Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 12
Perinatal Outcomes in Mexican Women with Untreated Mild Gestational Diabetes Mellitus Diagnosed by the International Association of Diabetes and Pregnancy Study Groups Criteria
Authors Martínez-Cruz N , Rapisarda AMC, Soriano-Ortega KP, Arce-Sánchez L, Cianci A, Ortega-Gonzalez C, Torres-Herrera U, Espino-Y-Sosa S , Estrada-Gutierrez G , Montoya-Estrada A , Romo-Yañez J , Reyes-Muñoz E
Received 3 September 2019
Accepted for publication 13 November 2019
Published 16 December 2019 Volume 2019:12 Pages 2667—2674
DOI https://doi.org/10.2147/DMSO.S229671
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Antonio Brunetti
Nayeli Martínez-Cruz,1 Agnese Maria Chiara Rapisarda,2 Karla Patricia Soriano-Ortega,3 Lidia Arce-Sánchez,1 Antonio Cianci,2 Carlos Ortega-Gonzalez,1 Ursula Torres-Herrera,1 Salvador Espino-Y-Sosa,3 Guadalupe Estrada-Gutierrez,4 Araceli Montoya-Estrada,5 José Romo-Yañez,5 Enrique Reyes-Muñoz5
1Department of Endocrinology, Instituto Nacional de Perinatología “Isidro Espinosa de los Reyes”, Mexico City, México; 2Department of General Surgery and Medical Surgical Specialties, University of Catania, Catania, Italy; 3Division of Clinical Research, Instituto Nacional de Perinatología “Isidro Espinosa de los Reyes”, Mexico City, México; 4Direction of Research, Instituto Nacional de Perinatología “Isidro Espinosa de los Reyes”, Mexico City, México; 5Department of Gynecological and Perinatal Endocrinology, Instituto Nacional de Perinatología “Isidro Espinosa de los Reyes”, Mexico City, México
Correspondence: Enrique Reyes-Muñoz
Department of Gynecological and Perinatal Endocrinology, Instituto Nacional de Perinatología Isidro Espinosa de los Reyes, Montes Urales 800 Lomas Virreyes, Miguel, Hidalgo 11000, Mexico City Mexico
Tel +52 5555209900 Ext 307
Email [email protected]
Purpose: To compare the risk of adverse perinatal outcomes (APO) between pregnant women with mild gestational diabetes mellitus (GDM) diagnosed by the International Association of Diabetes and Pregnancy Study Groups (IADPSG) criteria, on no specific treatment, versus pregnant women without GDM.
Patients and Methods: A retrospective cohort study of pregnant women referred to the Instituto Nacional de Perinatología, in Mexico City, for prenatal care and delivery. Eligibility criteria were singleton pregnancy, age >18 years, gestational age 20–28 weeks, and no history of pre-gestational diabetes. The study population was divided into two groups: Group 1, comprising women with mild GDM defined by one abnormal glucose value at the oral glucose tolerance test (OGTT) according to IADPSG criteria [fasting: 5.1–5.2 mmol/L (92–94 mg/dL) or 2h 8.5–8.56 mmol/L (153–154 mg/dL)], who did not receive specific treatment for GDM, and Group 2, comprising women without GDM, matched for maternal age and pre-gestational body mass index (BMI). Women with two or more abnormal OGTT values, pre-gestational diabetes, any chronic disease, or multiple pregnancies were excluded.
Results: As many as 282 women were included in each group. There were no significant differences in basal characteristics between groups. APO analysis showed that newborn weight was significantly higher in Group 1 (3042.4±499g) vs Group 2 (2910±565g) p=0.003; conversely, the incidence of large for gestational age (LGA) and macrosomic neonates was similar in both groups (6 vs 5.7% and 2.1 vs 2.2%, respectively). There were no differences in rates of preeclampsia and gestational hypertension, cesarean and preterm delivery, or premature rupture of membranes. A sub-analysis by maternal pre-gestational BMI showed that LGA incidence was significantly higher among babies born to women with pre-gestational BMI ≥30 kg/m2 in both groups.
