Back to Journals » Journal of Inflammation Research » Volume 13
Peripheral Blood Inflammatory-Immune Cells as a Predictor of Infertility in Women with Polycystic Ovary Syndrome
Authors He S, Mao X , Lei H , Dong B, Guo D, Zheng B, Sun P
Received 7 May 2020
Accepted for publication 29 July 2020
Published 18 August 2020 Volume 2020:13 Pages 441—450
DOI https://doi.org/10.2147/JIR.S260770
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
ShuQiong He,1,* XiaoDan Mao,2,3,* HuiFang Lei,2,3 BinHua Dong,2,3 DanHua Guo,1 BeiHong Zheng,4 PengMing Sun2,3
1Department of Prenatal Diagnosis, Fujian Maternity and Child Health Hospital, Affiliated Hospital of Fujian Medical University, Fuzhou, 350001, People’s Republic of China; 2Laboratory of Gynecologic Oncology, Department of Gynecology, Fujian Maternity and Child Health Hospital, Affiliated Hospital of Fujian Medical University, Fuzhou, 350001, People’s Republic of China; 3Fujian Key Laboratory of Women and Children’s Critical Diseases Research, Fujian Maternity and Child Health Hospital, Fuzhou, 350001, People’s Republic of China; 4Department of Reproduction, Fujian, Affiliated Hospital of Fujian Medical University, Fuzhou, 350001, People’s Republic of China
*These authors contributed equally to this work
Correspondence: PengMing Sun
Laboratory of Gynecologic Oncology, Department of Gynecology, Fujian Maternity and Child Health Hospital, Affiliated Hospital of Fujian Medical University, No. 18 Daoshan Road, Fuzhou 350001, Fujian, P.R. of China
Email [email protected]
Purpose: This study aimed to investigate the inflammatory-immune cells in the peripheral blood of women with polycystic ovary syndrome (PCOS) and assessed the potential correlation between inflammatory-immune cells and infertility in PCOS women.
Materials and Methods: In this case–control study, the profiles of lymphocyte subsets were analyzed by flow cytometry. White blood cells (WBC), neutrophils (Neu), lymphocytes, Ferriman–Gallwey (F–G) score, testosterone, prolactin, follicle-stimulating hormone, luteinizing hormone, fasting blood glucose, and fasting plasma insulin were measured, together with body mass index. Association between inflammatory-immune cells and PCOS was evaluated. Moreover, inflammatory-immune cells of the PCOS women with infertility were evaluated, and the relative operating characteristic (ROC) curve and cutoff values were calculated.
Results: The number of WBC, Neu, and lymphocytes was higher in PCOS women than controls (P< 0.05). The percentages of total T lymphocytes, CD4+T, and NK were significantly increased in the PCOS group (P< 0.001). The CD4/CD8 ratio was obviously elevated for increasing CD4+T (P< 0.05). Consequently, T%, CD4+T%, and NK% were found to be the independent risk factors of PCOS by ROC curve and multivariate logistic regression analysis. Furthermore, only NK% was significantly higher in PCOS women with infertility than those who had PCOS without infertility (P< 0.001). To diagnose infertility in PCOS, the cutoff value of NK% was calculated as 16.43%.
Conclusion: These findings suggest that the pathogenesis of PCOS is related to immune cells including T, CD4+T, and NK cells. NK cells are likely to be a potential predictive factor for PCOS women with infertility.
Keywords: lymphocyte subsets, inflammatory cells, natural killer cells, immunocompetent cells, pathogenesis
Introduction
Polycystic ovary syndrome (PCOS) is an endocrine disorder characterized by hyperandrogenemia, anovulation or rare ovulation, and polycystic ovary,1,2 which is considered as the most common cause of female infertility.3 Furthermore, PCOS is a powerful risk factor for insulin resistance (IR), type 2 diabetes mellitus (T2DM), cardiovascular disease, gestational diabetes, and endometrial cancer.4,5 In recent years, a plethora of studies have assessed its management, ranging from the psychosocial aspects of the conditions to the treatment of anovulatory infertility and reproductive consequences.6,7 Besides, recent studies have shown that IR and hyperandrogenemia have potential effects on the pathogenesis of PCOS.8,9 However, the mechanism of ovulatory dysfunction and pathogenesis in PCOS remain elusive, thus limiting the development of therapeutics. As the 2003 Rotterdam Criteria have been accepted internationally, the diagnostic of PCOS is undisputed. However, there is no objective, accurate biomarker to assess the pregnancy outcomes in patients with PCOS.
