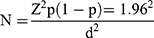Back to Journals » Clinical, Cosmetic and Investigational Dentistry » Volume 12
Periodontal Disease Status and Associated Risk Factors in Patients Attending a Tertiary Hospital in Northwest Ethiopia
Received 20 September 2020
Accepted for publication 31 October 2020
Published 10 November 2020 Volume 2020:12 Pages 485—492
DOI https://doi.org/10.2147/CCIDE.S282727
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Christopher E. Okunseri
Amare Tefera, Biruk Bekele
Department of Dentistry, School of Medicine, College of Medicine and Health Science, University of Gondar, Gondar, Ethiopia
Correspondence: Amare Tefera
Department of Dentistry, School of Medicine, College of Medicine and Health Science, University of Gondar, P. O. Box: 196, Gondar, Ethiopia
Tel +251-910-517002
Email [email protected]
Purpose: Information on periodontal disease and its predictors in sub-Saharan Africa is sparse. This study aimed to determine the prevalence of periodontal disease and assess the relationship with associated factors in patients who attended the University of Gondar comprehensive hospital.
Patients and Methods: A hospital-based cross-sectional study was conducted from October 1, 2019, to March 10, 2020, at the University of Gondar comprehensive Hospital. Participants were recruited with a systematic random sampling technique, and interviewed for sociodemographic and medical information through a structured questionnaire. Two examiners evaluated the periodontal status of the study participants using the community periodontal index (CPI).
Results: Four hundred twenty participants were involved in the study. The mean age of the study participants was 29.87 (± 7. 76). The majority of the study participants had a habit of tooth brushing (72.1%) and almost half of them did not have a fixed time to brush their teeth. Periodontal change (CPI> 0) was noted in 42.4% of the participants and among that 21.7% had shallow pocket (pocket depth of 4– 5 mm) and 5.95% had a deep periodontal pocket (≥ 6 mm). The prevalence was higher in 20– 34 years (57.3%), females (58.98%), monthly income of < 2500 ETB (82.02%), and frequent carbohydrate intakes (65.17%). Age (AOR=9.61, 95% CI: 6.42, 13.04), gender (AOR=2.00, 95% CI: 136, 2.97), educational status (AOR=3.25), residency (AOR= 1.66), monthly income (AOR=2.13), oral hygiene habit (AOR=4.85) and systemic disease (AOR=1.96) were significantly associated with periodontitis.
Conclusion: In the present study, 42.4% of the study participants encountered periodontal disease. The study confirmed that various sociodemographic risk factors/indicators are associated with an increased risk of periodontitis.
Keywords: clinical attachment loss, periodontal disease, periodontal pocket, community periodontal index
Background
Periodontal disease is the second common oral health problem next to dental caries and highly prevalent in African populations.1 The global burden of disease (1990–2010) revealed that periodontal disease as the 6th most prevalent disease in the world with a prevalence of 11.2%.2 The prevalence was differed significantly in low (28.7%), lower-middle (10%), upper-middle (42.5%), and high-income countries (43.7%).3 The prevalence was different in East Africa with a prevalence of 89.8% in Egypt,4 63.9% in Sudan,5 and 53.4% in Ethiopia.6
Periodontal disease has been associated with poor lifestyle, low-socioeconomic status, poor access to health care, and health-related risk behaviors (smoking, obesity, alcohol intake, carbohydrate diets, and poor oral hygiene practice) in developing countries.7–12 A cross-sectional study in Ethiopia found males were affected by periodontal disease,6 and also the prevalence of periodontal disease increases as the age of the participants increases.13 The prevalence of severe periodontal diseases was low in sub-Saharan African countries.14–16 Gingival bleeding on probing (BOP), calculus deposition, probing depth, and loss of clinical attachment are the indicators of periodontal disease and provide data to diagnose the presence of periodontal disease.17
The presence of advanced periodontal disease increases the risk of multiple tooth loss, masticatory dysfunction, and edentulous, which affects nutritional habits, quality of life, and self-esteem of the patients.18–21 In addition to the orofacial outcomes, advanced periodontal disease has been linked to multiple systemic diseases such as; diabetes mellitus, cardiovascular diseases, adverse pregnancy outcomes, respiratory diseases, renal diseases, and increased risk of malignancies of the head and neck.22,23
Because of the sparsity of data on periodontal disease among patients in Northwest Ethiopia, and the hypothesis that factors such as oral hygiene practice, sociodemographic behavior, and perception of oral health would be determinant factors for periodontal disease in adults. The present study is, aimed to determine the periodontal disease status and its potential determinant factors among a population in Northwest Ethiopia, Ethiopia.
