that are driven by genetic predisposition, but also environmental factors and the gut microbiota. This would suggest that potential benefit can be reaped from combining anti-inflammatory drugs with environmental and/or gut microbial interventions. Such a therapeutic approach is in line (yet different) with the current trend in IBD towards combination of advanced therapies (ie biologics and small molecules), which remain hindered by safety concerns and immense costs.34
Moreover, the potential of dietary interventions to modulate inflammation in IBD is increasingly accepted, and already adopted in the standard of care in induction therapy for paediatric CD.35,36
Personalized diets have been studied in IBD (such as various IgG and IgG4 exclusion diets and symptom-based diets), but unfortunately these studies were of low overall quality or yielded negative results in terms of inflammation control.35,37,38
Other studies, although without a clear individualized approach, tested dietary regimens specifically designed for IBD and have shown to be successful in inducing or maintaining remission (with improvement in symptoms combined with positive endoscopy results or inflammatory markers).35 Most notably, the CDED (Crohn Disease Exclusion Diet), CD-TREAT (Treatment-with-Eating Diet), IBD-AID (Inflammatory Bowel Disease-Anti-Inflammatory Diet), and the Autoimmune Protocol Diet (AIP) have been studied with positive results in small (pilot) trials and warrant further investigation before translation to the clinic can be pursuit.39–42
These types of diets typically eliminate many food groups and/or ingredients which complicates long-term adherence and sustainability. This is reminiscent of the better-known low Fermentable Oligo-, Di-, Monosaccharides and Polyols (FODMAP) diet for IBS patients, where FODMAP intake is dramatically reduced in the first 4- to 8-week restriction period, after which FODMAP containing foods are reintroduced as dietary challenges with the opportunity to personalize guided by gastrointestinal symptoms.43 After this reintroduction period of 6–10 weeks, successfully challenged FODMAP-containing foods that did not provoke symptoms, can be consumed over the long term to increase dietary variety. Similarly, this type of approach could be applied for IBD-, and gut inflammation-targeted interventions in the design of a personalized nutrition plan; howeverto date, this has not been adopted in current IBD-focused dietary trials.
On the other hand, the relative lack of overlap between these diets, showcased by contradicting ingredients or nutrients that are encouraged or avoided in these trials,44 is the perfect example of the complexity of dietary interventions. For example, the Mediterranean diet promotes the intake of vegetables, fruits, breads, cereals and legumes, whereas the AIP dictates to avoid grains and legumes, and the CD-TREAT and CDED both exclude gluten. Furthermore, the AIP excludes eggs and dairy products, whereas the Mediterranean diet promotes dairy foods and the CDED entails a mandatory consumption of eggs as part of the diet. Moreover, the list of “permitted” foods is often rather short, making it difficult for patients to adhere to these diets for an extended period of time due to taste fatigue and social aspects. It would be interesting to assess both patients who responded and those who did not respond to the diet. As in drug trials, often the reason for non-response remains elusive and is associated with more severe disease phenotypes or prior use of biologics, but understanding the underlying mechanisms here might give us a hint towards personalized nutrition advice. Until then, it is still unclear if a one-size-fits all approach might be a realistic (or even preferred) goal, as various potentially effective diets offer an opportunity to personalize the dietary strategy based on patient preferences and biological reasons, once they have been fully understood. Of course, this could only be considered after correcting the nutritional status if needed and not in special situations such as stricturing disease, short bowel syndrome, an ileostomy in CD, or a pouch in UC, which would require more specific approaches or adaptations to the existing diets.
In the era of disease modification and search for more individualized treatments, biomarkers related to (epi)genetic information and gene expression are being validated to predict response or non-response to medical treatment.45 Similarly, as genes and molecular pathways are involved in the nutrient uptake, the daily patient-specific dietary requirements, and the metabolism of nutrients differ between individuals, these could theoretically be used to predict the efficacy of a dietary intervention or dietary deficiencies. Insights in different -omic levels from genomics to metabolomics (which encompasses the host metabolic response to the environment, taking into account the influence of the gut microbiota as well) will be key in developing precision nutritional approaches in IBD.
As an example, the intake of PUFAs might induce gut inflammation and worsen the course of CD, as shown in mice models, in human CD epithelial organoids and in two human cohort studies.46 Interestingly, an increase in interleukin-8 and TNF expression was only observed in organoids from patients with CD with impaired glutathione peroxidase 4 (GPX4) expression. Together with conflicting data from prospective cohort and randomized controlled trials, this suggests that the inflammatory potential of PUFAs depends not only on crude intake of PUFAs but also on the genetic profile of the host.47 However, this hypothesis needs further exploration, and to our knowledge, a nutritional intervention trial based on genetic makeup of individual patients has not been set up to date. Furthermore, PUFAs are essential nutrients, implying that the therapeutic window of opportunity to vastly modify PUFA-intake, might be rather limited.
Thus, treating the inflammation, with diet as a leverage should be the second goal of nutritional treatment in IBD.
Treat the Symptoms: Want or Need?
