Back to Journals » Journal of Inflammation Research » Volume 15
Peripheral Blood Lymphocyte Subsets as a Risk Predictor of Patients with Endometrioid Endometrial Cancer
Authors Su P , An J , Yu L , Lei H , Huang L, Mao X , Sun P
Received 7 September 2022
Accepted for publication 1 November 2022
Published 8 November 2022 Volume 2022:15 Pages 6153—6163
DOI https://doi.org/10.2147/JIR.S388993
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Pingping Su,1 Jian An,1,2 Lirui Yu,1 Huifang Lei,1 Lixiang Huang,1 Xiaodan Mao,1,3 Pengming Sun1,3
1Department of Gynecology, Laboratory of Gynecologic Oncology, Fujian Maternity and Child Health Hospital, College of Clinical Medicine for Obstetrics & Gynecology and Pediatrics, Fujian Medical University, Fuzhou, Fujian, 350001, People’s Republic of China; 2Department of Gynecology, Women and Children’s Hospital, School of Medicine, Xiamen University, Xiamen, People’s Republic of China; 3Fujian Key Laboratory of Women and Children’s Critical Diseases Research, Fujian Maternity and Child Health Hospital, College of Clinical Medicine for Obstetrics & Gynecology and Pediatrics, Fujian Medical University, Fuzhou, Fujian, 350001, People’s Republic of China
Correspondence: Pengming Sun; Xiaodan Mao, Fujian Maternity and Child Health Hospital, Affiliated Hospital of Fujian Medical University, Fuzhou, Fujian, 350001, People’s Republic of China, Tel +86-591-87558732, Fax +86-591-87551247, Email [email protected]; [email protected]
Purpose: This study aimed to explore lymphocyte subsets for the personalized prediction of endometrioid endometrial cancer (EEC) risk and evaluated the correlation between immune cells and International Federation of Gynecology and Obstetrics (FIGO) staging in patients with EEC.
Patients and Methods: A case-control study was conducted to analyze the clinical data of 421 patients admitted to Fujian Maternity and Child Health Hospital from October 2017 to December 2021. t-tests or Mann–Whitney U-tests were used to analyze the percentages and absolute counts of peripheral blood lymphocyte subsets in patients with EEC and patients without cancer. The independent risk factors for ECC and FIGO stage were analyzed via multivariate binary logistic regression. The receiver operating characteristic curve was calculated to evaluate the prediction efficacy of risk factors on ECC.
Results: The CD4+ T% in the 121 patients with EEC was lower than in the 300 patients without cancer (P = 0.013). The absolute counts of peripheral CD4+ T (P = 0.002) and T cells (P = 0.007) in 37 patients with EEC were lower than in 51 patients without cancer. Multivariate binary logistic regression analysis showed that CD4+ T% and natural killer cell (NK)% were independent risk factors for FIGO staging in patients with EEC. NK% was significantly higher in patients with advanced stage (FIGO III and IV) than those with early EEC (FIGO I and II) (P = 0.004). To determine the early and advance FIGO stage of EEC, the cutoff value of NK% was calculated as 19.94%.
Conclusion: With the decrease of CD4+ T counts, the immune status of patients with EEC is impaired. NK cells may help in the evaluation of the prognosis of patients with EEC and are likely to be an independent risk factor for FIGO staging in patients with EEC.
Keywords: CD4+ T lymphocyte, NK cells, endometrioid endometrial cancer, lymphocyte subsets, immune
Introduction
Endometrial cancer (EC) is the most common malignant tumor of the female reproductive system in developed countries, and it is the second most common malignant tumor in China; the average age of patients is increasingly younger than previously observed.1 The prognosis of patients with early EC is good; however, patients with advanced EC have poor prognoses, and the 5-year survival rate for advanced stage patients is only 17%.2 According to statistics from the National Cancer Center in 2019, the incidence of EC in China was 10.28/100,000, and the mortality rate was 1.9/100,000.3
Immune escape plays a critical role in the occurrence, progression, and metastasis of EC. The detection of lymphocyte subsets in peripheral blood reflects the current immune status of the body; thus, they may be key to the treatment and prognosis evaluation of malignant tumors.4 Different subsets of lymphocytes and their homeostasis play important roles in the regulation of the immune system. Therefore, accurate evaluation of the percentages and absolute counts of lymphocyte subsets in peripheral blood as non-invasive risk markers may have favorable prospects for immunotherapy of EC.
