Back to Journals » Cancer Management and Research » Volume 13
Peripheral Blood Markers Associated with Immune-Related Adverse Effects in Patients Who Had Advanced Non-Small Cell Lung Cancer Treated with PD-1 Inhibitors
Authors Liu W, Liu Y, Ma F, Sun B, Wang Y, Luo J, Liu M, Luo Z
Received 24 November 2020
Accepted for publication 13 January 2021
Published 27 January 2021 Volume 2021:13 Pages 765—771
DOI https://doi.org/10.2147/CMAR.S293200
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Eileen O'Reilly
Wenhui Liu,1,2 Yiping Liu,1,2 Fang Ma,3 Bao Sun,1,2 Ying Wang,1,2 Jianquan Luo,1,2 Mouze Liu,1,2 Zhiying Luo1,2
1Department of Pharmacy, The Second Xiangya Hospital, Central South University, Changsha, People’s Republic of China; 2Institute of Clinical Pharmacy, Central South University, Changsha, People’s Republic of China; 3Department of Oncology, The Second Xiangya Hospital, Central South University, Changsha, People’s Republic of China
Correspondence: Zhiying Luo No. 139, People’s Middle Street, Furong District, Changsha City, Hunan Province 40013, People’s Republic of China
Tel +86 13787034276
Fax +86 0731 85294099
Email [email protected]
Background: Immune checkpoint inhibitors (ICIs) have transformed the treatment landscape for patients with advanced non-small-cell lung cancer (aNSCLC), but immune-related adverse events (irAEs) have been evidenced curtailed the clinical use of them.
Purpose: The aim of this study was to research the influences of inflammation-related peripheral blood markers, including neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) levels, on anti-PD-1 inhibitor-induced irAEs.
Patients and Methods: A retrospective analysis was conducted of patients treated with PD-1 inhibitors for stage III–IV NSCLC at a single center from 2017 to 2020 were included. Clinical characteristics, peripheral blood markers at the baseline and before subsequent treatment cycles were collected. NLR and PLR were calculated by division of neutrophil and platelet by lymphocyte measured in peripheral blood. The development of irAEs was evaluated and monitored from the therapy start based on CTCAE V4.03.
Results: A total of 150 patients were included. Fifty-seven patients had occurred at least one irAEs during follow-up, and mainly grade 1– 2 (73.68%). Pruritus, rash and thyroiditis were the most commonly irAEs. Low NLR, PLR and neutrophil at baseline were significantly associated with the development of severe irAEs (P-values were 0.023, 0.0016 and 0.009). The levels of neutrophil, NLR and PLR also significantly decreased when occurred irAEs compared with baseline (P-values were 0.0069, 0.017 and 1.18E-5, respectively).
Conclusion: The levels of NLR, PLR and neutrophil were associated with the increased risk of severe irAEs when baseline levels were low. NLR, PLR, and neutrophil are simple and available biomarkers that can be used to help predict severe adverse effects in NSCLC patients treated with anti-PD-1 inhibitors.
Keywords: immune checkpoint inhibitors, NSCLC, irAE, neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio
Introduction
Lung cancer continues to be the most frequent cause of cancer-related death worldwide.1 In the last decade, immunotherapy has prompted a paradigm shift in NSCLC treatment. Immune checkpoint therapies were rapidly developed owing to their inspiring clinical efficacy in specific cancer types.2,3 Anti-PD-1 and PD-L1 inhibitors have become the points of interest in immune checkpoint therapies. Unfortunately, the efficiencies of anti-PD-1 or anti-PD-L1 inhibitors were usually heterogeneous, the response rates range from 15% to 30% in most solid tumors.4 However, the response rate is higher (40–60%) in patients who receive combination therapy. These immune checkpoint inhibitors (ICIs) treatment also associated with the occurrence of immune-mediated toxicities, known as immune-related adverse events (irAEs), which may involve most organs like the gut, skin, endocrine glands, lung, heart and so on.4 IrAEs are often different from the classic chemotherapy-related toxicities, and they are off-target effects of an excessively activated immune system.5
Results from multiple clinical trials showed a high rate of serious or fatal anti-PD-1 or anti-PD-L1 inhibitors induced irAEs (grade 3–4).6 Several clinical studies had elucidated that immune checkpoint therapies plus chemotherapy induced a higher rate of serious irAEs.7,8 The precise mechanisms for anti-PD-1 or anti-PD-L1 inhibitors induced irAEs have not been fully elucidated, and scientists speculate that it may be consistent with the action of ICIs, through the bystander effects from activated T-cells.9 Numbers of studies had evidenced that patients who experienced irAEs had improved clinical outcomes, measured using the overall response rate (ORR), progression-free survival (PFS) and overall survival (OS).10,11 However, serious irAEs are also the main reason for patients die or withdrawing treatment.12
The absolute concentration of peripheral blood lymphocytes and absolute monocyte count can be used as an inexpensive and clinical estimate of systemic immunity in humans.13 The neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) are new biomarkers of systemic inflammation that can be readily obtained from a peripheral complete blood count.14 Previous researches had found that pre-treatment NLR and PLR are prognostic markers associated with worse overall survival and death of ICIs in several tumor types.15,16 An increased NLR and PLR may reflect an innately dysfunctional host immune response, a by-product of tumor-related immune suppression, or some combination of both.