Many IBD patients report avoiding certain foods or adhering to an exclusive diet, believing in beneficial effects on symptom severity and inflammatory episodes. Unfortunately, the dietary choices leading towards the newly adopted dietary patterns are often based on personal experience of symptoms, advice from other patients, popular diet books or the internet, which might lead to or exacerbate nutritional deficiencies.48–50
Although dietary fiber intake is safe in IBD patients (if overt gastrointestinal obstruction has been excluded), fibers are such a food component that is often avoided by patients due to increased abdominal symptoms.17 Dietary fibers are not digested by the human host, but rather fermented by the gut microbiota.51
There are various subtypes of dietary fibers described to date, categorized as soluble or insoluble, with varying chemical structures, and large differences in their fermentation profiles and thus potential (beneficial) effects on human health.51 Pectin, for example, is a complex polysaccharide and a soluble fiber that can be found in the cell wall of fruits and vegetables, such as apple (skins). Although different types of dietary pectins exist, studies have found positive effects of pectins in fortifying the gastro-intestinal barrier by strengthening the mucus layer, enhancing epithelial integrity, and activating or inhibiting dendritic cell and macrophage responses.52 In addition, the direct interaction of pectins with the gastrointestinal immune barrier may be governed through pattern recognition receptors, such as Toll-like receptors 2 and 4 or Galectin-3.52 Lastly, specific pectins can stimulate the diversity and abundance of beneficial microbial communities.52 Interestingly, apples are obligatory components in the CDED, a CD-specific diet that showed to have anti-inflammatory effects.39
Should we advise patients to eat lots of apples then? Unfortunately, apples (and many other fruits and vegetables) are also a source of FODMAPS, and a low-FODMAP diet is probably one of the most consistently reported diets to improve symptoms and QoL in IBD patients in an RCT setting.53,54 However, a clear improvement on inflammatory markers has not been shown, and the diet was even associated with worsening dysbiosis.53,54 Together with the restrictive nature of the dietary approach which carries a nutritional risk in patients already at risk for malnutrition, this diet should only be undertaken under strict professional guidance for a short period of time, aimed at controlling symptoms only. Without downplaying the importance of symptoms and QoL, it should remain the clinicians’ responsibility to guide patients towards a safe therapy that induces disease control (need), while ideally also addressing persistent abdominal complaints (want).
Thus, personalized and patient-centered dietary regimens in IBD should aim to ameliorate IBD-related symptoms and (food-related) quality of life, while maintain an anti-inflammatory, disease-controlling effect.
Are Personalized Dietary Strategies Within Reach?
The response of an individual to a specific diet results from the interaction of metabolic, environmental, social and genetic factors, suggesting that different individuals will respond differently to the same interventions.20 For example, in a recent RCT of over 600 individuals, a low-fat diet for 12 months led to weight loss of more than 30 kg in some, but to a weight gain of over 10 kg in others, indicating that no single diet is effective for all, and that personalized nutrition could be more efficient.55 However, after accounting for total energy intake and expenditure, neither genotype-pattern nor baseline insulin secretion was associated with the dietary effects of weight loss, implicating other factors are at play.56 Since the authors report good retention, adherence, and differentiation to the diet, these factors might be explained by unmeasured elements such as the microbiome.
In the Food4Me study, 1607 adults from seven European countries were recruited to an internet delivered RCT that provided 1) conventional dietary advice (control) or personalized advice based on 2) the individual baseline diet; 3) the individual baseline diet plus phenotype (anthropometry and blood biomarkers); or 4) the individual baseline diet plus phenotype plus genotype (five diet-responsive genetic variants).19 Although personalization based on the baseline diet improved key lifestyle factors relevant to a wide range of health outcomes, including phenotypic or genotypic information did not seem to have any additional effect. Although this study did not show any benefit of using genomic data (or even anthropometric data) in improving dietary outcomes, it does suggest personalized advice (even when internet-delivered) is superior to generic advice in inducing dietary changes.
In contrast, in a landmark trial by Zeevi et al, 800 people received continuous glucose level measurements for a week, which measured responses to 46,898 meals.57 Next to the observation that a high variability in glucose response between individuals was measured, a machine-learning algorithm that integrated those data with blood parameters, dietary habits, anthropometrics, physical activity, and the gut microbiota was able to accurately predict personalized postprandial glycemic responses to real-life meals. In a subsequent RCT based on the algorithm, significantly lower postprandial responses and consistent alterations to the gut microbiota profile were observed.57
Although interesting and promisingto date, such trials in specific to IBD are non-existing.
How to Predict Response to Diet in IBD?
The trial from Zeevi et al suggests that precision nutrition is indeed possible, when combining robust trial designs, relevant patient data (including several -omic layers if necessary), and advanced bioinformatic tools (Figure 3).
 |
Figure 3 The three pillars to predict response to dietary interventions in IBD. Created with Biorender.com. |
However, this raises the question which data or factors or combination thereof are most important to consider in developing personalized nutritional strategies in IBD? Although the jury is still out, there are several types of data that hold special promise.
The role of genetics in IBD has been widely recognized with currently over 240 common susceptibility loci identified, and the importance of these findings is reflected in clinical translational applications.58
A typical example where genetics influence diet outside the field of IBD is phenylketonuria, where affected patients need to avoid foods rich in phenylalanine due to a mutation in the gene that encodes the enzyme phenylalanine hydroxylase.20 Although the evidence for similar pathways in IBD is rather scarce and is derived from observational trials that need validation, some examples have been described. Specifically for IBD, the TXNIP gene has been linked to bloating, abdominal pain and diarrhea upon fructose consumption, and a variant of organic cation transporter gene OCTN1 was found to be associated with mushroom intolerance in CD.59–61 Since the links between genetic makeup and dietary elements did not affect inflammation and need validation, they are far from applicable in tomorrow’s clinic. However, such studies are promising as they can inform on which medications may benefit or harm patients. A similar approach could be used in finding effective dietary therapies tailored to individual IBD patients.