Conclusion: The risk of APO was similar among Mexican women with mild untreated GDM diagnosed by IADPSG criteria, compared to pregnant women without GDM. Pre-gestational BMI was an independent risk factor for LGA.
Keywords: gestational diabetes, large for gestational age, IADPSG, hyperglycemia, pregnancy
Introduction
Gestational diabetes mellitus (GDM) is a state of carbohydrate intolerance initially diagnosed during the second or third trimester of pregnancy, which is not overt diabetes before gestation.1 GDM is associated with increased risk of macrosomia and birth complications, and of maternal diabetes after pregnancy.1–3 The diagnostic criteria for GDM have been controversial for many decades; in fact, the first criteria were based on the risk of the mother to develop future diabetes,4 whereas newly proposed criteria are based on the risk of adverse perinatal outcomes, as reported by the Hyperglycemia and Adverse Pregnancy Outcomes (HAPO) Study.5
The one-step approach recommended by the International Association of Diabetes and Pregnancy Study Groups (IADPSG),6 and supported by the World Health Organization (WHO),7 is to base the diagnosis of GDM on one or more abnormal glucose values during a 75g-2h oral glucose tolerance test (OGTT) with fasting values 5.1–6.9 mmol/L (92–125 mg/dL), 1h ≥10 mmol/L (180 mg/dL) or 2h 8.5–11 mmol/L (153–199 mg/dL) as threshold levels. Using this approach, the incidence of GDM among the 15 centers participating in the HAPO study showed a higher variability from 9.3% to 25.5%.8 A recent systematic review showed that the prevalence of GDM among studies that used the IADPSG criteria was significantly higher (6–11 fold) than other diagnostic criteria subgroups.9 The increase in the number of women with GDM has implications for health services and human and material resources, and could “medicalize” pregnancies previously categorized as normal; consequently, some health services throughout the world, especially in developing countries, may have difficulties meeting the ensuing health-care demands.10,11 There is little clinical evidence of the benefit of treating GDM defined according to the new IADPSG criteria, and systematic reviews and meta-analysis on the benefits or drawbacks of treating GDM12 include studies with different diagnostic criteria, such as the National Diabetes Data Group criteria, WHO 1999’s, and Carpenter and Coustan’s.13–16 Therefore, the efficacy of treating GDM could not be extrapolated to women with lesser degrees of hyperglycemia. Even when treatment has been associated with a lower incidence of preeclampsia, macrosomia, LGA newborns and shoulder dystocia, the risk that these outcomes could be ascribed to GDM is low, mainly when glucose levels are modest high.3,12,17
In this regard, in one study, the prevalence of GDM increased from 10.3% to 30.1% among Mexican women comparing Carpenter and Coustan vs IADPSG criteria.18 In the same study, as many as 66% of GDM cases could be identified using only fasting glucose, and changing the cut-off of fasting levels from 5.3 to 5.1 mmol/L (95 to 92 mg/dL) increased the GDM prevalence by 5.5%; likewise, decreasing the cut-off levels for 2-h OGTT values from 8.6 to 8.5 mmol/L (155 to 153 mg/dL) increased GDM prevalence by 0.8%, posing a problem for the Mexican health-care system.18
In a recent study in Canadian women concluded that women who meet the IADPSG criteria for GDM but who were not diagnosed with GDM based on the two-step diagnostic strategy have an increased risk for adverse perinatal outcomes (APO) compared with women without GDM.19 However, another study in Japanese women reported no significant differences in the rates of GDM-related composite complications between women who were diagnosed with GDM according to the IADPSG criteria, but not by the old criteria (without treatment) and women with standard glucose tolerance according to both criteria.20
Against this background, this study aimed to compare the risk of APO between pregnant women with mild untreated GDM defined by IADPSG criteria versus women without GDM, matched for maternal age and pre-gestational BMI.