Recently, PCOS has also been found to be associated with chronic low-grade inflammation, including high levels of leukocytes, endothelial dysfunction, and disordered pro-inflammatory cytokines (such as interferon-gamma [IFN-γ] and interleukin-2 [IL-2]).10,11 Moreover, immune dysregulation in PCOS has also received increasing recognition. Accumulating evidence suggests that immunological mechanisms are involved in the regulation of PCOS. Of note, large amounts of immunocompetent cells, including T cells, B cells, and macrophages, have been found in human preovulatory follicles.12 Besides, recent studies have shown that several cytokines, including IFN-γ, tumor necrosis factor-α (TNF-α), IL-2, IL-4, IL-5, and IL-10, were produced by immunocompetent cells in the blood from PCOS with infertility in vitro,13 which indicate a possible correlation between immune function and female infertility.
In this study, we aim to evaluate the immunocompetent cells to predict infertility in PCOS women. As immunocompetent cells play a vital role in the occurrence and development of PCOS, knowledge regarding its role in PCOS is helpful for further understanding the molecular mechanism of immunocompetent cells in PCOS pathogenesis and providing new predictor for the infertility of females with PCOS.
Materials and Methods
Subjects
All subjects participating in this study gave written informed consent, and the Ethics Committee of Fujian Maternity and Child Health Hospital approved the study. The present study was designed as a case–control study conducted in a single center: consecutive outpatients of the fertility counseling department of Fujian Maternity and Child Health Hospital during the period January 2017 to January 2018. Finally, 175 patients with PCOS and 196 age- and body mass index (BMI)-matched healthy controls were included. All PCOS patients were diagnosed and divided into four phenotypes according to the Rotterdam Criteria: 107 patients with complete phenotype with hyperandrogenism (H) + oligomenorrhea (O) + the observation of polycystic ovaries on a sonogram (PCO) (A phenotype); 20 patients with H + O (B phenotype); 26 patients with H + PCO (C phenotype); and 22 patients with O + PCO (D phenotype). Women with PCOS were diagnosed according to the Rotterdam Criteria1 and must have at least two of the following three criteria: oligomenorrhea and/or anovulation, clinical and/or biochemical signs of hyperandrogenism, and polycystic ovaries (defined by the presence of 12 or more follicles measuring 2 ± 9 mm in diameter in each ovary and/or an ovarian volume >10 cm3, diagnosed by Voluson™ E10 [GE Healthcare, IL, USA]). In this study, the inclusion criteria of PCOS subjects are as follows: age ranging from 20 to 40 years old, PCOS diagnosis, and with the fertility requirements in recent 1 year. The exclusion criteria are as follows: hyperprolactinemia, congenital adrenal hyperplasia, Cushing’s syndrome, androgen-secreting tumors, hypophysoma, autoimmune disease, thyroid disease, history of abortion/infertility, and other inflammatory states. On the other hand, the control group was designed approximately 1:1 population of age- and BMI-matched cases with regular ovulatory menstrual cycles (26–32 days), without hirsutism and acne, with normal ovarian morphology on ultrasound examination, without a personal or family history of infertility, and without other chronic diseases of inflammatory origin and were in general good health. None of the women in the study was pregnant or was on immune-suppressive/modulatory treatment, hormonal therapy (including thyroxine replacement and/or contraceptive pills), or antibiotics at the time of laboratory assays. The reproductive outcomes of all PCOS subjects were followed up for 1 year. Infertility is defined as women with normal sexual life cohabiting for 1 year and not pregnant14 (excluding male infertility, fallopian tube factor, or factors of uterine anatomy). Moreover, pregnancy is defined as women with gestation ≥12 weeks. Finally, 105 PCOS women were diagnosed as infertile, and 67 PCOS women were pregnant (Figure 1).