Taking into account high treatment costs and potential severe toxicity, cheap and readily available biomarkers for irAEs is of interest17 Based on previous literature, we hypothesis that patients with decreased baseline NLR and PLR levels are more likely to benefit from PD-1 inhibitors and occur irAEs. Hence, we primarily aimed to evaluate the association between peripheral blood markers and the onset of irAEs. The secondary aims were to evaluate the changing trend of peripheral blood markers, based on the occurrence of irAEs.
Method
Study Population
A unicentric and prospective observational study was conducted to search the effect of NLR, PLR on severe irAEs interindividual difference in Chinese population. This study was approved by the Ethics Committee of Second Xiangya Hospital at Central South University, and all procedures were carried out in accordance with the Declaration of Helsinki. All patients were informed of this study and consented to participate. Patients who were diagnosed with advanced stage cancers (III–IV stage) and treated with anti-PD-1 between October 2017 and January 2020 were enrolled. The anti-PD-1 inhibitors (pembrolizumab or nivolumab) were administered intravenously every 3 weeks at a dose of 5 or 10mg/kg.
Patients included according to the following criteria: (1) clinical symptoms, physical signs and imaging examination consistent with the diagnostic criteria for advanced stage NSCLC; (2) no use of antibiotics or microbial ecological agents for at least 4 weeks before anti-PD-1 inhibitors therapy; (3) without autoimmune diseases; (4) normal parameters of blood routine, hepatorenal function before and after anti-PD-1 inhibitor therapy.
Clinical Characteristics Collection
Clinical factors, including demographic variables (age, sex, height, weight, smoking and drinking habits), clinical characteristics (disease condition, PD-L1 expression level and pre-treatment PFS), were mainly obtained from the electronic medical records. All patients underwent blood routine examination before and after they received anti-PD-1 inhibitor treatment, unless the patient absolutely refused. Baseline blood counts data (defined as the most recent blood count within 1 week before ICI initiation) and blood counts before each treatment cycle were reviewed. We collected data including absolute neutrophil count, absolute lymphocyte count, and platelet count to calculate NLR and PLR. The NLR was calculated by dividing the absolute number of neutrophils by the absolute number of lymphocytes obtained from peripheral blood before the start of PD-1 inhibitor treatment. PLR was calculated by the division of thrombocytes and lymphocytes.
Patient Follow-Up and Definition of irAEs
The data from patients receiving anti-PD-1 antibodies indicated that most irAEs occurred within the first 6 months of treatment. Each patient followed-up for at least 6 months by regular clinic visits in this research. An oncologist and a pharmacist conducted the follow-up regularly. The occurrence of irAEs was followed-up and monitored from the therapy start. It was included in this study if the onset was before January 2020. Adverse events were evaluated by two oncologists independently and categorized based on National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE V4.04). These adverse events included rash, generalised pruritus, vitiligo, colitis, pneumonitis, hepatitis, thyroiditis, hypophysitis, arthritis and others (asthenia, fatigue, nephritis and Guillain-Barre´ syndrome).