Based on the case data of a single center, we analyzed the difference of percentages and absolute counts of B cells, T cells, CD4+ T, CD8+ T, and natural killer (NK) cells in the peripheral blood of patients with endometrioid endometrial cancer (EEC) and patients without cancer, and we analyzed the difference of lymphocyte subsets in different International Federation of Gynecology and Obstetrics stages (FIGO), histologic tumor grades, depth of myometrial invasion (DMI), lymphovascular space invasion (LVSI), and lymphatic metastasis in EEC. Some studies have shown that the lymphocyte count in cancer tissue specimens can effectively predict the survival and prognosis of patients with EC.5 However, it is difficult and inconvenient to obtain tumor-infiltrating lymphocytes, and few clinics can perform this test. Pascual-Garcia et al showed that the number of CD8+ T cells in the peripheral blood of patients with Grade 3 EEC was lower than that in the normal group.6 For patients with an abnormal lymphocyte composition, it may be possible to evaluate the occurrence and development of EC. Therefore, we evaluated the immune status of patients by detecting peripheral blood lymphocyte subsets. Evaluating the percentages of lymphocyte subsets in patients with EEC at different FIGO stages is of important clinical significance. According to the differences in lymphocyte subsets, we can evaluate the treatment and prognosis of EC, hence providing a reference for clinical diagnosis, treatment, and prognosis judgment.
Methods
Study Population
In this case-control study, we examined the data of 421 patients admitted to the Department of Gynecology, Fujian Maternity and Child Health Hospital, from October 2017 to December 2021. Based on the pathological results, inclusion criteria, and exclusion criteria (defined in detail below), the study enrolled a total of 121 patients with EEC and 300 patients without cancer.
The inclusion criteria for the EEC group were as follows: 1) pathologically confirmed endometrioid adenocarcinoma; 2) not receiving any preoperative radiotherapy or chemotherapy; and 3) having complete clinical and pathological data. The inclusion criteria for the non-cancer group were as follows: 1) no malignant lesions in the endometrium, as confirmed by surgical pathology; and 2) having complete clinical and pathological data. The exclusion criteria for the study were as follows: 1) having an incomplete pathological diagnosis; 2) having other malignant tumors or precancerous lesions; and 3) having chronic inflammatory disease and immune system diseases (eg, autoimmune diseases, acquired immunodeficiency syndrome). All study participants provided informed consent, and the study design was approved by the ethics review board. This study was conducted in accordance with the principles of the Declaration of Helsinki.
Clinical data were collected to identify potential risk factors for EEC, including age, height, weight, menopausal status, reproductive history, parity, comprehensive medical history (eg, hypertension, diabetes), results of the pelvic ultrasounds performed at our hospital within 3 months before the operation, serum tumor marker levels within 3 months before the operation, and levels of blood lipids and lymphocyte subsets (B/T/CD4+/CD8+/NK cells) in the peripheral blood within 1 week prior to the operation. A body mass index (BMI) was dichotomized at 25 kg/m2 and analyzed as a categorical variable. Based on the results of the preoperative pelvic ultrasound performed at our hospital, endometrial thickness can be graded as follows: 0–0.5 cm, Grade 0; >0.5cm, Grade 1.7 The reference ranges for serum total triglyceride and cholesterol (CHOL) levels were 1–1.70 mmol/L and 0–5.20 mmol/L, respectively. The normal reference ranges for serum tumor marker carbohydrate antigen 125 (CA125) was 0–35.00 U/mL. The upper limit of each serological index was used as the threshold for statistical analyses.