Sample Size
In our study, a sample size of 175 cases in PCOS group (Infertility group (n=103), Non-infertility group (n=65)) and 196 cases in control group were obtained from the two groups whose NK cell frequencies were compared. We performed a sample size calculation according to two independent design data calculation formulas: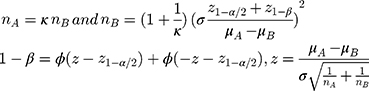
The result we got was that each group needed 46 cases at least. The sample size calculated was enough, and the power was 0.8. In addition, in our study, among the 175 PCOS patients retrospectively screened, 107 women exhibited complete phenotype with H + O + PCO (A group), 24 had H + O (B group), 26 had H + PCO (C group), 22 had O + PCO (D group). The result of sample size calculation showed that each group needed 60 cases at least. We tried our best to collect the total number of cases close to our expected sample size. Due to the large number of testing items, we have collected relatively few complete testing cases. However, the data were true and reliable. Owing to the small sample size of our study, we could not exclude that a type 1 error might occur in our statistical analysis.
Outcomes
Anthropometric measurements (weight and height) and age were recorded. All patients were investigated during the follicular phase of their menstrual cycle (between days 3 and 7). For women with PCOS who had not had a spontaneous bleeding episode for >90 days, 100 mg of micronized progesterone (Utrogestan, Faran Laboratories s. a., Athens, Greece) was administered to induce a bleeding episode, and serum blood samples were subsequently collected. Baseline blood samples were drawn from all patients for determination of total testosterone (T), prolactin (PRL), luteinizing hormone (LH), follicle-stimulating hormone (FSH), fasting blood glucose (FBG), and fasting plasma insulin (FINS) concentrations. FBG and FINS were measured by the glucose oxidase method (Randox, UK). Serum T, PRL, LH, and FSH were measured by radioimmunoassay (Testo-CT2 and SHBG-RIACT, respectively; CIS Bio International, Gif-sur-Yvette, France). Routine blood indices, including white blood cell (WBC), neutrophil (Neu), and lymphocyte (Lym) were analyzed for all patients. A second venous blood sample was obtained simultaneously to analyze Lym subset frequencies. Total WBC and differential counts and Lym subset distributions including T lymphocyte, B lymphocyte, natural killer cells (NK cells), CD4+T lymphocyte, and CD8+T lymphocyte were analyzed from fresh blood samples with a Bayer H-3 hematology analyzer using automated cytochemistry in flow and BD Canto II flow cytometry, respectively. Hirsutism was evaluated with Ferriman–Gallwey (F–G) map scoring system (hirsutism was diagnosed if FG ≥5).15 IR was determined by calculation of the homeostasis model assessment (HOMA-IR) score as fasting plasma glucose (mmol/L)× FINS (mIU/L)/22.5.16
Statistical Analysis
Data distribution was assessed by the Kolmogorov–Smirnov test. Continuous variables were presented as means ± standard deviations or as medians (interquartile ranges) as appropriate, depending on whether the data were normally distributed; dichotomous variables were expressed as percentages. Continuous variables were compared among the three groups in the study population with analyses of variance, Kruskal–Wallis tests, unpaired t-tests, or Mann–Whitney U-tests, as appropriate. Categorical variables were compared with chi-squared tests or Fisher’s exact tests, as appropriate. To identify factors associated with PCOS, receiver operating characteristic (ROC) curves were generated for each continuous variable found to be significantly associated with PCOS by the unpaired t-test or Mann–Whitney U-test. The areas under the curve are provided with their sensitivity, specificity, and 95% confidence intervals. The significance of the obtained cutoff values associated with PCOS was tested by performing both univariate and multivariate binary logistic regression analyses. Adjusted risk estimates were obtained with logistic regression models and accounted for the variables used for matching. Significance was set at ≤0.05. All analyses were performed with SPSS v. 22.0 software (IBM Corp., Armonk, NY, USA).
Results
Baseline Characteristics in PCOS Women with or without Infertility and Controls
As shown in Table 1, among the 175 PCOS patients retrospectively screened, 107 women (60.7%) exhibited complete phenotype with H + O + PCO (A phenotype), 24 (11.43%) had H + O (B phenotype), 26 (15.43%) had H + PCO (C phenotype), and 22 (12.57%) had O + PCO (D phenotype). No differences were observed among the four phenotypes regarding age, BMI, T, LH/FSH, PRL, HOMA-IR, and FBG (all P >0.05). On the other hand, Table 2 summarizes the baseline characteristics of the base levels of plasma hormones and biochemical indicators in this study. In general, there were no differences observed among all PCOS groups and controls with respect to age, BMI, total T, and PRL (all P >0.05). However, compared to women in the control group, women with PCOS had significantly elevated F–G Score, FBG, HOMA-IR, and LH/FSH (both P <0.001). Of note, no significant differences in age, BMI, T, F–G Score, LH/FSH, PRL, HOMA-IR, and FBG were found between PCOS women with infertility and without infertility (all P >0.05).