The totality of environmental exposures to which an individual is subjected over the course of a lifetime is described by the “exposome” and might be able to complement the genome and explain the heritability gap that is encountered in IBD.62,63
Although different sensors integrated in smart watches or other wearable devices to measure air pollution, humidity and other factors can be considered to capture these environmental stimuli, mapping the complete exposome is challenging, if not impossible, to date. However, many options exist to capture “intermediary” -omics layers that can bridge the gap between genome and exposome.
One such a mediator could be epigenetics.64 Epigenetics are heritable changes in gene expression and chromatin organization that do not depend on the DNA sequence itself, and may impact on the transcription of genes.65 Epigenetic changes can be found in a variety of chronic diseases such as type 2 diabetes, cardiovascular disease, cancer, but also in IBD.64,65 In recent years, it has become increasingly apparent that environmental factors, and most notably, dietary elements can mediate such epigenetic changes.65 In the field of cancer and cancer prevention, dietary polyphenols such as curcumin (which can be found in curry) and resveratrol (which is present in grapes), are able to inhibit DNA methyltransferase and act as histone modifiers.65 Another example of bioactive phytochemicals with anticancer properties through epigenetic modification would be isothiocyanates including sulforaphane (present in broccoli) and are known to act as a histone deacetylase inhibitor.65 However, the evidence remains limited to the field of cancer and cannot be translated to IBD.
Another key player in the pathogenesis of IBD is the gut microbiota. It is known that diet is an important driver of the gut microbial composition and function, and conversely, that the gut microbial response to dietary intervention varies between individuals.66 The modulation of the gut microbiota through dietary therapy is still at its infancy; however, promising results have been obtained already.55 It was shown, for example, that a low-FODMAP diet induced changes in the gut microbial composition in irritable bowel syndrome patients, and that responsiveness to this type of diet could be predicted by baseline faecal bacterial profiles.67
More specifically for IBD, beneficial responses to dietary treatment were reflected in the gut microbial composition as well. For the CDED, children receiving the diet and partial enteral nutrition (PEN) that reached corticosteroid-free remission showed sustained reductions in faecal proteobacteria.39 The CD-TREAT diet in healthy volunteers induced similar changes in the gut microbiome and faecal metabolome (including short chain fatty acids) as EEN.40 However, promising, care should be taken not to overinterpret these data as merely entering disease remission on its own leads to a shift in the microbiota towards a “healthier” composition. Nonetheless, these data suggest that the microbiome might serve as a predictor for dietary response in IBD, and pave the road to a more individualized approach.
Lastly, metabolomics is the study of metabolites in the human host, focuses on changes in the biochemical profile of biological fluids, can be considered to be the analytical end-point of the body response to dietary alterations, and should therefore be considered as well.20
As an illustration, in a recent large-scale population study, a higher milk intake among lactose intolerant individuals, and higher fiber intake were associated with a favorable profile of circulating tryptophan metabolites for type 2 diabetes mellitus, potentially through the host–microbial crosstalk shifting tryptophan metabolism towards gut microbial indole propionate production.68 Next to the appealing functional readout and the possibility to investigate the host–microbiome interface, metabolomics have shown promising results in dietary intake biomarker discovery and dietary intervention assessment, as has been shown for the intake of red meat, coffee, tea, and wine.69 However, these examples have not been independently validated in large-scale studies to date. Moreover, metabolomic changes in urine are often transient, which precludes the use of many of these candidates for long-term dietary intervention assessment. Lastly, before implementation into clinical practice can be considered, evidence should be gathered that changing dietary intake of a certain food product 1) changes the metabolites of interest, and that this 2) results in meaningful changes of clinical outcomes. For example, elegant human and animal trials have demonstrated that the intestinal microbiota metabolism of L-carnitine, which is abundant in red meat, produces trimethylamine-N-oxide (TMAO), which was found to accelerate atherosclerosis.70,71 Subsequently, plasma L-carnitine levels in subjects undergoing cardiac evaluation, predicted increased risks for both prevalent cardiovascular disease (CVD) and incident major adverse cardiac events (myocardial infarction, stroke or death), but only among subjects with concurrently high TMAO levels.70,71 Similar associations could lead to personalized advices regarding red meat intake depending on gut microbial composition and metabolism, after ascertainment of causality and reversibility, ideally in randomized controlled setting. The PREVENTOMICs study, as an example, which used metabolomic and genetic information to classify individuals into different “metabolic clusters” and created personalized dietary plans, was unable to show any additional benefit regarding weight loss over a generic, healthy diet, illustrating the necessity of experimental validation before implementation can be considered.72
Although more research is needed, these data clearly indicate that the combination of phenotypical data, (epi)genomics data, metabolomics, and the gut microbiota will be indispensable in achieving precision nutrition in IBD.
Future Perspectives
Personalized dietary strategies based on a combination of phenotypic characteristics and multiple -omic layers holds promise in IBD to support current treatments in reducing inflammation, as well as symptoms. Such integration of dietary strategies in our current treatment paradigm could have an additive effect to immunosuppressive treatments by targeting other drivers of disease, namely the environment and microbiota. Furthermore, it would be a less costly, and probably safer alternative than combination of advanced therapies for many patients.
The ability to design a dietary plan for an individual patient in this case would probably be 1) more effective at treating the disease, 2) increase compliance as personalized strategies are more acceptable by patients, 3) be less restrictive. Concerning the latter, many dietary approaches that are currently under investigation are not only more general approaches for IBD patients but also very restrictive, which complicates long-term adherence. More in-depth and personalized approaches could identify a minimal set of dietary changes that would be needed to achieve efficacy, which is needed for large-scale and long-term application.
To achieve these goals, several evidence gaps need closing.