Methods
Setting
A hospital-based cross-sectional study was conducted from October 1, 2019, to March 10, 2020, at the University of Gondar Comprehensive Hospital Dental clinic. The dental clinic is the only available governmental dental center in the area that provides oral health-care services for more than 5 million people in the catchment area. The clinic has both inpatient and outpatient services, and periodontal cases were evaluated and managed in the outpatient department.
Study Participants
The source populations were all residents living in Northwest Ethiopia and those who visited the dental clinic of the University of Gondar Comprehensive Hospital during the study period would be included in the study.
Exclusion Criteria
A participant with the following condition was excluded from the study; A patient with a dental emergency or critically ill, a Participant refused to sign a consent, a patient on orthodontic treatment, and a patient on periodontal therapy or treated with periodontal disease in the last 6months.
Sampling Technique and Procedure
A sample size of 420 participants was selected using the single population proportion formula.
With the following assumptions, the prevalence of periodontal disease to be (P) 53.4% a study done in Addis Ababa, Ethiopia.6 Confidence level (CI) of 95%, marginal error (d=5).
The sample size would be 382 and with a 10% non-response rate, the final sample would be 420.
Sampling Procedure
Systematic random sampling would be used; the first participant was selected using random sampling and the remaining would be every third patient (k=3) until the sample size was reached.
Data Collection
Structured interviews were designed, adopted from the WHO oral health surveys,24 and a face to face interview was conducted with the participants at the dental clinic. The interview questionnaire was developed in English, translated into the local language of Amharic, and back translated into English. Two dental professionals reviewed the questionnaire for its clearness, sensitivity to culture, and the presence of appropriate words. A pilot study was done in 42 patients attending the dental clinic, university of Gondar hospital, before the administration. Qualified two dental surgeons (KD and MT) did data collection. The collected data were sociodemographic, tooth brushing habits, dietary habits, and medical conditions. Moreover, the principal investigator (PI) trained the data collectors for 3 days on how to maintain confidentiality, approach, interview, and research ethics. A day-to-day supervision was made by the PI to check the completeness of the questionnaire.
Intra-Oral Examination
Two dental professionals (AT and BG) carried out all periodontal examinations in the dental clinic of the University of Gondar Hospital Dental Clinic based on the WHO oral health survey guideline24 using CPI periodontal probe, mouth mirror, dental light source, and tissue forceps. Third molars were excluded from scoring due to its delayed eruption. A periodontal examination, for each participant, was performed with subjects seated in the dental chair. The sextant of each tooth was examined using a CPI probe around the whole circumference of the tooth, pocket depths were measured at two sites per tooth (buccal ad lingual), and the highest sextant score was recorded. Finally, the participants were categorized based on their CPI results (CPI code: 0 = healthy periodontal tissue; 1 = bleeding on probing; 2=calculus; 3=periodontal pocket (PD) 4–5mm; 4=PD (≥ 6mm)).
Data Analysis
Each questionnaire was checked for completeness and coded before fed into the computer, and entered into Epi-info 7.0 software, and transferred to SPSS version 20 for analysis. Categorical variables were presented using frequencies and percentages. The association between the independent variables and periodontal disease was initially investigated using bivariate analysis and those with a p-value of ≤0.2 were included in the multivariable analysis. Results were presented using OR with its 95% CI.
