Back to Journals » Journal of Multidisciplinary Healthcare » Volume 13
Persian Adaptation of Actionable Bladder Symptom Screening Tool Among Patients with Multiple Sclerosis
Authors Azadvari M , Naser Moghadasi A, Azimi AR, Sharifiaghdas F, Emami Razavi SZ , Eskandarieh S, Hosseini M, Rahimi-Dehgolan S , Sahraian MA
Received 27 October 2019
Accepted for publication 30 December 2019
Published 17 January 2020 Volume 2020:13 Pages 79—83
DOI https://doi.org/10.2147/JMDH.S236275
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Mohaddeseh Azadvari, 1 Abdorreza Naser Moghadasi, 1 Amir Reza Azimi, 1 Farzaneh Sharifiaghdas, 2 Seyede Zahra Emami Razavi, 1 Sharareh Eskandarieh, 1 Maryam Hosseini, 1 Shahram Rahimi-Dehgolan, 1 Mohammad Ali Sahraian 1
1Multiple Sclerosis Research Center, Neuroscience Institute, Tehran University of Medical Sciences, Tehran, I. R. of Iran; 2Urology Department, Shahid Labbafinejad Hospital, Urology Nephrology Research Center (UNRC), Shahid Beheshti University of Medical Science(SBMU), Tehran, I. R. of Iran
Correspondence: Seyede Zahra Emami Razavi
Tel +98 2161192291
Fax +98 21 66581604
Email [email protected]
Purpose: Multiple sclerosis (MS) is the most neurologic disease among individuals of 20– 45 years. About 75% of MS patients report bladder problems that have a moderate-high impact on their life. The present study aimed to translate and determine the validity and reliability of the Persian version of ABSST questionnaire.
Methods: The standard validation process for preparing the Persian version of ABSST was performed by means of an expert committee. After a pilot study and confirming the harmonized translated form, we tested the final version of questionnaire on 40 patients with a definite diagnosis of MS symptoms, once at the baseline and another after two weeks in order to prevent recall-induced agreement. Test-retest reliability was calculated as Spearman’s correlation coefficient. Also, content validity indices (CVIs), as well as internal consistency were analyzed in STATA.
Results: Forty participants with a mean age of 38.8 years were included in this study. Only 20% of them were male. The Persian version achieved a good internal consistency with a Cronbach-α value of 0.91, relatively similar to the original version. The coefficients for measuring the correlation between each item score with the total score of our questionnaire were between 0.58– 0.89. This value confirmed an appropriate validity for the Persian version of ABSST. Regarding test-retest reliability assessment, total Spearman`s coefficient was 0.85; with 0.84 for the severity of symptoms and 0.87 for the impact of urologic disorders on patients` social life.
Conclusion: The current findings proved that this accurate questionnaire could be used to investigate urinary symptoms among Persian-speaking MS patients.
Keywords: reliability studies, reliability, questionnaires, urinary symptoms, multiple sclerosis
Introduction
Multiple Sclerosis (MS) is a demyelinating disorder of the central nervous system (CNS). It is the most neurologic disease among individuals of 20–45 years. A wide-spectrum of signs and symptoms might occur due to different loci of white matter plaques.1–4 Urinary problems are among the most important symptoms in MS patients.5–7 These problems include frequency and/or urgency of urination, hesitancy in starting urination, frequent nighttime urination (nocturia),8 urinary incontinence, and inability to empty the bladder completely.1 About 75% of MS subjects report urinary symptoms that have moderate to high impact on their life. Despite high prevalence of condition, many patients usually fail to talk about their urinary problems.9 In fact, a large survey found that only 8% of sufferers mentioned their symptoms.10 There are a few numbers of screening tools for urinary disorders.5–7 The Actionable Bladder Symptoms Screening Tool (ABSST) questionnaire is one of those which researchers advocate because of its brief and easy-to-use items. The ABSST was first developed to identify urinary symptoms, in order to screen MS patients who had corresponding problems.11 It includes items such as symptoms, coping strategies, and the effects of these symptoms on MS patients. Indeed, ABSST could facilitate communication between physicians and MS patients throughout describing their urinary symptoms.11
The reliability and validity of ABSST questionnaire had been previously determined among English-speaking patients with overactive bladder.12 Considering an increasing incidence of MS among Persian population with annual percentage change of 12% during last decade;13,14 and also due to the large psychosocial impact of urinary symptoms, the present study aimed to translate and determine the validity and reliability of Persian version of ABSST questionnaire. In this way, physicians would be able to use an accurate, practical and rapid screening tool based on patients` reported symptoms to facilitate the evaluation of urinary disorders in affected MS patients.