Epidemiological data (usually from large cohorts of healthy volunteers that are prospectively followed over time) provide probabilistic evidence, whereas the risk of chronic diseases is multifactorial in nature, combined with stochastic effects.18 However informative, the real benefit for an individual patient is not directly predictable from the mean outcome in populations. More in-depth studies focused on interindividual differences will be necessary to provide an evidence base to guide personalized strategies. Furthermore, most of our evidence today consists of observational studies that use surrogate outcomes, while a standard set of outcome measurements such as steroid use, hospitalization, weight, anemia, endoscopic response and remission, and calprotectin response should be the standard.73 Thus, properly performed, dedicated nutritional trials in IBD are needed.
The design of such well-performed dietary trials needs some consideration.74 However evident it might seem, many nutritional trials lack the same rigor that is applied for drug-trials, in terms of a randomized controlled design, controlling adherence and compliance, and the hard endpoints (such as endoscopic and histologic remission) that are used. To get the high-quality evidence to assess the role of diet in disease management, we will need to apply the same standards.
Because dietary changes require a substantial effort for the patient and usually are supported by several consultations with dieticians, controlling for the placebo response might be even more important than for drug trials.75 When designing a trial that studies the response to a specific nutrient that can be delivered as a supplement, providing a placebo is relatively easy, and similar to drug trials. However, when investigating the effects of a whole food diet, it is far more complex (or even impossible) to design a proper control arm. Not in the least because of collinearity of diets, meaning that when increasing the amount of one (macro)nutrient, the consumption of other macronutrients or food groups usually decrease. It is also extremely difficult to blind both researchers and participants to the intervention when a substantial daily effort is required. A possible solution could be cluster randomized trials, although additional complexity due to multi-site collaborations and statistical inefficiency due to within-cluster correlation and between-cluster variability, should then be taken into consideration.
Similarly, when designing cross-over trials, if and how to implement a wash-out phase needs consideration.76
An interesting, somewhat alternative, clinical trial design that can be considered when investigating personal benefit to personalized dietary treatments is the N-of-1 approach. The N-of-1 design is part of the family of Single-Case Designs that involves multiple cross-over periods, so that every patient is exposed to various treatments, and serves as their own control. Results from these trials often generate results that are readily actionable for the study subject, and may be aggregated for population estimates of effectiveness.77 The PRODUCE trial in paediatric IBD investigated the effect of the SCD and modified SCD (MSCD) on abdominal symptoms and faecal calprotectin, using this particular design to account for the expected heterogeneity in response and the opportunity to receive individualized results.77 Interestingly, no significant effect was seen on average for the 54 included patients, but among full completers, >50% had a meaningful symptomatic improvement and reduced faecal calprotectin compared with baseline (50% and 35% reduction in faecal calprotectin on the SCD and MSCD, respectively) on the SCD and/or MSCD. It is noteworthy that withdrawal or early completion occurred commonly (lack of response [n = 11], adverse events [n = 11], and not desiring to continue [n = 6]), indicating that for a reasonable proportion the dietary intervention was insufficiently effective. Thus, this type of trial design might help in investigating personalized dietary treatments.
Another barrier that needs to be overcome to perform high-quality nutritional research, is financial. Indeed, the generation of multi-omic datasets that are integrated with clinical data seems indispensable to advance the field of personalized nutrition. However, the more data is generated, the larger the sample size ought to be to generate statistically robust results. Since nutritional science is most often academia driven, support from regulatory authorities and large, multicenter or multinational collaborations seems necessary.
Lastly, the analysis of data will necessitate further improvements in artificial intelligence, and especially machine learning to develop algorithms and to integrate different -omics layer to move beyond the former “one gene, one phenotype” paradigm.
In terms of prevention, given the global rise in disease incidence mostly in newly industrialized regions of the world and the paediatric population, dietary adjustments for the population at large are important to consider as well. Epidemiologic data or data from IBD trials can inform on what a healthy and sustainable food industry should look like. Regarding which food elements should be avoided or consumed for IBD prevention, this type of research might be more feasible and cost-effective to conduct in the setting of disease management, rather than prevention with the disease as outcome of interest. Since UPFs (that are usually low in fibers, high in refined sugars, saturated fats, and sugar additives), are appetizing, have a long shelf life, are easy to consume and cheap, they are a beloved element in our daily diet for many. Furthermore, most marketing money is spent on marketing unhealthy products, thereby even increasing the problem.78 In order to break the junk food cycle, authorities will be instrumental, for example by increasing taxes on sugar in food products.78
Conclusion
The ideal personalized dietary regimen for IBD patients should be aimed at disease control (inflammation and symptoms) while taking into account the nutritional status of the patient and patient preferences, thereby increasing adherence. To achieve this goal, more research is needed to identify which biological aspects are needed to identify a minimal set of dietary changes that are necessary on an individual basis, and to develop the biostatistical tools needed to integrate these data. Large and properly performed dietary research with control arms and access to the appropriate biological samples will be instrumental in providing answers to these questions. To do this, financial obstacles to enable high-quality dietary trials with multi-omic datasets need to be overcome.
In terms of public health and prevention, many current advice will depend on epidemiological data, as well as data generated by these trials. As far as population-wide recommendations go, the standard advice that entails increased intake of fruits and vegetables, decreased intakes of processed foods, red meat, and fatty acids should be reinforced by authorities.
Personalized nutrition is possible and underway, but until further notice, we will need to settle for the current “healthy eating” advice, supplemented by the current epidemiological data.