Statistical Analyses
The clinical results were expressed as medians with interquartile range (IQR) for continuous variables, and frequency and percentage for categorical variables. The T-test (for continuous variables) and χ2 test (for categorical variables) were used to analyze the clinical characteristic difference between groups. The relationship between blood markers and the occurrence of irAE was assessed using the Mann–Whitney test. All statistical analyses were performed with the R statistical Language (V3.1.1) and GraphPad Prism (v6.0e) software package. Differences were considered to be significant at P < 0.05.
Results
Clinical Characteristics of the Enrolled Patients
The basic clinical characteristics are summarized in Table 1. A total of 150 patients were included in the study and followed-up for at least 6 months. The age of the included patients ranged between 17 and 79 years. Most patients were male (79.7%). More than half of patients were former or active smokers (58.0%). Most patients had squamous carcinoma (57.3%), and all patients had advanced NSCLC (aNSCLC). A large majority (87.3%) of patients had a pretreatment ECOG performance status of 0 or 1. About half of patients (50.7%) received immune therapy after disease progression on platinum-based chemotherapy. More than half of patients (69.3%) were not detected the expression of PD-L1 gene in tumor tissue. Among them, 130 patients received anti-PD-1 combined with chemotherapy, and 20 patients received anti-PD-1 monotherapy. A total of 62 patients stopped taking anti-PD-1 inhibitor therapy because of different reasons. Among them, most patients (77.4%) ceased treatment because of disease progression, while the others due to adverse events. There are two patients were died during the follow-up period due to fatal irAEs (one immune-related pneumonitis and one treatment-related cardiac event).
 |
Table 1 Characteristics of Enrolled Patients |
IrAEs Development During Patient Follow-Up
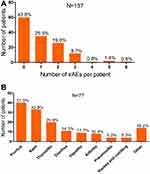
During the follow-up time, a total of 57 patients (38.0%) developed at least one type of irAEs (Figure 1A). Pruritus, rash and thyroiditis are the most commonly occurred irAEs (as shown in Figure 1B). IrAEs found in this research were mainly mild (grade 1–2 in 73.7% patients). We totally followed 15 severe irAEs (grade 3–4) and 2 fatal irAEs. Patients who had taken anti-PD-1 monotherapy had higher grade 1–2 irAEs than patients who under combination therapy (70.59% vs 42.50, P= 0.027). However, none of the patients in the monotherapy group occurred grade 3–4 irAEs (P=0.09).
Elevated Baseline NLR is Associated with Decreased Risk of Grade 3–4 irAEs
The baseline clinical features between patients with or without irAEs were not significantly different (shown in Supplemental Table 1). When dividing the population into three groups according to the occurrence of grade 3–4 irAEs, we observed lower levels of NLR, PLR and neutrophil in the grade 3–4 irAEs group (P-values were 0.023, 0.0016 and 0.009, respectively), as shown in Table 2. Since anti-PD-1 monotherapy patients had higher grade 1–2 irAEs than patients under combination therapy, we further compared the peripheral blood markers between the monotherapy and combination groups. No significant difference was found (data not shown).
 |
Table 2 Baseline Peripheral Blood Markers by the Degree of irAEs |
Seventy-six (50.7%) enrolled patients previously underwent platinum-based chemotherapy and had disease progression. Patients who had previously taken chemotherapy had a lower rate of irAEs compared to patients who did not (32.9% vs 43.2%, P=0.13). To evaluate the effects of previous treatments on baseline inflammation, we compared baseline blood markers between patients with and without prior chemotherapy. Patients who previously taken platinum-based chemotherapy had elevated baseline NLR, PLR, and lymphocyte levels (P-values were 0.029, 0.003 and 0.001, respectively), as shown in Supplemental Table 2.
NLR and PLR Level Decreased Before irAEs Occurred
To determine whether the change in NLR and PLR during treatment may serve as early biomarkers of irAEs, we compared the changes of blood markers at baseline and before subsequent cycles of treatment. The baseline and pretreatment cycle blood routine examination data of the patients who experienced irAEs were obtained. The NLR, PLR, and neutrophil counts were significantly decreased during the treatment cycle when irAEs occurred. The P-values were 0.0069, 0.013, and 1.18E-5, respectively, as shown in Figure 2.