Methods
Among patients presented to neurology clinic of MS center in Sina hospital, a tertiary referral center, of Tehran from June 2017 to the November 2017, 58 consequent subjects were enrolled. After explaining the methodology and excluding ineligible patients, 40 participants were included. Only subjects at the age of 20–50 years, who had a definite diagnosis of MS according to the 2017 revised McDonald MS diagnostic criteria for at least six months that have good cognitive state, vision and motor function in hand with EDSS < 7, were studied. Exclusion criteria were: Active phase of the disease, ongoing UTI, history of pelvic floor trauma or urologic surgery and any other major disease such as diabetes mellitus, hypertension. Written informed consent was obtained in accordance with the Declaration of Helsinki. This study approve by this number IR.TUMS.VCR.REC.1397.1104 in ethic committee of Tehran medical university.
The original ABSST questionnaire (Appendix-1) consisted of eight items; the first five questions were about micturition frequency, leakage, urgency, and nocturnal voiding; while another three items evaluated the impact of mentioned symptoms on patients` function on the work environment, social relationships, and embarrassment. The ABSST has a 4-point Likert scale (from 0–3; where 0 indicates no symptoms or impact; and 3 represent severe symptoms or large impact). The total ABSST score was calculated as the number of questions that patient had a serious problem (responses inside the blue section; Appendix-1). Therefore, total scores ranged from 0–8; a score of > 3 indicated a need for urological referral.2 The standard validation process was performed in four separatesteps. At first: the original English version was translated to Persian by two experienced bilingual translators. Secondly: they back-translated it to English; then the grammatical and semantic evaluation was done between two versions to assure that the original content was preserved. Then a final Persian questionnaire was provided by a committee of 12 experts including two translators, three physiatrists, three MS Fellowship, three urologists, and an epidemiologist. All 12 experts rated comments about the relevance, simplicity, and clarity of ABSST items were analyzed and expressed as three content validity indices (CVI-R for relevancy; CVI-S for simplicity; CVI-C for clarity of questions).15 Afterwards, in a pilot study, we administered the harmonized Persian version of ABSST to 10 participants. Almost all of them stated that ABSST was easy to fill out. Eventually, we tested the final version of the questionnaire (Appendix-2) among 40 patients with definite MS. Participants filled out the translated ABSST questionnaire, once at the baseline and another after two weeks in order to prevent recall-induced agreement.
Only data gathered from the entire population was analyzed and the pilot sample was not quantitatively evaluated. To confirm the properties of ABSST items, STATA® package version 11 was utilized. The descriptive findings were expressed as mean, standard deviation (SD), and relative frequency. Also, since the data did not show normal distribution, test-retest reliability was calculated as Spearman`s rank correlation. Psychometric validity was expressed as the aforementioned three indices, as well as content validity ratio (CVR). Moreover, internal consistency reliability was measured using Cronbach α coefficient. A p value less than 0.05 was considered as the significant level.