Abbreviations
IBD, inflammatory bowel disease; CD, Crohn’s disease; UC, ulcerative colitis; PUFAs, polyunsaturated fatty acids; UPF, ultra-processed food; TNF, tumor necrosis factor; CMC, carboxymethyl cellulose; GLIM, global clinical nutrition community; BMI, body mass index; QoL, quality of life; CDED, Crohn Disease Exclusion Diet; CD-TREAT, Treatment-with-Eating Diet; IBD-AID, Inflammatory Bowel Disease-Anti-Inflammatory Diet; AIP, Autoimmune Protocol Diet; FODMAP, Fermentable Oligo-, Di-, Monosaccharides and Polyols; GPX4, glutathione peroxidase 4; EEN, Exclusive enteral nutrition; PEN, partial enteral nutrition; TMAO, trimethylamine-N-oxide; CVD, cardiovascular disease; MSCD, modified SCD.
Acknowledgments
The ‘building icon’ was designed by Danil Polshin and downloaded from NounProject.com.
Funding
This work was supported by the Research Foundation Flanders (FWO), Belgium by a PhD Fellowship strategic basic research (SB) grant (1S06023N) for Judith Wellens.
Disclosure
Judith Wellens, Eva Vissers, and Christophe Matthys have no conflicts of interest to declare. Séverine Vermeire has received grants from AbbVie, J&J, Pfizer, Takeda and Galapagos; Séverine Vermeire has received consulting and/or speaking fees from AbbVie, AbolerIS Pharma, AgomAb, Alimentiv, Arena Pharmaceuticals, AstraZeneca, Avaxia, BMS, Boehringer Ingelheim, Celgene, CVasThera, Cytoki Pharma, Dr Falk Pharma, Ferring, Galapagos, Genentech-Roche, Gilead, GSK, Hospira, Imidomics, Janssen, J&J, Lilly, Materia Prima, MiroBio, Morphic, MrMHealth, Mundipharma, MSD, Pfizer, Prodigest, Progenity, Prometheus, Robarts Clinical Trials, Second Genome, Shire, Surrozen, Takeda, Theravance, Tillots Pharma AG, and Zealand Pharma. João Sabino has received Research support from Galapagos and Viatris; has received speaker’s fees from AbbVie, Ferring, Falk, Takeda, Janssen, and Fresenius; and has received consultancy fees from AbbVie, Janssen, Fresenius, Pharmacosmos, and Ferring. João Sabino also reports personal fees from Nestle, grants from The Research Foundation – Flanders and Helmsley Charitable Trust Fund, during the conduct of the study. The authors report no other conflicts of interest in this work.
References
1. Molodecky NA, Soon IS, Rabi DM, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142(1):46–54.e42. doi:10.1053/j.gastro.2011.10.001
2. Glade MJ, Meguid MM. A glance at. dietary emulsifiers, the human intestinal mucus and microbiome, and dietary fiber. Nutrition. 2016;32(5):609–614. doi:10.1016/j.nut.2015.12.036
3. Yang Y, Chung owyang GW, Wu GD. Summary of the Asia America Assembly of IBD (AAA IBD 2016): east meets west–the rising incidence of IBD in Asia as a paradigm for environmental effects on the pathogenesis of immune-mediated disease. Gastroenterology. 2016;151(6):e1–e5. doi:10.1016/j.physbeh.2017.03.040
4. Levine A, Sigall Boneh R, Wine E. Evolving role of diet in the pathogenesis and treatment of inflammatory bowel diseases. Gut. 2018;67(9):1726–1738. doi:10.1136/gutjnl-2017-315866
5. Hou JK, Abraham B, El-Serag H. Dietary intake and risk of developing inflammatory bowel disease: a systematic review of the literature. Am J Gastroenterol. 2011;106(4):563–573. doi:10.1038/ajg.2011.44
6. Wood JA, Halmos EP, Taylor KM, Gibson PR. The role of epidemiological evidence from prospective population studies in shaping dietary approaches to therapy in Crohn’s disease. Mol Nutr Food Res. 2021;65(5):2000294. doi:10.1002/mnfr.202000294
7. Monteiro CA, Moubarac JC, Cannon G, Ng SW, Popkin B. Ultra-processed products are becoming dominant in the global food system. Obes Rev. 2013;14(S2):21–28. doi:10.1111/obr.12107
8. Narula N, Wong ECL, Dehghan M, et al. Association of ultra-processed food intake with risk of inflammatory bowel disease: prospective cohort study. BMJ. 2021;374. doi:10.1136/bmj.n1554
9. Lo C-H, Khandpur N, Rossato SL, et al. Ultra-processed foods and risk of Crohn’s disease and ulcerative colitis: a prospective cohort study. Clin Gastroenterol Hepatol. 2021. doi:10.1016/j.cgh.2021.08.031
10. Halmos EP, Mack A, Gibson PR. Review article: emulsifiers in the food supply and implications for gastrointestinal disease. Aliment Pharmacol Ther. 2019;49(1):41–50. doi:10.1111/apt.15045
11. Dimitrijevic D, Shaw AJ, Florence A. Effects of some non-ionic surfactants on transepithelial permeability in Caco-2 cells. J Pharm Pharmacol. 2000;52(2):157–162. doi:10.1211/0022357001773805
12. Chassaing B, Koren O, Goodrich JK, et al. Dietary emulsifiers impact the mouse gut microbiota promoting colitis and metabolic syndrome. Nature. 2015;519(7541):92–96. doi:10.1038/nature14232