To compare the changes in blood markers between different grades of irAEs, we divided patients into two groups, based on the grade of their irAEs (grade 3–4 versus grade 1–2). In each subgroup, both NLR and PLR levels were decreased before the cycle when irAEs occurred, as shown in Table 3.
 |
Table 3 Baseline and Cycle of irAEs of Peripheral Blood Markers in Different irAE Groups |
Discussion
The emergence of PD-1 inhibitors has brought hope to patients with aNSCLC, but clinical studies have shown that no more than 20% of patients benefit from monotherapy. Regardless of PD-L1 expression, the combination of chemotherapy with PD-1 antibodies was more effective than chemotherapy alone as first-line therapy for NSCLC.7 The addition of anti-PD-1 to chemotherapy resulted in significantly higher response rates and longer progression-free survival than those from chemotherapy or anti-PD-1 alone. However, this was generally accompanied by a higher rate of adverse effects.12
As previous clinical studies elucidated that anti-PD-1 inhibitors plus chemotherapy induced a higher rate of serious irAEs, the results in our study showed that the rate of grade 1–2 irAEs in the monotherapy group is higher than that in the combination therapy group. This inconsistent result may be due to the small sample size of the monotherapy group (20 versus 130). Hence, this result cannot reflect the real clinical relevance.
Treatment with immune checkpoint inhibitors may result in grade 3–4 irAEs, which necessitate patients to terminate anti-tumor therapy and acquired long-term steroids or endocrine treatment.18 Understanding the mechanisms of irAEs will not only help clinicians to manage them more effectively but also enable safety assessments of treatment resumption after irAEs resolution.19 A previous study showed that polymorphisms in human leukocyte antigen (HLA) genes were associated with the occurrence of irAEs in patients under immune checkpoint therapy.20 There was also evidence that pre-treatment intestinal microbiota can be used as biomarkers that correlate with protection against CTLA-4 blockade-associated colitis.21,22 Pre-treatment autoimmune markers, including rheumatoid factor, antinuclear antibody, antithyroglobulin, and antithyroid peroxidase were independently associated with anti-PD-1 induced irAEs.23 These factors were significantly associated with the risk of irAEs. However, the mechanism behind irAEs has not been fully elucidated.
The prognostic significance of elevated NLR and PLR is observed with remarkable consistency across various cancer types. What remains unclear is whether decreased NLR and PLR levels represent a reaction to irAEs causing an aggressive clinical course. In our study population, the rate of irAE was similar to that reported in large real-life studies. Decreased baseline NLR and PLR were significantly associated with risk of severe irAEs (grade 3/4), but nor mild irAEs (grade 1/2), among aNSCLC patients under anti-PD-1 therapy. This finding agrees with previous studies, which associated lower inflammation-related blood markers with a higher risk of irAEs in NSCLC patients.24,25 While patients who had irAEs were more likely to have good treatment outcomes with anti-PD-1, they were also more likely to have lower baseline NLR and PLR levels. This result was consistent with the previous observation, that lower NLR and PLR were associated with good clinical outcomes in NSCLC patients treated with immune checkpoint inhibitors or platinum-based first-line chemotherapy.15,26,27
A novel finding in our research was that the levels of blood markers, including NLR, PLR and neutrophil, were dramatically decreased during the treatment cycle when irAEs occurred. The mechanism behind the ability of NLR and PLR to predict the clinical course during ICI treatment remains unclear. Alberto et al discussed the possible reasons for this. Fiamma et al previously reported that T cells recognized shared lung tumor and skin antigens simultaneously; thus, they target both organs, resulting in autoimmune skin toxic effects during immune checkpoint inhibitor therapy.28
Due to the association between blood markers and ICIs treatment outcomes, it is quite important to found factors that impact the balance between nonspecific inflammation and immunoreaction. In this study, we found that prior chemotherapy affected baseline levels of blood markers. In the clinical setting, oncologists should assess the potential risk of irAEs in patients who previously underwent chemotherapy.