Materials and Methods
Research Design and Study Population
This retrospective cohort study was approved by the Internal Review Board of the National Institute of Perinatology (INPer) in Mexico City (Register number 212250-3402-10102-02-14). The study was performed from January 2010 to December 2014, and included women with singleton pregnancies, maternal age >18 years, referred to INPer for prenatal care and delivery. Women with two or more abnormal OGTT values, pre-gestational diabetes, and autoimmune, immunosuppressive, kidney, or heart diseases were excluded from the study. The study population was divided into two groups: Group 1 comprising women with mild GDM defined by one abnormal glucose value during a 75g-OGTT, with fasting levels between 5.1 and 5.2 mmol/L (92–94 mg/dL), 1h ≤10 mmol/L (180 mg/dL) or 2h between 8.5 and 8.56 mmol/L (153–154 mg/dL) according to IADPSG criteria, who were not on any specific treatment (i.e., diet, exercise or antidiabetic medications); Group 2 comprising women without GDM matched 1:1 for maternal age and pre-gestational body mass index (BMI) with Group 1.
Procedures
During the study period, women underwent universal screening for GDM using a one-step approach with a 75g 2h-OGTT on the first visit for prenatal care; the diagnosis of GDM was performed using the Fifth International Workshop-Conference on Gestational Diabetes Mellitus criteria.21 Women with two or more abnormal OGTT values, i.e., fasting ≥ 5.3 mmol/L (95 mg/dL), 1h ≥ 10 mmol/L (180 mg/dL) and 2h ≥ 8.6 mmol/L (155 mg/dL), were diagnosed as having GDM and were prescribed specific treatment, in agreement with the Departments of Nutrition, Maternal-Fetal Medicine, Endocrinology and Obstetrics. Specific treatment for GDM consisted of an educational course, medical nutrition therapy (MNT), self-monitoring of capillary glucose, and subsequent evaluation of glycemic control at 2–4 week intervals. Women who did not achieve glycemic control with MNT were given 1500–2550 mg metformin and/or insulin therapy (0.3–1.0 U/kg of body weight) to achieve capillary glucose levels of 5.3 mmol/L (<95 mg/dL) in the fasting state and <7.8 mmol/L (<140 mg/dL) 1-h post-prandially.
Women who had a single abnormal value were not considered as having GDM and only underwent an educational course with nutritional counseling and were referred to an obstetrician for regular prenatal care.
However, women with one abnormal OGTT value between 5.1 and 5.2 mmol/L (92–94 mg/dL) in the fasting state, ≤10 mmol/L (<180 mg/dL) 1h post-prandially or between 8.5 and 8.56 mmol/L (153–154mg/dL) 2h post-prandially were considered as having normal OGTT and continued their regular obstetric care. Data regarding these mothers and their neonates were collected from clinical records.
Outcome Variables
The primary study outcome was to compare APO risk between mild GDM women and no-GDM women. For this study, the APOs considered were LGA newborn—defined as birth weight at or above the 90th percentile of the gestational age-specific week.22 Macrosomia was defined as a newborn with birth weight above 4000 g. Preeclampsia was ascertained as hypertension associated with proteinuria after the 20th gestational week, following the National High Blood Pressure Education Program Working Group recommendations.23 Preterm delivery was defined as any birth between 20 and 36.6 gestational weeks and premature rupture of membranes as water breaking before the onset of labor.24
Pre-gestational BMI was calculated according to self-reported pre-gestational weight during the first clinical interview and was classified as normal (BMI ≤25 kg/m2) or obese (BMI ≥30 kg/m2) for analysis.
Sample Size
The sample size was calculated based on a 12% prevalence of LGA newborns in women with mild untreated GDM; considering a 6% incidence of LGA in our population without GDM, an alpha of 0.05, an 80% power and a one-sided test, a sample size of 281 women per group was required.
Statistical Analysis
Continuous variables were expressed as mean ± standard deviation, and categorical variables were expressed as frequencies and proportions. Student’s t- or Mann–Whitney U-tests were used to compare continuous variables according to the variable distribution, and the chi-square test or Fisher’s exact test were used to evaluate differences in proportions. Statistical significance was considered if p ≤ 0.05. Logistic regression was used to calculate the odds ratio (OR) for APO with 95% confidence intervals (CI). Pearson’s correlation analysis was used to assess a linear association between birth weight and other continuous variables. We used the Statistical Package for Social Science Software to conduct data analysis (SPSS 15, Chicago, IL, USA).