 |
Table 1 Baseline Characteristics of the Study Population According to Polycystic Ovary Syndrome (PCOS) Phenotypes and in Control Subjects |
 |
Table 2 Baseline of the PCOS and Control Subjects on Biochemical Data |
The Characteristics of Inflammatory-Immune Cells in PCOS Women
To clarify the role of inflammatory-immune cells in PCOS, we investigated inflammatory cells and lymphocyte subsets in the peripheral blood (PB) of all PCOS groups and control female subjects. Interestingly, Table 3 shows that WBC, Neu, and Lym counts were significantly higher in women with PCOS (all P <0.05), which indicated patients with PCOS might be in a state of chronic low-degree inflammation. Further, the lymphocyte subsets were also analyzed to evaluate the level of inflammatory-immune in PCOS. Compared to the control group, women with PCOS had significantly higher percentages of T, CD4+T, and NK cells and a higher ratio of CD4/CD8 cells (all P >0.05). There were no statistically significant between-group differences in the percentages of CD8+T cells and B cells (P >0.05). The results showed that PCOS patients’ inflammatory cells kept a relatively high level to a state of mild immune stress. Moreover, they were likely to be in an immune imbalance state, which mainly due to the dysregulation of CD4+T cells and NK cells. In other words, increased inflammatory-immune cells indicated the immune system played an important role in the pathogenesis of PCOS.
 |
Table 3 The Characteristics of Inflammatory-Immune Cells in the PCOS and Control Subjects |
Immune Cells Were the Independent Risk Factors in Women with PCOS
To explore the independent high-risk factors of infertility in PCOS women, the significantly different inflammatory immune cells were evaluated by ROC curve analyses. The cutoff values for the statistically significant PCOS indicators were identified (Figure 2A–G). Based on the cutoff values, T cells, CD4+T cells, and NK cells were found to be independent risk factors for PCOS by the univariate and multivariate binary logistic regression analyses (P <0.05, Figure 3).
NK Cells Play an Important Role in the PCOS Women with Infertility
The fertility outcome was evaluated in the PCOS women. They were divided into two groups according to whether infertility occurs to analyze the relationship between these factors and infertility. There were no statistically significant differences between PCOS women with infertility and without infertility in the percentage of all inflammatory-immune cells except NK% (P <0.001, Table 3). The NK% was chosen to make an ROC curve, and the cutoff values were calculated as 16.43% (Figure 4).
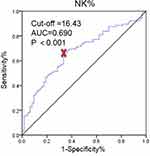 |
Figure 4 ROC curve analysis of NK% between PCOS with and without infertility groups. |
Discussion
This study focused on inflammation and immune reaction in PCOS women. In this study, we investigated the inflammatory indicators in PB, especially lymphocyte subsets in women with PCOS, and explored the relationship between immune function and female infertility in PCOS. The women enrolled in this study were matched for age and BMI. According to the Rotterdam Criteria, all the 175 PCOS patients could be divided into four groups, and no differences were observed among the four phenotypes regarding age, BMI, T, LH/FSH, PRL, HOMA, and FBG. Our results for the basic hormonal profiles for women with PCOS compared to women in the control group were concordant with well-established evidence of the fundamental characteristics of the syndrome.2 Although there were no differences between women with PCOS and controls regarding T, the F–G Score, which could be used to assess the clinical hyperandrogenism symptom, was significantly higher than that of the controls. Our data showed disparities compared with previous reports. This discrepancy might be the result of the heterogeneity of PCOS. Hyperandrogenism in PCOS could be reflected as clinical symptoms like hirsutism and acne and/or biochemical elevation of androgen, which result in the non-differential T between PCOS and control and higher F–G Score in PCOS. Li et al also show that there is no difference in T between PCOS and controls.17 Consistent with previous reports, women with PCOS have higher WBC, Neu, and Lym counts than the control group in our study. PCOS shares a state of low-grade inflammation with other non-transmissible chronic diseases, such as obesity, T2DM, and cardiovascular diseases.3 Markers of low-grade inflammation in PCOS reportedly include C-reactive protein, leukocytes, and cytokines.10,18 In addition, Orio et al also suggested that women with PCOS have increased WBC counts.10 Meanwhile, they claimed that the increased WBC count was mainly attributable to an increased number of peripheral blood mononuclear cells.