We are aware of the limitations of this study. First, this is a retrospective analysis with manual data extraction and a relatively small sample size. However, no missing data concerning laboratory values and follow-up data were encountered in our analysis. There may have also been the risk of a patient selection bias. Second, this was a mono-centre and real-world study, and most of the enrolled patients received PD-1 inhibitors as their second-line treatment or beyond. Although prior treatment did not affect the risk of irAEs, the baseline blood markers may have been affected by previous treatment. Third, as most studies divided NLR and PLR values into categorical variables, the absolute values of blood marker were used during the statistical analysis process. Despite these limitations, the study was unique as the first to investigate the dynamic changes in blood markers from the beginning of therapy to the occurrence of irAEs.
In conclusion, we have demonstrated an association between baseline NLR and PLR and the probability of severe irAEs in aNSCLC through a real-world study. Both NLR and PLR dramatically decreased before irAEs occurred. NLR and PLR are cheap and readily available biomarkers for identifying patients who can benefit from anti-PD-1 antibody treatment. Further prospective studies with adequate sample sizes are needed to validate our results.
Ethical Conduct of Research
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
Acknowledgments
The authors thank all the patients and their families. We thank the support from the doctors of the Department of Oncology, The Second Xiangya Hospital of Central South University.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the National Natural Scientific Foundation of China (82003883). Natural Science Foundation of Hunan Province China (Grant No: 2017JJ3462, 2020JJ5822).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34. doi:10.3322/caac.21551
2. Hamid O, Robert C, Daud A, et al. Five-year survival outcomes for patients with advanced melanoma treated with pembrolizumab in KEYNOTE-001. Ann Oncol. 2019;30(4):582–588. doi:10.1093/annonc/mdz011
3. Sato Y, Fukuda N, Wang X, et al. Efficacy of nivolumab for head and neck cancer patients with primary sites and histological subtypes excluded from the checkmate-141 trial. Cancer Manag Res. 2020;12:4161–4168. doi:10.2147/CMAR.S249393
4. Baxi S, Yang A, Gennarelli RL, et al. Immune-related adverse events for anti-PD-1 and anti-PD-L1 drugs: systematic review and meta-analysis. BMJ. 2018;360:k793. doi:10.1136/bmj.k793
5. Martins F, Sofiya L, Sykiotis GP, et al. Adverse effects of immune-checkpoint inhibitors: epidemiology, management and surveillance. Nat Rev Clin Oncol. 2019;16(9):563–580. doi:10.1038/s41571-019-0218-0
6. Moslehi JJ, Salem JE, Sosman JA, Lebrun-Vignes B, Johnson DB. Increased reporting of fatal immune checkpoint inhibitor-associated myocarditis. Lancet. 2018;391(10124):933. doi:10.1016/S0140-6736(18)30533-6
7. Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378(22):2078–2092. doi:10.1056/NEJMoa1801005
8. Horn L, Mansfield AS, Szczęsna A, et al. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med. 2018;379(23):2220–2229. doi:10.1056/NEJMoa1809064
9. Passat T, Touchefeu Y, Gervois N, Jarry A, Bossard C, Bennouna J. Physiopathological mechanisms of immune-related adverse events induced by anti-CTLA-4, anti-PD-1 and anti-PD-L1 antibodies in cancer treatment. Bull Cancer. 2018;105(11):1033–1041. doi:10.1016/j.bulcan.2018.07.005
10. Das S, Johnson DB. Immune-related adverse events and anti-tumor efficacy of immune checkpoint inhibitors. J Immunother Cancer. 2019;7(1):306. doi:10.1186/s40425-019-0805-8
11. Buder-Bakhaya K, Hassel JC. Biomarkers for clinical benefit of immune checkpoint inhibitor treatment-a review from the melanoma perspective and beyond. Front Immunol. 2018;9:1474. doi:10.3389/fimmu.2018.01474