Ethical Consideration
Ethical approval was sought from the University of Gondar ethical review board (IRB) before the commencement of the study (V/P/RCS/05/149/2019). Besides, a study permit was acquired from the University of Gondar Department of Dentistry. Written consent was obtained from each participant.
Results
Sociodemographic data of the study participants are shown in Table 1. Fifty-one percent of the subjects were males, 35.2% were students and 78.1% were urban residents. The mean age of the participants was 29.87 (±7.76). The majority (78.3%) attended formal education, and 73.1% were from the age group 20 to 34 years.
 |
Table 1 The Sociodemographic Characteristics of the Study Participants (n=420) |
Table 2 presents the oral hygiene practice and carbohydrate intake habits of the study participants. The majority of the study participants had a habit of tooth brushing (72.2%). However, 62.7% had no regular tooth brushing habits. 43.6% of the participants brushed their teeth in the early morning and 81.9% used horizontal stroke to brush their teeth. Only 6.9% of the participants brushed their teeth twice and 43.2% had no fixed time to brush their teeth. In addition, 72.9% of the participants consumed fermentable carbohydrates (See Table 2).
 |
Table 2 Frequency Distribution of the Associated Factors for Periodontal Disease |
Prevalence of Periodontal Changes and Associated Factors
Periodontal inflammation (CPI>0) was observed in 42.4% of the participants, bleeding on probing (BOP), calculus deposition and shallow and deep pockets were the commonly detected changes. The presence of a shallow pocket was found in 21.7% of the participants, and 5.9% had a deep periodontal pocket (Table 3). The prevalence was higher in 20–34 years (57.3%), females (58.98%), monthly income of <2500 ETB (82.02%), frequent carbohydrate intakes (65.17%), and self-perceived halitosis patients (56.17%) (See Table 4).
 |
Table 3 The Periodontal Status of the Study Participants Based on the Independent Variables |
 |
Table 4 Multivariate Analysis of Variables Associated with Periodontal Disease Among Patients Visited the Dental Clinic of the University of Gondar Comprehensive Hospital, Gondar, Ethiopia |
Logistic Regression Analysis
Table 4 shows the results of the adjusted analysis. Factors that had a significant association in the bivariate analysis were entered into the multivariate logistic regression model as an independent variable for the outcome of periodontal disease. The analysis found a significant association between periodontal disease and age (AOR=9.61 (6.42, 13.04); P=0.0000), gender (AOR=2.00 (1.36, 2.97); P=0.000), educational status (AOR=3.25 (2.00, 5.29)), residency (AOR=1.66 (1.04, 2.65)) and poor oral hygiene practice (AOR=4.85 (3.059, 7.69)).
A patient with self-perceived halitosis were 1.64 times at risk of developing periodontal disease (AOR=1.64 (1.11, 2.43); P= 0.012), and the presence of the systemic disease, has a significant effect on the occurrence of malocclusion (AOR=1.96 (1.02, 3.79), P=0.041) (See Table 4).
Discussion
The habit of maintaining day to day oral hygiene was found to be significantly correlated with better periodontal health25 and this perception was confirmed in the current study as the number of patients without tooth brushing habit showed more periodontal disease. The presence of periodontal disease is a reality in adult populations and affects the quality of their life.18–21 The present study has identified a significant amount (42.38%) of the participants had some form of periodontal inflammation, which corresponds with a study done in Addis Ababa (53.4%),6 and Arusi (52%).26 However, this result is low compared with a study done in Egypt (89.8%)4 and Sudan (63.9%).5
A cross-sectional study done in Sudan among pregnant women revealed that 8.9% of the participants had moderate periodontal disease and 3.0% had a severe periodontal disease, which is supported by the present study where 5.95% had severe periodontal disease. However, the findings of the study were found to be higher in the study done in Brazil (2.9%).27 This difference might be due to the oral health-care system and awareness difference between the two countries.