Detection of Lymphocyte Subpopulations in Peripheral Blood
Two millilitres of fresh peripheral blood were obtained from patients with EEC and patients without cancer who had just been admitted to hospital, and were stored in the EDTA blood collection tubes. The manipulation was referred to BD operating instruction.
Staining the Cells and Analyzing
- For each patient sample, label a BD Trucount tube with the sample identification number.
- Pipette 20 µL of BD Multitest 6-color TBNK reagent (BD Catalog No. 337166) into the bottom of the tube. Pipette just above the stainless steel retainer of the BD Trucount tube. Do not touch the pellet.
- Pipette 50 µL of well-mixed, anticoagulated whole blood into the bottom of the tube.
- Cap the tube and vortex gently to mix. Incubate for 15 minutes in the dark at room temperature (20° to 25°C).
- Add 450 µL of 1X BD FACS lysing solution to the tube. Use care to protect the tubes from direct light. Perform the procedure at room temperature (20° to 25°C).
- Cap the tube and vortex gently to mix. Incubate for 15 minutes in the dark at room temperature (20° to 25°C).
- Samples were analyzed on the flow cytometer.
Statistical Analyses
All data were analyzed using SPSS version 25.0 and GraphPad Prism version 7.0. According to whether the data were normal distribution or not, the continuous variables were represented by mean ± standard deviation or median (interquartile ranges) and analyzed via t-tests for normal distributed data and Mann–Whitney U for non-normally distributed data. Categorical variables were expressed as percentages and compared using the Chi-squared test, Fisher’s exact probability test, and binary logistic regression. We implemented univariate logistic analysis to evaluate the independent risk factors for ECC. All variables with a two-sided P-value < 0.05 were included in the multivariate logistic regression, with correlations estimated through odds ratios (ORs) and 95% confidence intervals (CIs). Receiver operating characteristic (ROC) curves and the areas under the curve (AUCs) were implemented to obtain the cutoff value related to FIGO staging of EEC. P-values < 0.05 were considered statistically significant.
Results
Baseline Characteristics in Patients with Endometrioid Endometrial Cancer and Patients Without Cancer
The clinical characteristics of the study participants are shown in Table 1. The proportions of patients with overweight BMI and who were postmenopausal were higher in the EEC group than in the non-cancer group. Compared with the proportions in the non-cancer group, the proportions of patients with diabetes, hypertension, hyperlipidemia, and elevated total cholesterol were higher in the EEC group. The proportions of patients with a thicker endometrium (> 0.5 cm) and elevated serum CA125 levels were higher in the EEC group than in the non-cancer group. There were no significant differences associated with age, number of pregnancies, or parturition between the two groups. In the univariate analysis, BMI, menopausal status, diabetes, hypertension, endometrial thickness, serum triglyceride level, serum total cholesterol levels, and serum CA125 levels were significantly associated with the risk of developing EEC. Multivariate logistic regression analysis showed that menopausal status, endometrial thickness, CHOL, and high CA125 were independent risk factors for EEC (Figure 1).
 |
Table 1 Univariate Logistic Regression Analysis of the Onset of Endometrioid Endometrial Cancer in the Study Population |
Characteristics of the Percentages and Absolute Counts of Peripheral Blood Lymphocyte Subsets Between Patients with Endometrioid Endometrial Cancer and Patients Without Cancer
In order to clarify the role of immune cells in EEC, we investigated the peripheral blood lymphocyte subsets’ percentages between patients with EEC and patients without cancer. Interestingly, Table 2 and Figure 2 show that the percentages of CD4+ T cells in patients with EEC and patients without cancer were 39.90 (34.90–44.98) and 42.84 (37.10–47.41), respectively (P = 0.013). There were no significant differences in the percentages of B cells, T cells, CD8+ T cells, NK cells, and CD4/CD8 in patients with EEC and patients without cancer. Next, we analyzed the absolute lymphocyte counts of 37 patients with EEC and 51 patients without cancer (Figure 3). The absolute counts of CD4+ T (P = 0.002) and T cells (P = 0.007) in patients with EEC were lower than those in patients without cancer; however, there were no significant differences in the absolute counts of B cells, CD8+ T cells, and NK cells, or the ratio of CD4+/CD8+. The results of percentages and absolute lymphocyte counts showed that lymphocyte proliferation in patients with EEC was significantly impaired. This suggests that the peripheral blood CD4+ T cells play an important role in the occurrence of EC.