Results
During the study period, 320 women fulfilled the criteria of mild GDM; 38 of them were excluded for comorbidities. The 282 women with mild GDM who fulfilled the inclusion criteria (group 1) were matched for maternal age and pre-gestational BMI, with as many women without GDM (group 2). The characteristics of the participants are shown in Table 1. There was no difference in terms of maternal age, weight, and BMI at admission to prenatal care, parity, gestational age at OGTT and pre-gestational BMI at the time of enrollment. The mean of three OGTT values was significantly higher in group 1 vs group 2 (p = 0.0001).
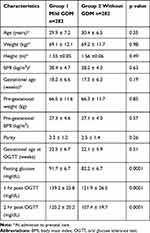 |
Table 1 Clinical Characteristics at Enrollment of Mexican Women with and Without Mild Gestational Diabetes Mellitus (GDM) |
For the diagnosis of mild GDM (group 1), a fasting glucose value was the most frequently abnormal value in 90.7% of the women, with only 9.3% having abnormal 2h glucose values.
As to personal history, the group with mild GDM had a higher frequency of family history of diabetes, abortion, and infertility (Table 2).
 |
Table 2 Personal History of Mexican Women with and Without Mild Gestational Diabetes Mellitus (GDM) |
Among perinatal outcomes (Table 3), gestational ages at delivery were similar in both groups, 38.3±2.3 and 38.5±2.4 for groups 1 and 2, respectively. Birthweight was significantly higher in babies born to women in group 1 with mild GDM (3042.4 ± 499 gr vs 2910 ± 565 gr, p=0.003), whereas the frequency of LGA and macrosomia was similar in both groups (6 vs 5.7% and 2.1 vs 2.2%, respectively). No differences were found in the frequency of other adverse perinatal outcomes such as preeclampsia, gestational hypertension, cesarean delivery, preterm delivery, or premature rupture of membranes.
 |
Table 3 Perinatal Outcomes of Mexican Women with and Without Mild Gestational Diabetes Mellitus (GDM) |
As many as 27% of the women in group 1 presented with pre-gestational obesity (BMI ≥30 kg/m2) compared with 25% in group 2. In a sub-group analysis by maternal pre-gestational BMI (Table 4), the frequency of LGA was significantly higher when the mother presented with pre-gestational BMI ≥30 kg/m2 in both groups, 4.5 vs 13.6% p = 0.01 OR: 3.31 (95% CI 1.22─9.00) in the group with mild GDM and 4.1 vs 12.3% p = 0.02 OR: 3.24 (95% CI 1.08─9.68) in the no-GDM group but no differences were found in the frequency of macrosomic newborns. Only in the group with mild GDM did pre-gestational BMI ≥30 kg/m2 increase the prevalence of cesarean delivery 69.3% vs 84.8% p = 0.01 OR: 2.47 (95% CI 1.17─5.22).
 |
Table 4 Perinatal Outcomes by Maternal Pre-Gestational BMI of Mexican Women with and Without Mild Gestational Diabetes Mellitus (GDM) |
A correlation analysis was performed between newborn’s birth weight, and the three biochemical parameters examined, i.e., fasting, 1h, and 2h OGTT, and clinical parameters such as maternal weight and pre-gestational BMI, using a Bonferroni correction to control for risk of type 1 error; a p-value of <0.05 was considered as significant. The results showed a correlation between newborn weight and fasting glucose with r=0.132 (p=0.002), and between maternal pre-gestational weight and BMI with r=0.235 (p=0.0001) and r= 0.124 (p=0.007), respectively.
Discussion
The results of this retrospective cohort study do not show significant differences in the risk of LGA or macrosomia in babies born to women with mild untreated GDM compared to those born to women without GDM; however, birthweight was significantly higher in babies born to women with mild GDM. There were no differences in the risk of adverse perinatal outcomes such as preeclampsia, gestational hypertension, cesarean delivery, preterm delivery, or premature rupture of membranes.