13. Watt J, Marcus R. Carrageenan-induced ulceration of the large intestine in the Guinea pig. Gut. 1971;12(2):164–171. doi:10.1136/gut.12.2.164
14. Bhattacharyya S, Dudeja PK, Tobacman JK. Tumor necrosis factor α-induced inflammation is increased but apoptosis is inhibited by common food additive carrageenan. J Biol Chem. 2010;285(50):39511–39522. doi:10.1074/jbc.M110.159681
15. Chassaing B, Compher C, Bonhomme B, et al. Randomized controlled-feeding study of dietary emulsifier carboxymethyl cellulose reveals detrimental impacts on the gut microbiota and metabolome. Gastroenterology. 2021. doi:10.1053/j.gastro.2021.11.006
16. Roberts CL, Keita ÅV, Duncan SH, et al. Translocation of Crohn’s disease Escherichia coli across M-cells: contrasting effects of soluble plant fibres and emulsifiers. Gut. 2010;59(10):1331–1339. doi:10.1136/gut.2009.195370
17. Wedlake L, Slack N, Andreyev HJN, Whelan K. Fiber in the treatment and maintenance of inflammatory bowel disease: a systematic review of randomized controlled trials. Inflamm Bowel Dis. 2014;20(3):576–586. doi:10.1097/01.MIB.0000437984.92565.31
18. Ordovas JM, Ferguson LR, Tai ES, Mathers JC. Personalised nutrition and health. BMJ. 2018;361(k2173):1–7. doi:10.1136/bmj.k2173
19. Celis-Morales C, Livingstone KM, Marsaux CFM, et al. Effect of personalized nutrition on health-related behaviour change: evidence from the Food4Me European randomized controlled trial. Int J Epidemiol. 2017;46(2):578–588. doi:10.1093/ije/dyw186
20. Ferguson LR, De Caterina R, Görman U, et al. Guide and position of the international society of nutrigenetics/nutrigenomics on personalised nutrition: part 1 - fields of precision nutrition. J Nutrigenet Nutrigenomics. 2016;9(1):12–27. doi:10.1159/000445350
21. Wyatt P, Berry SE, Finlayson G, et al. Postprandial glycaemic dips predict appetite and energy intake in healthy individuals. Nat Metab. 2021;3(4):523–529. doi:10.1038/s42255-021-00383-x
22. Moore SE, McMullan M, McEvoy CT, McKinley MC, Woodside JV. The effectiveness of peer-supported interventions for encouraging dietary behaviour change in adults: a systematic review. Public Health Nutr. 2019;22. doi:10.1017/S1368980018003294
23. Cederholm T, Jensen GL, Correia MI, et al. GLIM criteria for the diagnosis of malnutrition – a consensus report from the global clinical nutrition community. Clin Nutr. 2019;38(1):1–9. doi:10.1016/j.clnu.2018.08.002
24. Filippi J, Al-Jaouni R, Wiroth JB, Hébuterne X, Schneider SM. Nutritional deficiencies in patients with Crohn’s disease in remission. Inflamm Bowel Dis. 2006;12(3):185–191. doi:10.1097/01.MIB.0000206541.15963.c3
25. Kondrup J, Rasmussen H, Hamberg O, Stanga Z. Nutritional risk screening (NRS 2002): a new method based on an analysis of controlled clinical trials. Clin Nutr. 2003;22:321–336. doi:10.1016/S0261-5614(02)00214-5
26. BAPEN. The “MUST” explanatory booklet: a guide to the “Malnutrition Universal Screening Tool” (‘MUST’) for adults; 2011. Available from: https://www.health.gov.il/download/ng/N500-19.pdf.
27. Day AS, Yao CK, Costello SP, Andrews JM, Bryant RV. Food avoidance, restrictive eating behaviour and association with quality of life in adults with inflammatory bowel disease: a systematic scoping review. Appetite. 2021;167:105650. doi:10.1016/j.appet.2021.105650
28. Czuber-Dochan W, Morgan M, Hughes LD, Lomer MCE, Lindsay JO, Whelan K. Perceptions and psychosocial impact of food, nutrition, eating and drinking in people with inflammatory bowel disease: a qualitative investigation of food-related quality of life. J Hum Nutr Diet. 2020;33(1):115–127. doi:10.1111/jhn.12668
29. Dunleavy K, Ungaro R, Manning L, Novak J, Gold S, Colombel J. Vitamin C deficiency in inflammatory bowel disease: the forgotten micronutrient. J Crohns Colitis. 2020;14(Supplement_1):S209–S209. doi:10.1093/ecco-jcc/jjz203.272
30. Bischoff SC, Escher J, Hébuterne X, et al. ESPEN practical guideline: clinical Nutrition in inflammatory bowel disease. Clin Nutr. 2020;39(3):632–653. doi:10.1016/j.clnu.2019.11.002
31. Torres J, Bonovas S, Doherty G, et al. ECCO guidelines on therapeutics in Crohn’s disease: medical treatment. J Crohns Colitis. 2020;14(1):4–22. doi:10.1093/ecco-jcc/jjz180
32. Raine T, Spinelli A, Bonovas S, et al. ECCO guidelines on therapeutics in ulcerative colitis: surgical treatment. J Crohns Colitis. 2022;16(2):179–189. doi:10.1093/ecco-jcc/jjab177
33. Chiu E, Oleynick C, Raman M, Bielawska B. Optimizing inpatient nutrition care of adult patients with inflammatory bowel disease in the 21st century. Nutrients. 2021;13(5):1581. doi:10.3390/nu13051581
34. Danese S, Solitano V, Jairath V, Biroulet LP. The future of drug development for inflammatory bowel disease: the need to ACT (advanced combination treatment). Gut. 2022;71:2380–2387. doi:10.1136/gutjnl-2022-327025