 |
Table 2 Percentages and Absolute Counts of of Lymphocyte Subsets in Patients with Endometrioid Endometrial Cancer and Patients Without Cancer |
Analysis of the Percentages of Peripheral Blood Lymphocyte Subsets in Patients with Different Clinicopathological Features
Figure 4 shows the differences of lymphocyte subsets in different FIGO stages, histologic tumor grades, DMI, LVSI, and lymphatic metastasis in EEC. The proportion of NK cells in patients with EEC with III–IV stage, DMI≥50%, LVSI (+) and lymph node metastasis (+) was higher than that in FIGO I–II stage, DMI<50%, LVSI (-), and lymph node metastasis (-) (P < 0.05). However, we observed no difference in the percentages of B, T, CD4+ T, and CD8+ T cells, or the ratios of CD4+/CD8+ in FIGO stages, histologic tumor grades, DMI, LVSI, or lymphatic metastasis of EEC (P > 0.05).
Role of NK Cells in Malignant Progression of Endometrioid Endometrial Cancer
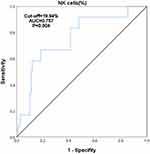
To investigate the effect of immune cell subsets on patients with early (FIGO I and II) and advance stage (FIGO III and IV) of EEC, univariate logistic regression analysis showed that there were no significant differences in the percentage of immune cell subsets except NK cells between patients with early EEC (FIGO I and II) and patients with advanced stage EEC (FIGO III and IV) (P = 0.004). Multivariate binary logistic regression analysis showed that CD4+ T and NK cells were independent risk factors for FIGO staging in patients with EEC after adjusting for the confounding factors of age and BMI (Figure 5). Therefore, we used the ROC curve to determine the critical value of NK cells in the FIGO stages of EEC, and we calculated the cut-off value as 19.94% (Figure 6).
Discussion
The study focused on the changes of immune cell subsets in patients with EEC and explored the relationship between immune cells and the FIGO staging in EEC patients. All the women included in the study were matched in age. At present, the clinical significance levels of the percentages and absolute counts of lymphocyte subsets differ. The proportion, or composition of lymphocyte subsets, indicates the development and differentiation of lymphocytes, while the absolute lymphocyte count shows the exact number of lymphocyte subsets in the peripheral blood, indicating the proliferation of progenitor lymphoid cells. As we have shown, the percentage and absolute count of CD4+ T cells in patients with EEC were lower than those in patients without cancer; however, the other lymphocyte percentages were normal. Xia et al also found that the absolute number of CD3+CD4+ cells is an independent factor for predicting progression-free survival in patients with non-small cell lung cancer.8 To some extent, a decrease in CD4+ T cells in the peripheral blood may weaken the body’s anti-tumor immunity and increase the risk of occurrence and development of cancer.