The perinatal outcomes analyzed by maternal pre-gestational BMI showed that the frequency of LGA was significantly higher in both groups when maternal pre-gestational BMI was ≥30 kg/m2; this finding is in agreement with the correlation analysis showing a positive correlation between maternal pre-gestational weight and BMI, and neonatal birth weight. These findings are in accordance with those from other authors who demonstrated that maternal pre-gestational BMI and gestational weight gain are more closely associated in women diagnosed with GDM at lower glucose thresholds, and both maternal GDM and obesity are independently associated with adverse pregnancy outcomes.25,26
Therefore, these results show that there is no higher frequency of APO in women with mild GDM, not undergoing any specific treatment (diet, exercise, or antidiabetic medications). The condition is defined according to the IADPSG criteria by one abnormal value at a 75g OGTT, with fasting levels between 5.1 and 5.2 mmol/L (92–94 mg/dL) or 2h post load between 8.5 and 8.56 mmol/L (153–154 mg/dL), allowing these women to undergo regular obstetric follow-up.
One of the strengths of our study is the inclusion of women matched 1:1 for maternal age and pre-gestational BMI, which rules out the possible effect of the main confounding factors on the incidence of APO. In addition to its retrospective design, the main limitation of our study is the inclusion of only women with a limited spectrum of hyperglycemic episodes. We defined this condition as “mild” GDM, to identify a group of women diagnosed with GDM according to new IADPSG criteria, which set lower levels of hyperglycemia than those defined by Carpenter and Coustan. This might make these findings specific for this subgroup of women; indeed, we should consider that over 60% of our population is at high risk for GDM because of ethnicity, 80% is overweight or obese, and 50% has a family history for type 2 diabetes. Considering these epidemiological cornerstones, in our setting, women undergo the first prenatal visit before the 20th gestational week—and OGTT is requested as a baseline evaluation. Besides, this population underwent OGTT between the 24th and the 28th gestational week again, following the IADPSG recommendations.
Other limitations of this study were that each participant reported pregestational weight. However, the BMI at admission to prenatal care was similar in both groups that could decrease this possible bias. Finally, in this study, we did not evaluate the efficacy of the treatment of women with mild GDM on the incidence of APO. Randomized clinical trials are required for it.
In 2013, a WHO panel recommended the use of IADPSG cut-off levels; however, the level of these recommendations was graded as weak, and the quality of the evidence very low.7 Other authors suggested some modifications in different settings, for instance, when health services cannot reasonably be expected to handle the higher number of GDM cases that would be detected by the IADPSG criteria.10
On the other hand, the possible problems of overdiagnosing GDM could be more intensive surveillance during pregnancy and a higher overall number of primary cesarean deliveries, diagnosis and management, and more significant maternal anxiety.27,28 As for cost-effectiveness, the information available is still limited; in fact, two studies reported that screening using IADPSG criteria was cost-effective only when the long-term benefits of preventing diabetes development in the mother were considered, but not for the prevention of major perinatal outcomes.29,30
In this scenario, GDM diagnostic criteria in Mexican women with “mild GDM” may be re-evaluated, and women with only one abnormal value could be considered just as being “at risk” of developing GDM.
Although a mild increase in glucose levels of Mexican pregnant women did not increase the risk of giving birth to LGA or macrosomic newborns, more prospective and randomized clinical trials are required to corroborate these findings. Likewise, studies to assess the long-term risk of women with “mild GDM” are needed to predict the risk of developing DM2 and other cardiovascular comorbidities, given the available evidence that women with GDM diagnosed by Carpenter and Coustan’s criterion have a seven-fold higher risk to develop DM2 compared with those who experience a normoglycemic pregnancy (RR 7.43, 95% CI 4.79–11.51).31
Conclusion
The risk of APO was similar among Mexican women with mild untreated GDM diagnosed by IADPSG criteria, compared to pregnant women without GDM. Pre-gestational BMI > 30 kg/m2 was an independent risk factor for LGA.