35. Wellens J, Vermeire S, Sabino J. Let food be thy medicine — its role in Crohn’s disease. Nutrients. 2021;13(832):1–16. doi:10.3390/nu13030832
36. Dziechciarz P, Horvath A, Shamir R, Szajewska H. Meta-analysis: enteral nutrition in active Crohn’s disease in children. Aliment Pharmacol Ther. 2007;26(6):795–806. doi:10.1111/j.1365-2036.2007.03431.x
37. Gunasekeera V, Mendall MA, Chan D, Kumar D. Treatment of Crohn’s disease with an IgG4-guided exclusion diet: a randomized controlled trial. Dig Dis Sci. 2016;61(4):1148–1157. doi:10.1007/s10620-015-3987-z
38. Komperød MJ, Sommer C, Mellin-Olsen T, Iversen PO, Røseth AG, Valeur J. Persistent symptoms in patients with Crohn’s disease in remission: an exploratory study on the role of diet. Scand J Gastroenterol. 2018;53(5):573–578. doi:10.1080/00365521.2017.1397736
39. Levine A, Wine E, Assa A, et al. Crohn’s disease exclusion diet plus partial enteral nutrition induces sustained remission in a randomized controlled trial. Gastroenterology. 2019;157(2):440–450.e8. doi:10.1053/j.gastro.2019.04.021
40. Svolos V, Hansen R, Nichols B, et al. Treatment of active Crohn’s disease with an ordinary food-based diet that replicates exclusive enteral nutrition. Gastroenterology. 2019;156(5):1354–1367.e6. doi:10.1053/j.gastro.2018.12.002
41. Olendzki BC, Silverstein TD, Persuitte GM, Ma Y, Baldwin KR, Cave D. An anti-inflammatory diet as treatment for inflammatory bowel disease: discovery service for Endeavour College of Natural Health Library. Nutr J. 2014;13(5):1–7. doi:10.1186/1475-2891-13-5
42. Konijeti GG, Kim N, Lewis JD, et al. Efficacy of the autoimmune protocol diet for inflammatory bowel disease. Inflamm Bowel Dis. 2017;23(11):2054–2060. doi:10.1097/MIB.0000000000001221
43. Whelan K, Martin LD, Staudacher HM, Lomer MCE. The low FODMAP diet in the management of irritable bowel syndrome: an evidence-based review of FODMAP restriction, reintroduction and personalisation in clinical practice. J Hum Nutr Diet. 2018;31(2):239–255. doi:10.1111/jhn.12530
44. Sabino J, Lewis JD, Colombel JF. Treating inflammatory bowel disease with diet: a taste test. Gastroenterology. 2019;157(2):295–297. doi:10.1053/j.gastro.2019.06.027
45. Noor NM, Verstockt B, Parkes M, Lee JC. Personalised medicine in Crohn’s disease. lancet Gastroenterol Hepatol. 2020;5(1):80–92. doi:10.1016/S2468-1253(19)30340-1
46. Schwärzler J, Mayr L, Vich Vila A, et al. PUFA-induced metabolic enteritis as a fuel for Crohn’s disease. Gastroenterology. 2022;162(6):1690–1704. doi:10.1053/j.gastro.2022.01.004
47. Sabino J. Understanding the role of PUFAs in Crohn’s disease. Gastroenterology. 2022;162(6):1590–1591. doi:10.1053/j.gastro.2022.02.048
48. Triggs CM, Munday K, Hu R, et al. Dietary factors in chronic inflammation: food tolerances and intolerances of a New Zealand Caucasian Crohn’s disease population. Mutat Res. 2010;690(1–2):123–138. doi:10.1016/j.mrfmmm.2010.01.020
49. Cohen AB, Lee D, Long MD, et al. Dietary patterns and self-reported associations of diet with symptoms of inflammatory bowel disease. Dig Dis Sci. 2013;58(5):1322–1328. doi:10.1007/s10620-012-2373-3.Dietary
50. Pittet V, Vaucher C, Maillard MH, et al. Information needs and concerns of patients with inflammatory bowel disease: what can we learn from participants in a bilingual clinical cohort? PLoS One. 2016;11(3):1–17. doi:10.1371/journal.pone.0150620
51. Armstrong H, Mander I, Zhang Z, Armstrong D, Wine E. Not all fibers are born equal; variable response to dietary fiber subtypes in IBD. Front Pediatr. 2021;8:1–15. doi:10.3389/fped.2020.620189
52. Beukema M, Faas MM, de Vos P. The effects of different dietary fiber pectin structures on the gastrointestinal immune barrier: impact via gut microbiota and direct effects on immune cells. Exp Mol Med. 2020;52(9):1364–1376. doi:10.1038/s12276-020-0449-2
53. Cox SR, Lindsay JO, Fromentin S, et al. Effects of low FODMAP diet on symptoms, fecal microbiome, and markers of inflammation in patients with quiescent inflammatory bowel disease in a randomized trial. Gastroenterology. 2020;158(1):176–188.e7. doi:10.1053/j.gastro.2019.09.024
54. Halmos EP, Christophersen CT, Bird AR, Shepherd SJ, Muir JG, Gibson PR. Consistent prebiotic effect on gut microbiota with altered fodmap intake in patients with crohn s disease: a randomised, controlled cross-over trial of well-defined diets. Clin Transl Gastroenterol. 2016;7(4):e164–10. doi:10.1038/ctg.2016.22
55. Loughman A, Staudacher HM. Treating the individual with diet: is gut microbiome testing the answer? Lancet Gastroenterol Hepatol. 2020;5(5):437. doi:10.1016/S2468-1253(20)30023-6