When T lymphocytes were inhibited, the proportion of NK cells increased significantly, indicating that the immune response ability of NK cells was enhanced, which may be because cellular immune function promoted the proliferation of NK cells. According to the FIGO staging system, patients with early EC (stages I and II) usually experience relatively good results after surgery and adjuvant radiotherapy/chemotherapy.9,10 In contrast, patients with advanced EC (FIGO III and IV) have metastasis to ovaries, lymph nodes, and other parts of the body.11 Even with multi-dimensional and high-intensity treatment, it is easy to relapse, and the overall survival is low.12 In this study, we found that NK cells have an advantage in distinguishing FIGO stages of EEC. Calculating the cutoff value of NK cells may help clinicians identify the disease state. However, there are still many controversies about the role of NK cells in different tumor types. Lorenzo-Herrero et al reported that increased NK cell infiltration is a good prognostic factor for prostate, gastric, and esophageal cancers.13 In contrast, some studies have reported a negative correlation between tumor infiltrating lymphocytes, including NK cells and advanced cancer.14,15 This may be related to the different expression of NK cell subsets and their functional molecules (cytotoxicity, regulation, and tolerance) in tumor patients under different clinicopathological conditions, or the imbalance in the expression of activated or inhibitory receptors in NK cells, resulting in the decrease of NK cell cytotoxicity.16 As the results showed that patients with EEC may be in a state of immune imbalance, which is mainly due to the dysregulation of NK cells. In the future, the classification of NK cell subsets in EC patients can be further clarified, which may have implications for immunotherapy based on tumor NK cells.
Immune function is closely related to the occurrence and development of tumors, the efficacy of treatments, and the survival rate of patients. High expression of CD4+ T tumor-infiltrating immune cells is associated with a better survival prognosis and a longer treatment-free interval,17 and CD4+ helper T cells may be an effective target for improving survivorship (by regulating chemotherapy sensitivity).18 Thus, these study findings provide medical guidance for EC immunotherapy. The percentages and absolute counts of CD4+ T cells in patients with EEC are low, indicating that the immune function of the patients is impaired. The decrease of CD4+T cells did not significantly affect the FIGO stage of EEC, but after adjusting the confounding factors of age and BMI, this decrease played a potential role in promoting the malignant progression of EEC. This finding should be verified in further populations before fully recognizing the influence of CD4+ T cells on the malignant progress of EEC.
Damage to the immune system can promote tumor immune escape, which in turn leads to tumor progression.19 David et al reported the long-term follow-up results of pembrolizumab for second-line treatment of microsatellite instability (MSI-H)/mismatch repair defect (dMMR) in patients with advanced EC, which showed the long-term survival benefits of immunotherapy.20 Therefore, during clinical treatment, attention should be paid to the immune status of patients, as the improvement of immune function can enhance anti-tumor ability. Low immune function is a key factor in the recurrence and metastasis of malignant tumors. Through the detection of lymphocyte subsets, especially CD4+ T and NK cells, the immune status of patients in the population can be identified early, which can help patients regulate their immunity and enhance immune function.
Currently, the mechanisms of occurrence and development of EC remain unclear. Multivariate logistic regression analysis revealed that menopausal status, endometrial thickness, CHOL, and high CA125 were independent risk factors for the onset of EEC. These findings concur with the findings of previous research.21–24 For example, Smith-Bindman et al observed a cancer risk of approximately 7.3% in postmenopausal women with abnormal vaginal bleeding and an endometrial thickness > 5 mm (if the endometrial thickness was < 5 mm, the risk of cancer was < 0.07%).7 Our results further confirmed the correlation between endometrial thickness and EC. Thus, the diagnostic value of endometrial thickness in the detection of EC cannot be ignored and should be elucidated in future studies. The association between lipid metabolism disorders and the occurrence of EC has also attracted attention in recent years. Some studies have demonstrated that serum cholesterol levels and lipid metabolism disorders are correlated with the occurrence of EC.25 High total cholesterol level was also an independent risk factor for EC in this study. Future studies should explore the pathogenic mechanism mediating this association. The prognosis of cancer patients is based not only on tumor-related factors, but also on the activation of the host’s systemic immune cells.26 It is necessary to expand the number of clinical observations and further clarify the differences in lymphocyte subsets in patients with EEC. Therefore, in this study, we emphasized the need to detect lymphocyte subsets at the clinic, which will help clinicians to understand the changes in patients’ immune function, analyze the clinical situation, and predict curative effects on patients.