The present study revealed that 57.30% of periodontal disease was found within the 20–34 years age group and 24.15% in elderly patients. More than two-thirds of elder patients developed a periodontal disease which supports the findings of the previous studies.13,28,29 However, age itself does not affect the periodontal condition, rather it is the cumulative effect of untreated disease reflecting the effect of age on disease severity.30 Moreover, females were 2 times at risk of developing periodontal disease than males which is similar to a study in India.31 However, a study done in Spain revealed a higher prevalence of periodontal disease among males than females.32 This might be males using more oral hygiene practice compared with females due to the cultural influence in the country and the hormonal changes during pregnancy and lactation increase the incidence of periodontal change.
On analysis of the socioeconomic variables, a statistically significant association was found between low educational level, low social class, and a higher prevalence of periodontal disease. This agrees with previous studies.20,27,32,33 Participants who had no formal education were 3.25 times at risk of developing periodontal disease (AOR=3.25, 95% CI: 2.00, 5.29). Moreover, participants with a monthly income of <2500Ethiopian birr were at high risk of developing periodontal disease (AOR=2.13, 95% CI: 1.33, 3.40). The present study found a non-significant association between smoking and periodontal disease (OR= 1.01, p=0.52) which is against a study done in Spain where smokers were twice more likely to develop periodontal disease (OR=2.0).32 The possible reason for the absence of a significant association in the present study included cultural influence on smoking and a small sample of the participants were smokers.
There are literatures that found a significant correlation between halitosis and increases prevalence and depth of periodontal pockets, increased radiographic bone loss, and the presence of periodontal pathogens.34,35 In the present study, there was a significant correlation between self-perceived halitosis and periodontal disease. A participant with self-perceived halitosis was 1.65 times more likely to develop periodontal change which corresponds with a study done in Spain, where the unfavorable periodontal condition was higher among those who evaluated their oral health negatively (e.g. Presence of halitosis).27 Similarly, a significant association was found between periodontal disease and the presence of systemic diseases. A participant who had other systemic disease was 1.96 times more likely to develop periodontal changes which are similar to previous studies.36,37
Limitations of the Study
The present study had some inherent limitations. First, the study focused on patients who attended the dental clinic and we hope it increases the prevalence of the disease. Second, even if some factors worsened the periodontal condition, we only targeted individual-level factors. Hence, further research is recommended to focus on factors such as; service providers, barriers, community water fluoridation, and conduct on large-scale community surveys.
Strengths of the Study
The study used systematic random sampling during sample selection, subsequently, reduces the selection bias. Moreover, the questionnaire was adapted from the WHO oral health survey 5th edition and the pretest was done in 10% of the total sample size before the actual study.
Conclusion
In the present study, 42.4% of the study participants encountered periodontal disease. The study confirmed that; age, gender, residency, monthly income, and presence of self-perceived halitosis were significant predictors associated with periodontal disease. To reduce the prevalence of periodontal disease and minimize its impact on the community oral health education should be given at the school level and community-based oral health education programs should be designed.
Abbreviations
CI, confidence interval; CPI, community periodontal index; AOR, adjusted odds ratio; PD, pocket depth.
Data Sharing Statement
All the supporting information were included in the main manuscript.
Ethics Approval and Consent to Participate
The study was approved by the institutional review board (IRB) of the University of Gondar and Complied with the Declaration of Helsinki Ethical Principles for Medical Research. Before the commencement of the study, a formal letter was given to the department of Dentistry and written consent was obtained from each study participant after explaining the purpose of the study. All the participants were informed about the confidentiality of their information (IRB number: V/P/RCS/05/149/2019).
Acknowledgments
Our earnest gratitude goes to the University of Gondar IRB for their review and approval of this research paper. Our appreciation is also forwarded to data collectors and participants, without their involvement, it would not have been realized.
Author Contributions
Both authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
There was no external funding for this study.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Grappin G, Lancour M. Periodontal disease; a grave socio-economic problem in black Africa. Odontostomatol Trop. 1978;11:7–28.