12. Michot JM, Bigenwald C, Champiat S, et al. Immune-related adverse events with immune checkpoint blockade: a comprehensive review. Eur J Cancer. 2016;54:139–148. doi:10.1016/j.ejca.2015.11.016
13. Wang Y, Zhou S, Yang F, et al. Treatment-related adverse events of PD-1 and PD-L1 inhibitors in clinical trials: a systematic review and meta-analysis. JAMA Oncol. 2019;5(7):1008–1019. doi:10.1001/jamaoncol.2019.0393
14. Sun Y, Zhang L. The clinical use of pretreatment NLR, PLR, and LMR in patients with esophageal squamous cell carcinoma: evidence from a meta-analysis. Cancer Manag Res. 2018;10:6167–6179. doi:10.2147/CMAR.S171035
15. Diem S, Schmid S, Krapf M, et al. Neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) as prognostic markers in patients with non-small cell lung cancer (NSCLC) treated with nivolumab. Lung Cancer. 2017;111:176–181. doi:10.1016/j.lungcan.2017.07.024
16. Bartlett EK, Flynn JR, Panageas KS, et al. High neutrophil-to-lymphocyte ratio (NLR) is associated with treatment failure and death in patients who have melanoma treated with PD-1 inhibitor monotherapy. Cancer. 2020;126(1):76–85. doi:10.1002/cncr.32506
17. Giuliani J, Bonetti A. Financial toxicity and non-small cell lung cancer treatment: the optimization in the choice of immune check point inhibitors. Anticancer Res. 2019;39(7):3961–3965. doi:10.21873/anticanres.13550
18. Friedman CF, Proverbs-Singh TA, Postow MA. Treatment of the immune-related adverse effects of immune checkpoint inhibitors: a review. JAMA Oncol. 2016;2(10):1346–1353. doi:10.1001/jamaoncol.2016.1051
19. Giuliani J, Bonetti A. Immune-checkpoint inhibitors in head and neck squamous cell carcinoma: cost-efficacy in second-line treatment based on programmed death-ligand 1 (PD-L1) level. Oral Oncol. 2019;97:143–145. doi:10.1016/j.oraloncology.2019.08.010
20. Hasan Ali O, Berner F, Bomze D, et al. Human leukocyte antigen variation is associated with adverse events of checkpoint inhibitors. Eur J Cancer. 2019;107:8–14. doi:10.1016/j.ejca.2018.11.009
21. Dubin K, Callahan MK, Ren B, et al. Intestinal microbiome analyses identify melanoma patients at risk for checkpoint-blockade-induced colitis. Nat Commun. 2016;7(1):10391. doi:10.1038/ncomms10391
22. Chaput N, Lepage P, Coutzac C, et al. Baseline gut microbiota predicts clinical response and colitis in metastatic melanoma patients treated with ipilimumab. Ann Oncol. 2017;28(6):1368–1379. doi:10.1093/annonc/mdx108
23. Toi Y, Sugawara S, Sugisaka J, et al. Profiling preexisting antibodies in patients treated with anti-PD-1 therapy for advanced non-small cell lung cancer. JAMA Oncol. 2019;5(3):376–383. doi:10.1001/jamaoncol.2018.5860
24. Pavan A, Calvetti L, Dal Maso A, et al. Peripheral blood markers identify risk of immune-related toxicity in advanced non-small cell lung cancer treated with immune-checkpoint inhibitors. Oncol. 2019;24(8):1128–1136. doi:10.1634/theoncologist.2018-0563
25. Peng L, Wang Y, Liu F, et al. Peripheral blood markers predictive of outcome and immune-related adverse events in advanced non-small cell lung cancer treated with PD-1 inhibitors. Cancer Immunol Immunother. 2020;69(9):1813–1822. doi:10.1007/s00262-020-02585-w
26. Gu XB, Tian T, Tian XJ, Zhang XJ. Prognostic significance of neutrophil-to-lymphocyte ratio in non-small cell lung cancer: a meta-analysis. Sci Rep. 2015;5(1):12493. doi:10.1038/srep12493
27. Liu ZL, Zeng TT, Zhou XJ, et al. Neutrophil-lymphocyte ratio as a prognostic marker for chemotherapy in advanced lung cancer. Int J Biol Markers. 2016;31(4):e395–e401. doi:10.5301/jbm.5000222
28. Berner F, Bomze D, Diem S, et al. Association of checkpoint inhibitor-induced toxic effects with shared cancer and tissue antigens in non-small cell lung cancer. JAMA Oncol. 2019;5(7):1043–1047. doi:10.1001/jamaoncol.2019.0402
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.