Monitoring the changes of immune cell subsets in peripheral blood is an effective strategy for optimizing tumor immunotherapy, and peripheral blood sampling is easy to obtain, minimally invasive, and repeatable. These characteristics can make up for the inherent limitation of tissue-based immune biomarkers during tumor immunotherapy. Limitations of this study include insufficient generalizability owing to the single-center nature of the study and the homogenous study population, as well as potential biases standard within epidemiologic investigations. Thus, it is necessary to verify our results in larger, highly powered multi-center studies. We need to confirm our findings within a larger study sample with more detailed prospective clinical data in order to clarify the associations between peripheral blood lymphocytes and the occurrence and progression of EC.
Conclusion
The CD4+ T cells of patients with EEC decreased significantly compared to those in patients without cancer, indicating that the immune function of patients was impaired. The NK% in patients with EEC was higher than 19.94%, which is related to advanced EEC and likely to be a potential predictive factor for FIGO staging in patients with EEC. By paying attention to the balance between patients’ immune cells’ composition, we may be able to better evaluate patients’ prognoses and improve their treatment plans.
Abbreviations
EC, Endometrial cancer; EEC, Endometrioid endometrial cancer; FIGO, International Federation of Gynecology and Obstetrics; NK, Natural Killer cell; LVSI, Lymphovascular space invasion; DMI, Depth of myometrial invasion; BMI, Body mass index; ORs, Odds ratios; CIs, Confidence intervals; TG, Triglyceride; CHOL, Cholesterol; CA125, Carbohydrate antigen 125; ROC, Receiver operating characteristic; AUC, Areas under the curve.
Data Sharing Statement
All data and material are contained within the manuscript. Data are available upon request from Professor Pengming Sun: ([email protected]).
Ethics Approval and Consent to Participate
The study design was approved by the ethics review board of Fujian Maternity and Child Health Hospital, with ethical approval code 2019-137. Informed consent was obtained from each patient.
Consent to Publication
All contributing authors consent to publication of the manuscript.
Acknowledgments
This work was supported by the Fund of Fujian Maternity and Child Health Hospital (Grant No. YCXM 19-10) and the National Key Research and Development Program of China (Grant No. 2022YFC2704300).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no competing interests.
References
1. Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.21492
2. Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2021. CA Cancer J Clin. 2021;71(1):7–33. doi:10.3322/caac.21654
3. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34. doi:10.3322/caac.21551
4. Li B, Wan X. Prognostic significance of immune landscape in tumor microenvironment of endometrial cancer. J Cell Mol Med. 2020;24(14):7767–7777. doi:10.1111/jcmm.15408
5. Bellone S, Centritto F, Black J, et al. Polymerase ε (POLE) ultra-mutated tumors induce robust tumor-specific CD4+ T cell responses in endometrial cancer patients. Gynecol Oncol. 2015;138(1):11–17. doi:10.1016/j.ygyno.2015.04.027
6. Pascual-García M, Bértolo C, Nieto JC, et al. CD8 down-regulation on cytotoxic T lymphocytes of patients with endometrioid endometrial carcinomas. Hum Pathol. 2016;56:180–188. doi:10.1016/j.humpath.2016.05.025
7. Smith-Bindman R, Weiss E, Feldstein V. How thick is too thick? When endometrial thickness should prompt biopsy in postmenopausal women without vaginal bleeding: endometrial thickness and cancer risk. Ultrasound Obstetr Gynecol. 2004;24(5):558–565. doi:10.1002/uog.1704
8. Xia Y, Li W, Li Y, et al. The clinical value of the changes of peripheral lymphocyte subsets absolute counts in patients with non-small cell lung cancer. Transl Oncol. 2020;13(12):100849. doi:10.1016/j.tranon.2020.100849
9. Emons G, Vordermark D. Adjuvant treatment for endometrial cancer:. Curr Opin Oncol. 2019;31(5):404–410. doi:10.1097/CCO.0000000000000558
10. Lu M, Zheng J, Xu N, et al. Postoperative chemotherapy as adjuvant treatment for endometrioid adenocarcinoma: early stage vs late stage. Cancer Chemother Pharmacol. 2019;84(2):299–305. doi:10.1007/s00280-019-03847-w
11. Bohîlțea R, Ancăr V, Rădoi V, et al. Project for the national program of early diagnosis of endometrial cancer part II. J Med Life. 2015;8(4):423–431.