2. Kassebaum NJ, Bernabé E, Dahiya M, Bhandari B, Murray CJL, Marcenes W. Global burden of severe periodontitis in 1990–2010: a systematic review and meta-regression. J Dent Res. 2014;93(11):1045–1053. doi:10.1177/0022034514552491
3. Nazir M, Al-Ansari A, Al-Khalifa K, Alhareky M, Gaffar B, Almas K. Global prevalence of periodontal disease and lack of its surveillance. ScientificWorldJournal. 2020;2020:2146160. doi:10.1155/2020/2146160
4. Abbass MM, Rady D, Radwan IA, et al. The occurrence of periodontal diseases and its correlation with different risk factors among a convenient sample of adult Egyptian population: a cross-sectional study. F1000Research. 2020;8:1740. doi:10.12688/f1000research.20310.2
5. Khalifa N, Allen PF, Abu-bakr NH, Abdel-Rahman ME, Abdelghafar KO. A survey of oral health in a Sudanese population. BMC Oral Health. 2012;12(1):5. doi:10.1186/1472-6831-12-5
6. Simon C, Tesfaye F, Berhane Y. Assessment of the oral health status of school children in Addis Ababa. Ethiop Med J. 2003;41(3):245–256.
7. Gugushe TS. The influence of socio-economic variables on the prevalence of periodontal disease in South Africa. SADJ. 1998;53(2):41–46.
8. Cruz SSD, Costa MDCN, Gomes Filho IS, Vianna MIP, Santos CT. Maternal periodontal disease as a factor associated with low birth weight. Rev Saude Publica. 2005;39:782–787. doi:10.1590/S0034-89102005000500013
9. Sakki TK, Knuuttila ML, Vimpari SS, Hartikainen MS. Association of lifestyle with periodontal health. Community Dent Oral Epidemiol. 1995;23(3):155–158. doi:10.1111/j.1600-0528.1995.tb00220.x
10. Haber J, Wattles J, Crowley M, Mandell R, Joshipura K, Kent RL. Evidence for cigarette smoking as a major risk factor for periodontitis. J Periodontol. 1993;64(1):16–23. doi:10.1902/jop.1993.64.1.16
11. Peck T, Price C, English P, Gill G. Oral health in rural South African type 2 diabetic patients. Trop Doct. 2006;36(2):111–112.
12. Kroidl A, Schaehen A, Oette M, Wettstein M, Herfordt A, Haussinger D. Prevalence of oral lesions and periodontal diseases in HIV-infected patients on antiretroviral therapy. Eur J Med Res. 2005;10(10):448.
13. Kim JK, Baker LA, Seirawan H, Crimmins EM. Prevalence of oral health problems in US adults, NHANES 1999–2004: exploring differences by age, education, and race/ethnicity. Spec Care Dentist. 2012;32(6):234–241. doi:10.1111/j.1754-4505.2012.00280.x
14. Mumghamba EG, Manji KP. Maternal oral health status and preterm low birth weight at Muhimbili National Hospital, Tanzania: a case-control study. BMC Oral Health. 2007;7(1):8. doi:10.1186/1472-6831-7-8
15. Baelum V, Scheutz F. Periodontal diseases in Africa. Periodontol 2000. 2002;29(1):79–103. doi:10.1034/j.1600-0757.2002.290105.x
16. Baelum V, Manji F, Fejerskov O, Wanzala P. Validity of CPITN’s assumptions of hierarchical occurrence of periodontal conditions in a Kenyan population aged 15–65 years. Community Dent Oral Epidemiol. 1993;21(6):347–353. doi:10.1111/j.1600-0528.1993.tb01097.x
17. Segundo TK, Ferreira EF, Costa JED. A doença periodontal na comunidade negra dos Arturo’s, Contagem, Minas Gerais, Brasil. Cad Saude Publica. 2004;20:596–603. doi:10.1590/S0102-311X2004000200029
18. Chapple IL. Time to take periodontitis seriously. BMJ Publishing Group. 2014. doi:10.1136/bmj.g2645
19. Chapple IL, Van der Weijden F, Doerfer C, et al. Primary prevention of periodontitis: managing gingivitis. J Clin Periodontol. 2015;42:S71–S76. doi:10.1111/jcpe.12366
20. Petersen PE, Ogawa H. The global burden of periodontal disease: towards integration with chronic disease prevention and control. Periodontol 2000. 2012;60(1):15–39. doi:10.1111/j.1600-0757.2011.00425.x
21. Pihlstrom BL, Michalowicz BS, Johnson NW. Periodontal diseases. lancet. 2005;366(9499):1809–1820. doi:10.1016/S0140-6736(05)67728-8
22. Nazir MA. Prevalence of periodontal disease, its association with systemic diseases and prevention. Int J Health Sci. 2017;11(2):72.