As we know, the immune system can promote chronic, low-grade systemic inflammation, which is usually defined by the occurrence of increased cytokines and acute reaction proteins.19,20 Limited to the technology of that time, we evaluated the percentage of lymphocyte subsets in the analysis of immune cell components in PB. Obviously, the structure of immune cells was changed. The proportion of T cells and NK cells was increased in women with PCOS. In addition, the increased T cell proportion was mainly caused by the increased proportion of CD4+T cells. Previous studies have revealed that CD4+T cells are elevated in women with PCOS,21,22 which was in accordance with our results. However, the changes in T lymphocyte subsets in patients with PCOS remain controversial at present. It is well known that CD4+T cells can be subdivided into several different subsets, including Th17 cells, Th1 cells, Th2 cells, and regulatory T cells (Tregs). The interaction among subtypes is very active. Recent studies have shown that, compared with those in women in the control group, the Treg cells in PB of women with PCOS were significantly decreased.23 Additionally, reduced Treg cells resulted in decreased secretion of anti-inflammatory factors, such as interleukin-10, in the body and ovaries and increased levels of some autoreactive antibodies, thus aggravating a chronic inflammatory state.23 Moreover, the expansion of cytotoxic CD4+CD28null T lymphocytes23,24 and increased Th1725 cells have also been found in PB of women with PCOS. Given the complexity of CD4+T cells, we speculated that the balance among each component of CD4+T cells might play an important role in the pathogenesis of PCOS according to our data. Meanwhile, T%, CD4+T%, and NK% were found to be the independent risk factors of PCOS, and the cutoff values were calculated to help clinicians identify the disease. Thus, PCOS is proved to be an immune inflammatory disease affected by the cytokines secreted from CD4+T cells again. Moreover, Sala Elpidio et al found that the killer-cell immunoglobulin-like receptors and their human leukocyte antigen class I ligands were associated with the development of PCOS.26 Currently, uterine NK cells are considered to be endometrial marker for PCOS.27
For women of childbearing age, PCOS is one of the leading causes of infertility. Previous studies showed that abnormal activation of T cells and cytokine production might lead to the abnormal oocyte development observed in PCOS patients, which was associated with women’s infertility.17,28 A recent study also found that the activation of T cells in PCOS patients with infertility might be suppressed by increased expression of PD-1. IL-10 markedly inhibits the functions of monocytes-macrophages, such as antigen presentation.17 Therefore, the lymphocyte subsets were evaluated and T cell subsets were focused in our research. Unexpectedly, T cell subsets and B cells did not seem to differ from infertile women to pregnant women with PCOS (P >0.05). However, NK cells showed a significant elevation in the PCOS women with infertility (P <0.001). Numerous studies claimed that NK cell played an important role in female infertility or recurrent miscarriage.29 Seshadri et al reported that peripheral NK cells as numbers showed significantly higher NK cell numbers in infertile women compared with fertile controls.29 NK cells are likely to be related to infertility or recurrent miscarriage and used to evaluate the risk of infertility or recurrent miscarriage at present. Currently, the data is sparse with regard to the ability of NK cells to predict infertility in women with PCOS. In this research, NK cells showed an advantage in distinguishing PCOS women with infertility. Above all, the cutoff value of NK% was obtained to give a better reminder to clinician. A higher prevalence of women with NK% ≥16.43% was observed in infertility in PCOS women. Triggianese et al discovered that the percentage of peripheral NK cells in infertile/recurrent spontaneous abortion women with non-autoimmune thyroid diseases was higher than those who are fertile women with a 15% cutoff value, which was consistent with our results.30 Zhang et al report for the first time the beneficial effect of intrauterine perfusion of dexamethasone for patients with repeated implantation failure characterized by high number of uterine NK (uNK) cells. The clinical pregnancy rate was decreased if the percentage of uNK cells was higher than the 75th percentile (18.06%), which was considered as the cutoff value for increased uNK cells.31 Although the discrepancies in reported results may be due to the different study populations and different analysis methods, the relevance of changes in T lymphocyte subsets in women with PCOS remains inconclusive. However, there is no denying that immune dysregulation is associated with PCOS and that unbalanced regulation of T lymphocyte subsets plays an important role in this process.