56. Gardner CD, Trepanowski JF, Gobbo LCD, et al. Effect of low-fat VS low-carbohydrate diet on 12-month weight loss in overweight adults and the association with genotype pattern or insulin secretion the DIETFITS randomized clinical trial. JAMA. 2018;319(7):667–679. doi:10.1001/jama.2018.0245
57. Zeevi D, Korem T, Zmora N, et al. Personalized nutrition by prediction of glycemic responses. Cell. 2015;163(5):1079–1094. doi:10.1016/j.cell.2015.11.001
58. Mirkov MU, Verstockt B, Cleynen I. Genetics of inflammatory bowel disease: beyond NOD2. Lancet Gastroenterol Hepatol. 2017;2(3):224–234. doi:10.1016/S2468-1253(16)30111-X
59. Petermann I, Triggs CM, Huebner C, et al. Mushroom intolerance: a novel diet gene interaction in Crohn’s disease. Br J Nutr. 2009;102(4):506–508. doi:10.1017/S0007114509276446
60. Takahashi Y, Masuda H, Ishii Y, Nishida Y, Kobayashi M, Asai S. Decreased expression of thioredoxin interacting protein mRNA in inflamed colonic mucosa in patients with ulcerative colitis. Oncol Rep. 2007;18(3):531–535. doi:10.3892/or.18.3.531
61. Laing BB, Lim AG, Ferguson LR. A personalised dietary approach-A way forward to manage nutrient deficiency, effects of the western diet, and food intolerances in inflammatory bowel disease. Nutrients. 2019;11(7):1–28. doi:10.3390/nu11071532
62. Wild CP. Complementing the genome with an “exposome”: the outstanding challenge of environmental exposure measurement in molecular epidemiology. Cancer Epidemiol Biomarkers Prev. 2005;14(8):1847–1850. doi:10.1158/1055-9965.EPI-05-0456
63. Sudhakar P, Alsoud D, Wellens J, Verstockt S. Tailoring multi-omics to inflammatory bowel diseases: all for one and one for all. JCC. 2022;16:1306–1320. doi:10.1016/S2468-1253(21)00065-0
64. Zeng Z, Mukherjee A, Zhang H. From genetics to epigenetics, roles of epigenetics in inflammatory bowel disease. Front Genet. 2019;10:1–17. doi:10.3389/fgene.2019.01017
65. Hardy TM, Tollefsbol TO. Epigenetic diet: impact on the epigenome and cancer. Epigenomics. 2011;3(4):503–518. doi:10.2217/epi.11.71
66. Biesiekierski JR, Jalanka J, Staudacher HM. Can gut microbiota composition predict response to dietary treatments? Nutrients. 2019;11(5):1–15. doi:10.3390/nu11051134
67. Bennet SMP, Böhn L, Störsrud S, et al. Multivariate modelling of faecal bacterial profiles of patients with IBS predicts responsiveness to a diet low in FODMAPs. Gut. 2018;67(5):872–881. doi:10.1136/gutjnl-2016-313128
68. Qi Q, Li J, Yu B, et al. Host and gut microbial tryptophan metabolism and type 2 diabetes: an integrative analysis of host genetics, diet, gut microbiome and circulating metabolites in cohort studies. Gut. 2021;71:1095–1105. doi:10.1136/gutjnl-2021-324053
69. Tebani A, Bekri S. Paving the way to precision nutrition through metabolomics. Front Nutr. 2019;6:1–10. doi:10.3389/fnut.2019.00041
70. Koeth RA, Wang Z, Levison BS, et al. Intestinal microbiota metabolism of l-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. 2013;19(5):576–585. doi:10.1038/nm.3145
71. Tang WHW, Wang Z, Levison BS, et al. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med. 2013;368(17):1575–1584. doi:10.1056/nejmoa1109400
72. Aldubayan MA, Pigsborg K, Gormsen SMO, et al. A double-blinded, randomized, parallel intervention to evaluate biomarker-based nutrition plans for weight loss: the PREVENTOMICS study. Clin Nutr. 2022;41:101890. doi:10.1016/j.clnu.2022.06.032
73. Kim AH, Roberts C, Feagan BG, et al. Developing a standard set of patient-centred outcomes for inflammatory bowel disease-an international, cross-disciplinary consensus. J Crohns Colitis. 2018;12(4):408–418. doi:10.1093/ecco-jcc/jjx161
74. Laville M, Segrestin B, Alligier M, et al. Evidence-based practice within nutrition: what are the barriers for improving the evidence and how can they be dealt with? Trials. 2017;18(1). doi:10.1186/s13063-017-2160-8
75. Staudacher HM, Irving PM, Lomer MCE, Whelan K. The challenges of control groups, placebos and blinding in clinical trials of dietary interventions. Proc Nutr Soc. 2017;76(3):203–212. doi:10.1017/S0029665117000350
76. Dron L, Taljaard M, Cheung YB, et al. The role and challenges of cluster randomised trials for global health. Lancet Glob Health. 2021;9(5):e701–e710. doi:10.1016/S2214-109X(20)30541-6
77. Kaplan HC, Opipari-Arrigan L, Yang J, et al. Personalized research on diet in ulcerative colitis and crohn’s disease: a series of N-of-1 diet trials. Am J Gastroenterol. 2022;117(6):902–917. doi:10.14309/ajg.0000000000001800
78. Dimbleby H. The national food strategy: the plan. Environ Rural Aff. 2021;290. doi:10.2307/j.ctt4cgqfz.3
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