23. Michaud DS, Fu Z, Shi J, Chung M. Periodontal disease, tooth loss, and cancer risk. Epidemiol Rev. 2017;39(1):49–58. doi:10.1093/epirev/mxx006
24. Organization WH. Oral Health Surveys: Basic Methods. World Health Organization; 2013.
25. Shah N, Sundaram KR. Impact of socio-demographic variables, oral hygiene practices and oral habits on periodontal health status of Indian elderly: a community-based study. Indian J Dent Res. 2003;14(4):289–297.
26. Olsson B. Periodontal disease and oral hygiene in Arussi province, Ethiopia. Community Dent Oral Epidemiol. 1978;6(3):139–145. doi:10.1111/j.1600-0528.1978.tb01138.x
27. Silveira MF, Freire RS, Brito MFSF, Martins Ame de B, Marcopito LF. Periodontal condition of adolescents and associated factors. Rev Gaucha Odontol. 2019;67.
28. Ababneh KT, Abu Hwaij ZMF, Khader YS. Prevalence and risk indicators of gingivitis and periodontitis in a multi-centre study in North Jordan: a cross sectional study. BMC Oral Health. 2012;12:1. doi:10.1186/1472-6831-12-1
29. Khader YS, Rice JC, Lefante JJ. Factors associated with periodontal diseases in a dental teaching clinic population in northern Jordan. J Periodontol. 2003;74(11):1610–1617. doi:10.1902/jop.2003.74.11.1610
30. Kumar S, Dagli RJ, Chandrakant D, Prabu D, Suhas K. Periodontal status of green marble mine labourers in Kesariyaji, Rajasthan, India. Oral Health Prev Dent. 2008;6(3):217–221.
31. Ramoji Rao MV, Katari PK, Vegi L, Bypureddy TT, Prabhakara Rao KS, Tejaswi KS. Prevalence of periodontal diseases among rural population of Mustabad, Krishna District. J Int Soc Prev Community Dent. 2016;6(Suppl 1):S59–S63. doi:10.4103/2231-0762.181169
32. Almerich-Silla J-M, Almiñana-Pastor PJ, Boronat-Catalá M, Bellot-Arcís C, Montiel-Company J-M. Socioeconomic factors and severity of periodontal disease in adults (35–44 years). A cross sectional study. J Clin Exp Dent. 2017;9(8):e988.
33. Carasol M, Llodra JC, Fernández-Meseguer A, et al. Periodontal conditions among employed adults in Spain. J Clin Periodontol. 2016;43(7):548–556. doi:10.1111/jcpe.12558
34. Yaegaki K, Sanada K. Biochemical and clinical factors influencing oral malodor in periodontal patients. J Periodontol. 1992;63(9):783–789. doi:10.1902/jop.1992.63.9.783
35. Morita M, Wang HL. Relationship between sulcular sulfide level and oral malodor in subjects with periodontal disease. J Periodontol. 2001;72(1):79–84. doi:10.1902/jop.2001.72.1.79
36. Teshome A, Yitayeh A. Relationship between periodontal disease and preterm low birth weight: systematic review. Pan Afr Med J. 2016;24:1. doi:10.11604/pamj.2016.24.215.8727
37. Winning L, Linden GJ. Periodontitis and systemic disease: association or causality? Curr Oral Health Rep. 2017;4(1):1–7. doi:10.1007/s40496-017-0121-7
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.