Results
A total of 40 participants with a mean age of 38.88 ± 9.0 years, were enrolled in this study. The average value of BMI was 25.27 ± 5.3 kg/m2, respectively. Other demographics have been described in Table 1. Eighty percent of the patients were female. Seventy percent were married, and about 40% were university graduated.
 |
Table 1 Baseline Characteristics of Participants |
Validity
The content validity of ABSST questionnaire has been presented in Table 2. All calculated CVRs were between 0.66–1. For the participation of 12 persons in the expert panel, CVR=0.56 was considered as the minimum. Then it would be concluded that all eight items of the questionnaire were acceptable. The content validity indices (CVI) for most items were above 0.75. The overall CVI for the entire questionnaire was 0.91, 0.86, and 0.92 in regard to relevancy, simplicity, and clarity, respectively. Also, the face validity of the Persian version was acceptable, since almost all of patients filling out the questionnaire stated that ABSST was easy and could be administered in a short time. Moreover, the result of existing correlation between each item with total score has been demonstrated. All eight items had a significant and direct correlation with total score of the tool [Table 3].
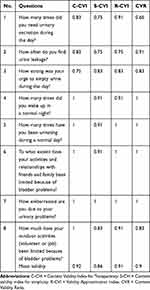 |
Table 2 Results of Content Validity for the Persian Version of ABSST |
 |
Table 3 Correlation of Each Item with Total ABSST Score |
Reliability
The Cronbach α coefficient for the Persian version of ABSST was 0.91 that confirmed the internal consistency. Spearman`s rank coefficients were 0.84 and 0.87 for two subscales of symptoms severity and their impact, respectively; with the total amount of 0.91 (p-value < 0.0001). Also, the intra-class correlation coefficient (ICC) was 0.86, again indicating excellent test-retest reliability for the Persian version of ABSST [Table 4].
 |
Table 4 Internal Consistency, Reliability and Test–Retest Reliability for the Persian ABSST Questionnaire |
Discussion
In the present study, 40 MS subjects were included; the majority was females. The Persian version revealed a good agreement, near to that of the original version. Generally, this survey confirmed an appropriate validity and acceptable reliability for the Persian version of ABSST in the review of urinary disorders among patients with MS. Cronbach α coefficient was calculated as 0.91, which was relatively similar to the original (α= 0.95) version.11 The coefficients for measuring the correlation between each item with the total score were between 0.58–0.89; which discovered an appropriate internal consistency for the Persian form of ABSST. Current findings are in accordance with an earlier study on 2014, conducted to assess the validity and reliability of the English version.12 The Cronbach α coefficient was reported as 0.9 in that research, and the correlation coefficients of items with total score were all between 0.28–0.81.12 In the present version, the correlation between items was determined using Spearman`s rank coefficient, which was 0.84 for the severity of urinary symptoms and 0.87 for the impact of the urologic disorder on patients' social life. This coefficient was 0.85 in total, approximately near to the original (p-value=0.782) version.11
The present study aimed to translate and determine the validity and reliability of the Persian version of the ABSST questionnaire. In this way, it would be possible to use a reliable, accurate, practical and rapid implementation tool based on the patient’s reported symptoms to facilitate the evaluation of overactive urinary disorders in affected patients. There are various screening tools for urinary disorders. The ABSST questionnaire has been selected because of the low number of questions and ease of completion by the patients, as well as attention focused on overactive bladder symptoms. The latter is the most common urinary tract disorder diagnosed among MS patients. The current questionnaire also evaluates the effect of urinary symptoms on the patient’s quality of life and function. This is a useful screening for physicians to detect urinary symptoms in MS patients who may be in need of treatment for lower urinary tract (LUT) symptoms related to the disease. Occasionally, referral to the urologist is delayed, which causes the symptoms to become more severe. ABSST offers valuable benefits to both neurologists and urologists. In this case, Persian questionnaire can help the physician to control the condition and manage the corresponding costs by earlier treatment.