Acknowledgments
The abstract of this paper was presented at the 20th European Congress of Endocrinology as a poster presentation with interim findings. The poster’s abstract was published in “Poster Abstracts” in Endocrine Abstracts (2018) 56 P481 | DOI: 10.1530/endoabs.56.P481. Also, the abstract of this paper was presented at the 2018 Annual Clinical and Scientific Meeting of The American College of Obstetricians and Gynecologists as a poster presentation with interim findings. The poster’s abstract was published in “Poster Abstracts” in Obstetrics & Gynecology. 2018;131:162S DOI:10.1097/01.AOG.0000533135.61739.1b
We are grateful to Instituto Nacional de Perinatología in Mexico City.
Ethics and Consent
The study was approved by the Internal Review Board of the National Institute of Perinatology (INPer) in Mexico City (Register number 212250-3402-10102-02-14). Written informed consent from participants is not required by the Internal Review Board at our institution for retrospective studies, because the data used in this study were obtained from medical records and information related to privacy was not extracted. All the patients at admission to our Institution gave their written consent to use medical records data for research studies. This study has been performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments.
Disclosure
Dr Enrique Reyes-Muñoz reports grants from National Council of Science and Technology, CONACYT, Mexico, during the conduct of the study. The authors have no other conflicts of interest to declare.
References
1. American Diabetes Association. Classification and diagnosis of diabetes: standards of medical care in diabetes—2019. Diabetes Care. 2019;42:S13–S28. doi:10.2337/dc19-S002
2. Budak MS, Kahramanoglu I, Vitale SG, et al. Maternal abdominal subcutaneous fat thickness as a simple predictor for gestational diabetes mellitus. J Perinat Med. 2019;47:605–610. doi:10.1515/jpm-2018-0431
3. Salman H, Shah M, Ali A, et al. Assessment of relationship of serum neurokinin-B level in the pathophysiology of pre-eclampsia: a case-control study. Adv Ther. 2018;35:1114–1121. doi:10.1007/s12325-018-0723-z
4. O’Sullivan JB, Mahan CM. Criteria for the oral glucose tolerance test in pregnancy. Diabetes. 1964;13:278–285.
5. HAPO Study Cooperative Research Group. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008;358:1991–2002.
6. Metzger BE, Gabbe SG, Persson B, et al. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care. 2010;33:676–682. doi:10.2337/dc10-0719
7. Diagnostic Criteria and Classification of Hyperglycaemia First Detected in Pregnancy. Geneva: World Health Organization; 2013.
8. Sacks DA, Hadden DR, Maresh M, et al. Frequency of gestational diabetes mellitus at collaborating centers based on IADPSG consensus panel recommended criteria: the Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study. Diabetes Care. 2012;35:526–528. doi:10.2337/dc11-1641
9. Behboudi-Gandevani S, Amiri M, Bidhendi Yarandi R, et al. The impact of diagnostic criteria for gestational diabetes on its prevalence: a systematic review and meta-analysis. Diabetol Metab Syndr. 2019;11:11. doi:10.1186/s13098-019-0406-1
10. Colagiuri S, Falavigna M, Agarwal MM, et al. Strategies for implementing the WHO diagnostic criteria and classification of hyperglycaemia first detected in pregnancy. Diabetes Res Clin Pract. 2014;103:364–372. doi:10.1016/j.diabres.2014.02.012
11. Sosa-Rubi SG, Dainelli L, Silva-Zolezzi I, et al. Short-term health and economic burden of gestational diabetes mellitus in Mexico: a modeling study. Diabetes Res Clin Pract. 2019;153:114–124. doi:10.1016/j.diabres.2019.05.014
12. Hartling L, Dryden DM, Guthrie A, et al. Benefits and harms of treating gestational diabetes mellitus: a systematic review and meta-analysis for the U.S. Preventive Services Task Force and the National Institutes of Health Office of Medical Applications of Research. Ann Intern Med. 2013;16:123–129. doi:10.7326/0003-4819-159-2-201307160-00661