12. Braun MM. Diagnosis and management of endometrial cancer. Endometrial Cancer. 2016;93(6):7.
13. Lorenzo-Herrero S, López-Soto A, Sordo-Bahamonde C, et al. NK cell-based immunotherapy in cancer metastasis. Cancers. 2018;11(1):29. doi:10.3390/cancers11010029
14. Vgenopoulou S, Lazaris AC, Markopoulos C, et al. Immunohistochemical evaluation of immune response in invasive ductal breast cancer of not-otherwise-specified type. Breast. 2003;12(3):172–178. doi:10.1016/S0960-9776(03)00004-3
15. Somayeh R, Safaei A, Talei A, et al. NK, NKT and invariant-NKT cells in tumor draining lymph nodes of patients with breast cancer. Iran J Immunol. 2019;16(4):291–298.
16. Rezaeifard S, Talei A, Shariat M, et al. Tumor infiltrating NK cell (TINK) subsets and functional molecules in patients with breast cancer. Mol Immunol. 2021;136:161–167. doi:10.1016/j.molimm.2021.03.003
17. Zhang S, Minaguchi T, Xu C, et al. PD-L1 and CD4 are independent prognostic factors for overall survival in endometrial carcinomas. BMC Cancer. 2020;20(1):127. doi:10.1186/s12885-020-6545-9
18. Guo F, Dong Y, Tan Q, et al. Tissue infiltrating immune cells as prognostic biomarkers in endometrial cancer: a meta-analysis. Dis Markers. 2020;2020:1–11. doi:10.1155/2020/1805764
19. Dunn GP, Bruce AT, Ikeda H, et al. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3(11):991–998. doi:10.1038/ni1102-991
20. O’Malley DM, Bariani GM, Cassier PA, et al. Pembrolizumab in patients with microsatellite instability-high advanced endometrial cancer: results from the KEYNOTE-158 study. J Clin Oncol. 2022;2022:JCO2101874.
21. Bacalbasa N, Diaconu C, Iliescu L, et al. The influence of the metabolic syndrome on early postoperative outcomes of patients with advanced-stage endometrial cancer. In Vivo. 2020;34(5):2913–2917. doi:10.21873/invivo.12120
22. Kokts-Porietis RL, McNeil J, Nelson G, et al. Prospective cohort study of metabolic syndrome and endometrial cancer survival. Gynecol Oncol. 2020;158(3):727–733. doi:10.1016/j.ygyno.2020.06.488
23. Reijnen C, Visser NC, Kasius JC, et al. Improved preoperative risk stratification with CA-125 in low-grade endometrial cancer: a multicenter prospective cohort study. J Gynecol Oncol. 2019;30(5):e70. doi:10.3802/jgo.2019.30.e70
24. Knific T, Osredkar J, Smrkolj Š, et al. Novel algorithm including CA-125, HE4 and body mass index in the diagnosis of endometrial cancer. Gynecol Oncol. 2017;147(1):126–132. doi:10.1016/j.ygyno.2017.07.130
25. Yasin HK, Taylor AH, Ayakannu T. A narrative review of the role of diet and lifestyle factors in the development and prevention of endometrial cancer. Cancers. 2021;13(9):2149. doi:10.3390/cancers13092149
26. Showe MK, Kossenkov AV, Showe LC. The peripheral immune response and lung cancer prognosis. OncoImmunology. 2012;1(8):1414–1416. doi:10.4161/onci.21096
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.