The shortcomings of the present study include the lack of patients' co-operation due to the misunderstanding of validation methodology, which cause some drop out during test–the retest evaluation. These limitations could be addressed in future studies by providing patients’ guide to the methodology and the beneficial effects of this research on improving their quality of life. Another limitation was the lack of a parallel gold standard test to assess the prediction performance of ABSST questionnaire. Based on the original version scores,11 ABSST could be used as a screening tool for detection of LUTdisorders among MS patients, with a minimum score=3, as the cut-off point. Therefore, further studies are needed to determine sensitivity, specificity, and overall accuracy of this translated version at different cut-off points.
Conclusion
The present study proved that a valid and reliable version of ABSST questionnaire could be used to investigate LUT symptoms among Persian-speaking MS patients.
Acknowledgments
The authors want to thank authorities of Tehran University of Medical Sciences for their comprehensives support for this study and also thank Ms. Taktem Firoozi for helping us in data gathering of this study.
Disclosure
The authors of this article declare that they have no conflict of interests.
References
1. Noseworthy JH, Lucchinetti C, Rodriguez M, et al. Multiple sclerosis. N Engl J Med. 2000;343(13):938–952. doi:10.1056/NEJM200009283431307
2. Compston A, Coles A. Multiple sclerosis. Lancet. 2002;359(9313):1221–1231. doi:10.1016/S0140-6736(02)08220-X
3. Lew-Starowicz M, Rola R. Prevalence of sexual dysfunctions among women with multiple sclerosis. Sex Disabil. 2013;31(2):141–153. doi:10.1007/s11195-013-9293-9
4. Borello-France D, Leng W, O’Leary M, et al. Bladder and sexual function among women with multiple sclerosis. Mult Scler. 2004;10(4):455–461. doi:10.1191/1352458504ms1060oa
5. de Seze M, Ruffion A, Denys P, et al. The neurogenic bladder in multiple sclerosis: review of the literature and proposal of management guidelines. Mult Scler. 2007;13(7):915–928. doi:10.1177/1352458506075651
6. Pappalardo A, Patti F, Reggio A. [Management of neuropathic bladder in multiple sclerosis]. Clin Ter. 2004;155(5):183–186.
7. Sammarco A, Mahajan S. The bladder in MS: a review. J Neurol Neurophysiol. 2014;5:200. doi:10.4172/2155-9562.1000200
8. Nakipoglu GF, Kaya AZ, Orhan G, et al. Urinary dysfunction in multiple sclerosis. J Clin Neurosci. 2009;16(10):1321–1324. doi:10.1016/j.jocn.2008.12.012
9. Brucker BM, Nitti VW, Kalra S, et al. Barriers experienced by patients with multiple sclerosis in seeking care for lower urinary tract symptoms. Neurourol Urodyn. 2017;36(4):1208–1213. doi:10.1002/nau.23101
10. Kommunikation K Nordic survey on urinary dysfunction 2011–2012. Available from: http://nikola.nu/sites/all/files/documents/natverk/2nordisk_undersokning_sinoba.pdf.
11. Burks J, Chancellor M, Bates D, et al. Development and validation of the actionable bladder symptom screening tool for multiple sclerosis patients. Int J MS Care. 2013;15(4):182–192. doi:10.7224/1537-2073.2012-049
12. Cardozo L, Staskin D, Currie B, et al. Validation of a bladder symptom screening tool in women with incontinence due to overactive bladder. Int Urogynecol J. 2014;25(12):1655–1663. doi:10.1007/s00192-014-2417-7
13. Eskandarieh S, Heydarpour P, Elhami S-R, et al. Prevalence and incidence of multiple sclerosis in Tehran, Iran. Iran J Public Health. 2017;46(5):699–704.
14. Samadzadeh S, Tabibian E, Sabokbar T, et al. HLA-DRB1 does not have a role in clinical response to interferon-beta among Iranian multiple sclerosis patients. J Neurol Sci. 2015;352(1–2):37–40. doi:10.1016/j.jns.2015.03.004
15. Waltz CF. Nursing Research: Design Statistics and Computer Analysis. F.A. Davis Company; 1981.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