13. Crowther CA, Hiller JE, Moss JR, et al. Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. N Engl J Med. 2005;352:2477–2486. doi:10.1056/NEJMoa042973
14. Landon MB, Spong CY, Thom E, et al. A multicenter, randomized trial of treatment for mild gestational diabetes. N Engl J Med. 2009;361:1339–1348. doi:10.1056/NEJMoa0902430
15. Bonomo M, Corica D, Mion E, et al. Evaluating the therapeutic approach in pregnancy complicated by borderline glucose intolerance a randomized clinical trial. Diabet Med. 2005;22:1536–1541. doi:10.1111/j.1464-5491.2005.01690.x
16. Garner P, Okun N, Keely E, et al. A randomized controlled trial of strict glycemic control and tertiary level obstetric care versus routine obstetric care in the management of gestational diabetes: a pilot study. Am J Obstet Gynecol. 1997;177:190–195. doi:10.1016/S0002-9378(97)70461-7
17. Laganà AS, Vitale SG, Sapia F, et al. miRNA expression for early diagnosis of preeclampsia onset: hope or hype? J Matern Fetal Neonatal Med. 2018;31:817–821. doi:10.1080/14767058.2017.1296426
18. Reyes Muñoz E, Parra A, Castillo Mora A, et al. Effect of the diagnostic criteria of the International Association of Diabetes and Pregnancy Study Groups on the prevalence of gestational diabetes mellitus in urban Mexican women: a cross-sectional study. Endocr Pract. 2012;18:146–151. doi:10.4158/EP11167.OR
19. Shah BR, Sharifi F. Perinatal outcomes for untreated women with gestational diabetes by IADPSG criteria: a population-based study. BJOG. 2019. doi:10.1111/1471-0528.15964.
20. Shindo R, Aoki S, Kasai J, et al. Impact of introducing the International Association of Diabetes and Pregnancy Study Groups (IADPSG) criteria on pregnancy outcomes in Japan. Endocr J. 2019. doi:10.1507/endocrj.EJ19-0279.
21. Proceedings of the 4th International Workshop-Conference on Gestational Diabetes Mellitus. Chicago, Illinois, USA. 14-16 March 1997. Diabetes Care. 1998;21:B1–B16.
22. Flores-Huerta S, Martínez-Salgado H. Birth weight of male and female infants born in hospitals affiliated with the Instituto Mexicano del Seguro Social. Bol Med Hosp Infant Mex. 2012;69:
23. National high blood pressure education program working group on high blood pressure in pregnancy. Am J Obstet Gynecol. 2000;183:
24. American College of Obstetricians and Gynecologists; Committee on Practice Bulletins‒ Obstetrics. ACOG practice bulletin no. 127: management of preterm labor. Obstet Gynecol. 2012;119:
25. Black MH, Sacks DA, Xiang AH, et al. The relative contribution of prepregnancy overweight and obesity, gestational weight gain, and IADPSG defined gestational diabetes mellitus to fetal overgrowth. Diabetes Care. 2013;36:56–62. doi:10.2337/dc12-0741
26. Catalano PM, McIntyre HD, Cruickshank JK; HAPO Study Cooperative Research Group. et al. The hyperglycemia and adverse pregnancy outcome study: associations of GDM and obesity with pregnancy outcomes. Diabetes Care. 2012;35:780–786. doi:10.2337/dc11-1790
27. Feig DS, Chen E, Naylor CD. Self-perceived health status of women three to five years after the diagnosis of gestational diabetes: a survey of cases and matched controls. Am J Obstet Gynecol. 1998;178:386–393. doi:10.1016/S0002-9378(98)80030-6
28. Rumbold AR, Crowther CA. Women’s experiences of being screened for gestational diabetes mellitus. Aust N Z J Obstet Gynaecol. 2002;42:131–137. doi:10.1111/ajo.2002.42.issue-2
29. Mission JF, Mika S, Ohno MS, et al. Gestational diabetes screening with the new IADPSG guidelines: a cost-effectiveness analysis. Obstet Gynecol. 2012;207:e1–e9.
30. Werner EF, Pettker CM, Zuckerwise L, et al. Screening for gestational diabetes mellitus: are the criteria proposed by the International Association of the diabetes and pregnancy study groups cost-effective? Diabetes Care. 2012;35:529–535. doi:10.2337/dc11-1643
31. Bellamy L, Casas JP, Hingorani AD, et al. Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta-analysis. Lancet. 2009;373:1773–1779. doi:10.1016/S0140-6736(09)60731-5
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