Conclusion
In summary, some routine indicators in PB about inflammatory-immune cells were significantly increased in women with PCOS. Increased CD4+T cells and NK cells were the independent risk factors of PCOS and were probably related to the pathogenesis of PCOS. Furthermore, the increased NK%, which was higher than 16.43%, is associated with the infertility of PCOS patients and is a good predictor in PB to identify infertility in PCOS.
Ethical Approval
This study was approved by the ethical committee of the Department of Gynecology of Fujian Provincial Maternity and Child Health Hospital (ethical no. 2019-137) and complied with the Declaration of Helsinki.
Informed Consent
The requirement to obtain informed consent from research subjects was waived due to the study’s retrospective nature.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no conflicts of interest for this work.
References
1. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertility and sterility. 2004;81(1):19–25. doi:10.1016/j.fertnstert.2003.10.004
2. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Human reproduction. 2004;19(1):41–47. doi:10.1093/humrep/deh098
3. Norman RJ, Dewailly D, Legro RS, Hickey TE. Polycystic ovary syndrome. Lancet. 2007;370(9588):685–697. doi:10.1016/s0140-6736(07)61345-2
4. Moran L, Teede H. Metabolic features of the reproductive phenotypes of polycystic ovary syndrome. Hum Reprod Update. 2009;15(4):477–488. doi:10.1093/humupd/dmp008
5. Niu Z, Lin N, Gu R, Sun Y, Feng Y. Associations between insulin resistance, free fatty acids, and oocyte quality in polycystic ovary syndrome during in vitro fertilization. J Clin Endocrinol Metab. 2014;99(11):E2269–76. doi:10.1210/jc.2013-3942
6. Wang W, Zheng J, Cui N, et al. Baicalin ameliorates polycystic ovary syndrome through AMP-activated protein kinase. J Ovarian Res. 2019;12(1):109. doi:10.1186/s13048-019-0585-2
7. Luo M, Huang JC, Yang ZQ, Wang YS, Guo B, Yue ZP. Hydroxysafflor yellow A exerts beneficial effects by restoring hormone secretion and alleviating oxidative stress in polycystic ovary syndrome mice. Exp Physiol. 2020;105(2):282–292. doi:10.1113/ep088147
8. Laganà AS, Rossetti P, Buscema M, et al. Metabolism and ovarian function in PCOS women: a therapeutic approach with inositols. Int J Endocrinol. 2016;2016:6306410. doi:10.1155/2016/6306410
9. Paul C, Laganà AS, Maniglio P, Triolo O, Brady DM. Inositol’s and other nutraceuticals’ synergistic actions counteract insulin resistance in polycystic ovarian syndrome and metabolic syndrome: state-of-the-art and future perspectives. Gynecol Endocrinol. 2016;32(6):431–438. doi:10.3109/09513590.2016.1144741
10. Orio F, Palomba S, Cascella T, et al. The increase of leukocytes as a new putative marker of low-grade chronic inflammation and early cardiovascular risk in polycystic ovary syndrome. J Clin Endocrinol Metab. 2005;90(1):2–5. doi:10.1210/jc.2004-0628
11. Harris HR, Terry KL. Polycystic ovary syndrome and risk of endometrial, ovarian, and breast cancer: a systematic review. Fertil Res Pract. 2016;2:14. doi:10.1186/s40738-016-0029-2
12. Saito S, Nakashima A, Shima T, Ito M. Th1/Th2/Th17 and regulatory T-cell paradigm in pregnancy. Am J Reprod Immunol. 2010;63(6):601–610. doi:10.1111/j.1600-0897.2010.00852.x
13. Benson S, Janssen OE, Hahn S. Obesity, depression, and chronic low-grade inflammation in women with polycystic ovary syndrome. Brain Behav Immun. 2008;22(2):177–184. doi:10.1016/j.bbi.2007.07.003
14. Zegers-Hochschild F, Adamson GD, Dyer S. The international glossary on infertility and fertility care, 2017. Fertil Steril. 2017;108(3):393–406. doi:10.1016/j.fertnstert.2017.06.005
15. Zhao X-M, Ni R-M, Huang J. [Study on the facial and body terminal hair growth in women in Guangdong by using modified Ferriman-Gallwey scoring system]. Zhonghua Fu Chan Ke Za Zhi. 2013;48(6):427–431.
16. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. doi:10.1007/bf00280883
17. Li Z, Peng A, Feng Y, et al. Detection of T lymphocyte subsets and related functional molecules in follicular fluid of patients with polycystic ovary syndrome. Sci Rep. 2019;9(1):6040. doi:10.1038/s41598-019-42631-x
18. Shi Y, Han T, Cui L, et al. White blood cell differential counts in patients with polycystic ovary syndrome: a pilot study on Chinese women. Eur J Obstet Gynecol Reprod Biol. 2013;170(1):162–164. doi:10.1016/j.ejogrb.2013.06.002
19. Petrikova J, Lazurova I, Yehuda S. Polycystic ovary syndrome and autoimmunity. Eur J Intern Med. 2010;21(5):369–371. doi:10.1016/j.ejim.2010.06.008
20. Benson S, Janssen OE, Hahn S, et al. Obesity, depression, and chronic low-grade inflammation in women with polycystic ovary syndrome. Brain Behav Immun. 2008;22(2):177–184. doi:10.1016/j.bbi.2007.07.003
21. Moro F, Morciano A, Tropea A, et al. CD4(+)CD28(null) T lymphocyte frequency, a new marker of cardiovascular risk: relationship with polycystic ovary syndrome phenotypes. Fertil Steril. 2012;98(6):1609–1615. doi:10.1016/j.fertnstert.2012.08.015
22. Qin L, Xu W, Li X, et al. Differential expression profile of immunological cytokines in local ovary in patients with polycystic ovarian syndrome: analysis by flow cytometry. Eur J Obstet Gynecol Reprod Biol. 2016;197:136–141. doi:10.1016/j.ejogrb.2015.12.003
23. Nasri F, Doroudchi M, Namavar Jahromi B, Gharesi-Fard B. T helper cells profile and CD4+CD25+Foxp3+regulatory T cells in polycystic ovary syndrome. Iran J Immunol. 2018;15(3):175–185. doi:10.22034/iji.2018.39387
24. Hu C, Pang B, Ma Z, Yi H. Immunophenotypic profiles in polycystic ovary syndrome. Mediators Inflamm. 2020;2020:5894768. doi:10.1155/2020/5894768
25. Ozcaka O, Buduneli N, Ceyhan BO, et al. Is interleukin-17 involved in the interaction between polycystic ovary syndrome and gingival inflammation? J Periodontol. 2013;84(12):1827–1837. doi:10.1902/jop.2013.120483
26. Sala Elpidio LN, de Alencar JB, Tsuneto PY, et al. Killer-cell immunoglobulin-like receptors associated with polycystic ovary syndrome. J Reprod Immunol. 2018;130:1–6. doi:10.1016/j.jri.2018.08.003
27. Piltonen TT. Polycystic ovary syndrome: endometrial markers. Best Pract Res Clin Obstet Gynaecol. 2016;37:66–79. doi:10.1016/j.bpobgyn.2016.03.008
28. Vujisic S, Lepej SZ, Aksamija A, et al. B- and T-cells in the follicular fluid and peripheral blood of patients undergoing IVF/ET procedures. Am J Reprod Immunol. 2004;52(6):379–385. doi:10.1111/j.1600-0897.2004.00238.x
29. Seshadri S, Sunkara SK. Natural killer cells in female infertility and recurrent miscarriage: a systematic review and meta-analysis. Hum Reprod Update. 2014;20(3):429–438. doi:10.1093/humupd/dmt056
30. Triggianese P, Perricone C, Conigliaro P, Chimenti MS, Perricone R, De Carolis C. Peripheral blood natural killer cells and mild thyroid abnormalities in women with reproductive failure. Int J Immunopathol Pharmacol. 2016;29(1):65–75. doi:10.1177/0394632015615130
31. Zhang T, Huang C, Du Y, et al. Successful treatment with intrauterine delivery of dexamethasone for repeated implantation failure. Am J Reprod Immunol. 2017;78(6). doi:10.1111/aji.12766
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



